Abstract
Severe fever with thrombocytopenia syndrome (SFTS) is a newly emerging and epidemic infectious disease in central and northeast China. It is caused by New Bunyavirus and carries an average 12% case fatality rate. Early and rapid detection is critical for prevention and control of New Bunyavirus infection, since no vaccine or antiviral drugs are currently available, and prevention requires careful attention to control of the suspected tick vector. In this study, a simple and sensitive reverse transcription–loop-mediated isothermal amplification (RT-LAMP) assay was developed for rapid detection of New Bunyavirus. The detection limit of the RT-LAMP assay was approximately 103 50% tissue culture infective doses/ml of New Bunyavirus in culture supernatants, and no cross-reactive amplification of other viruses known to cause similar clinical manifestations was observed. The assay was further evaluated using 138 specimens from clinically suspected SFTS and 40 laboratory-proven hantavirus infection with fever and renal syndrome patients, and the assay exhibited 97% agreement compared to real-time RT-PCR and conventional RT-PCR. Using real-time RT-PCR as the diagnostic gold standard, RT-LAMP was 99% sensitive and 100% specific. The RT-LAMP assay could become a useful alternative in clinical diagnosis of SFTS caused by New Bunyavirus, especially in resource-limited hospitals or rural clinics of China.
INTRODUCTION
Since 2007, many patients in China have presented with an illness with clinical characteristics that include acute onset of fever with leukopenia, thrombocytopenia, elevated serum hepatic enzyme levels, gastrointestinal manifestations, neurological symptoms, and bleeding tendencies. The disease was initially suspected to be human granulocytic anaplasmosis (HGA) (1), but in 2009, difficulty in proving that etiology prompted syndromic characterization of the patients as having “severe fever with thrombocytopenia syndrome” (SFTS), while other potential etiologies were investigated (2). A novel bunyavirus was isolated from patient acute-phase serum samples and is now known as New Bunyavirus (NBV), severe fever with thrombocytopenia syndrome bunyavirus (SFTSV), or Huaiyangshan virus (HYSV) (1–3).
As the clinical manifestations of SFTS are nonspecific, there is a need to develop assays that can differentiate this from various other infectious diseases, in particular, hemorrhagic fever with renal syndrome (HFRS) and HGA. Reliable rapid and accurate diagnostic tests are urgently required to identify and diagnose clinically suspected SFTS, to assess treatment efficacy, and for disease surveillance (4).
Recently, a novel DNA amplification method called loop-mediated isothermal amplification (LAMP) has been applied for diagnosis of infectious diseases (5). The addition of reverse transcriptase makes it possible to amplify RNA sequences by LAMP (reverse transcription–loop-mediated isothermal amplification, or RT-LAMP). RT-LAMP has already been applied to the detection of several RNA viruses, such as enterovirus 71 (EV71), swine-origin influenza A H1N1 virus (S-OIV), severe acute respiratory syndrome coronavirus (SARS-CoV), and most recently, SFTS virus (NBV) (6–9). In this study, we sought to confirm and extend findings for RT-LAMP as applied to NBV and to determine its diagnostic performance in clinical specimens from Henan Province.
MATERIALS AND METHODS
Virus strains and clinical samples.
Five NBV strains (HN01, HN20, YXX1, WZ69, and WZ87) isolated from NBV-infected patients, and the other viruses (Hantan virus [ZT10], Xinjiang hemorrhagic fever virus [BC04 strain], dengue virus I [GX43 strain], Japanese encephalitis virus [HW strain], and yellow fever virus [17D-204 strain]) that cause similar symptoms were used in this study. The titers of all viruses were determined based on the 50% tissue culture infective dose (TCID50).
Patients with SFTS were clinically diagnosed based on the criteria described by Xu et al. (1). Briefly, the epidemiological contact history included whether patients had a recent tick bite, worked in the mountains or the forest, or had had direct contact with the blood of patients with SFTS in the prior 2 weeks. Clinical manifestations included fever, headache, muscle aches, nausea, vomiting, diarrhea, skin bruising, bleeding, multiple organ injury, and disseminated intravascular coagulation. Laboratory tests included evaluation for leukopenia and thrombocytopenia. Patients with the above-described clinical presentation were categorized as SFTS and confirmed infected when NBV RNA was identified in acute-phase serum by a real-time RT-PCR assay.
A total of 138 acute-phase serum samples were collected in 2012 from patients in Henan province clinically diagnosed with SFTS. All 138 acute-phase serum samples were tested for HGA by indirect immunofluorescence serological assay (IFA) (1). Acute-phase serum samples from 40 HFRS patients, all confirmed by using the hantavirus IgG/IgM Combo test card (catalog number 1N03C2; Boson Biotech Co., Ltd.), were also included to evaluate specificity.
These virus strains and clinical samples were used to validate the RT-LAMP method. This research was approved by the Review Board of the Center for Disease Control and Prevention of Henan Province. All participants gave written informed consent for use of their samples in research.
RNA extraction.
Total RNA was extracted from 140-μl aliquots of viral culture supernatants or patient acute-phase serum samples by using the QIAamp viral RNA minikit (Qiagen, Germany), according to the instructions of the manufacturer. The RNA was eluted into a final volume of 60 μl of elution buffer and used immediately or stored at −80°C.
Design of RT-LAMP primers.
The primers for RT-LAMP amplification of NBV were designed to target the S segment sequence of the Henan 01 virus (GenBank accession number HQ642768). Primers F3, B3, FIP, BIP, FLP, and BLP were designed with the Primer Explorer version 4 software (http://primerexplorer.jp/elamp4.0.0/index.html; Eiken Chemical Co., Japan). The feasibility of all sets of primers was validated by BLAST analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and then empirically tested with different NBV isolates from China. The primer sequences are shown in Table 1.
TABLE 1.
RT-LAMP primer sequences used for detection of New Bunyavirus
| Primer | Sequence (5′–3′) | Length (bp) |
|---|---|---|
| F3 | GCAAGATGCCTTCACCAAGA | 20 |
| B3 | TGCTGCTTACAGGTTTCTGT | 20 |
| FIP | AGCATGGAGAGGGTCCCTGAAGTCAATGTGAAGATGCGTGGA | 42 |
| BIP | TGTTCGGGTGAAGTGGCTGAAGCAACCTCAGCAGCTCTG | 39 |
| FLP | AGTTATAAACTTCTGTCTTGCTGGC | 25 |
| BLP | CTTGGCCCAGATGGGGTTCC | 20 |
Optimization of RT-LAMP reaction conditions.
The RT-LAMP reaction mixture was prepared by using a Loopamp RNA amplification kit (Eiken Chemical Co., Ltd., Tokyo, Japan) in accordance with the manufacturer's instructions (10). Each 25-μl reaction mixture contained 12.5 μl of 2× reaction mix buffer, 0.2 μM (each) two outer primers (F3 and B3), 1.6 μM (each) two inner primers (FIP and BIP), 0.8 μM (each) two loop primers (FLP and BLP), 1 μl of enzyme mix, 1 μl of fluorescent detection reagent (FDR; Eiken Chemical Co., Ltd., Tokyo, Japan), and 5 μl of sample RNA. The reaction mixtures were incubated at 63°C for 40 min. A positive reaction was identified when the color of the reaction mixture changed from dark yellow to bright green; an unchanged dark yellow color indicated a negative reaction. This color change can be observed by the naked eye under natural light or with the aid of UV light. The reaction products were also analyzed by electrophoresis through 1.5% agarose gels.
Conventional RT-PCR and real-time RT-PCR.
Conventional RT-PCR was conducted using the Quant One Step RT-PCR kit (Tiangen Biotech, Beijing, Co., Ltd.) and primers (Stest-F1, 5′-ATGTCAGAGTGGTCCAGGATT-3′; Stest-R1, 5′-AAGGATTCCCTTGGCCTTCA-3′) as described previously (3). The RT-PCR mixtures contained 10 μl of 5× One Step RT-PCR buffer, 4 μl of One Step enzyme mix, 2 μl of Stest-F1 primer (10 μM), 2 μl of Stest-R1 primer (10 μM), 5 μl of RNA template, and RNase-free double-distilled water for a total volume of 50 μl. PCR amplification was conducted using a MyCyclerTM thermal cycler (Bio-Rad Laboratories, Inc.). The reaction mixtures were incubated at 50°C for 30 min, heated at 95°C for 15 min, and subjected to 35 cycles of 94°C for 30 s, 54°C for 30 s, and 72°C for 40 s, with a final extension at 72°C for 10 min. The RT-PCR products were analyzed by 2% agarose gel electrophoresis.
The real-time RT-PCR assay was performed using primers (S3-137F, 5′-ATGGATGCAAGGTCCAGAAT-3′; S3-268R, 5′-CTTCCTCAGAGCTGCTTGCT-3′), probe (C437-PROBE2, 5′-TGCCGCTGAGCAATTTCTCTCTCCA-3′) (11), and the Qiagen QuantiTect probe RT-PCR kit (Qiagen, Germany). The real-time RT-PCR conditions included 50°C for 30 s, 95°C for 15 min, and 45 cycles of 95°C for 15 s and 60°C for 45 s. Data analysis was conducted with the software supplied by the manufacturer.
Analytical sensitivity of New Bunyavirus RT-LAMP, conventional RT-PCR, and real-time RT-PCR.
Titers for culture supernatants of NBV strain Henan 01-infected Vero cells were determined, and the TCID50 was determined as described previously (12).Virus preparations were diluted 10-fold from 107 to 101 TCID50/ml, and genomic RNA was extracted from 140 μl of diluted viral preparation by using the QIAamp viral RNA kit (Qiagen, Germany) according to the manufacturer's instructions. Ten-fold serial dilutions of RNA were prepared to determine the analytical sensitivities of the RT-LAMP, conventional RT-PCR, and real-time RT-PCR assays.
Analytical specificity of RT-LAMP.
The specificity and potential cross-reactions of NBV RT-LAMP primers were examined by using RNA extracted from 5 different NBV strains and other viruses genetically related to NBV or those that typically elicit similar clinical manifestations.
Isolation of virus with Vero E6 cells.
All 138 patient acute-phase serum samples were inoculated onto Vero E6 cells. Briefly, 100 μl of serum was inoculated onto cell monolayers in 25-cm2 flasks and incubated for 14 days at 37°C, 5% CO2 in minimal essential medium (MEM)–2% fetal calf serum with a medium change after day 7. All cultures were monitored daily for cytopathic effect (CPE). Each sample underwent at least 3 cell culture passages in Vero E6 cells before being considered negative. Virus-infected cells and uninfected cells were also examined for NBV by real-time RT-PCR assay at each passage.
Evaluation of the RT-LAMP assay using clinical samples.
To evaluate the reliability of the RT-LAMP assay, acute-phase serum samples from 138 patients who met the clinical definition for SFTV and serum samples from 40 HFRS patients were collected and subjected to RT-LAMP, conventional RT-PCR, and real-time RT-PCR in parallel assays, along with reference negative and positive controls. The rate of detection in RT-LAMP assays was compared with that in conventional and real-time RT-PCR assays and also to the detection rate with in vitro viral cultivation.
Statistical analysis.
The statistical significance of the clinical study results were evaluated by using the χ2 test. A significant difference was considered when the P value was less than 0.05.
RESULTS
Identification of RT-LAMP amplification products.
RT-LAMP amplification products identified by electrophoresis yielded a ladder-like pattern characteristic of stem-loop structures (Fig. 1C). After adding FDR dye, amplification products exhibited a bright green color (Fig. 1A) and green fluorescence under UV light (Fig. 1B); however, negative control samples remained unchanged, had a dark yellow color with FDR, and did not fluoresce. Fluorescence detection results were consistent with the results of agarose gel electrophoresis.
FIG 1.
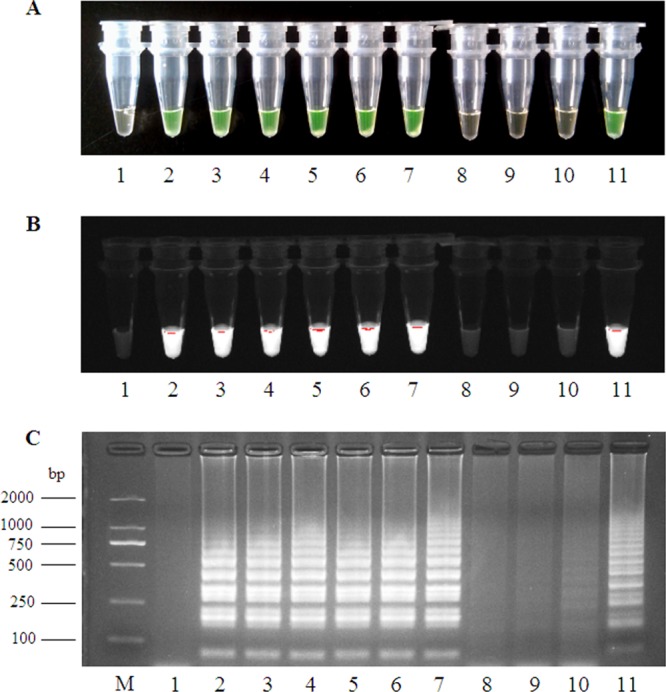
RT-LAMP products detected by visual observation (A), UV light (B), and agarose gel electrophoresis (C). Lane 1, negative control; lanes 2 to 4, different New Bunyavirus strains; lanes 5 to 7, positive samples; lanes 8 to 10, negative samples; lane 11, positive control; lane M, DL2000 molecular size marker.
RT-LAMP analytical sensitivity and specificity.
Using RNA from 10-fold serial dilutions of virus preparations, the lower limit of RT-LAMP detection was approximately 103 TCID50/ml, while conventional RT-PCR could only amplify 104 TCID50/ml and real-time RT-PCR could amplify 101 TCID50/ml (Fig. 2). Therefore, the analytical sensitivity of the RT-LAMP assay was less than that of real-time RT-PCR but 10-fold higher than that of conventional RT-PCR. During the evaluation for specificity, all five NBV strains were amplified by RT-LAMP while no other virus strains were detected, indicating that the RT-LAMP assay for NBV has a high level of analytical specificity.
FIG 2.
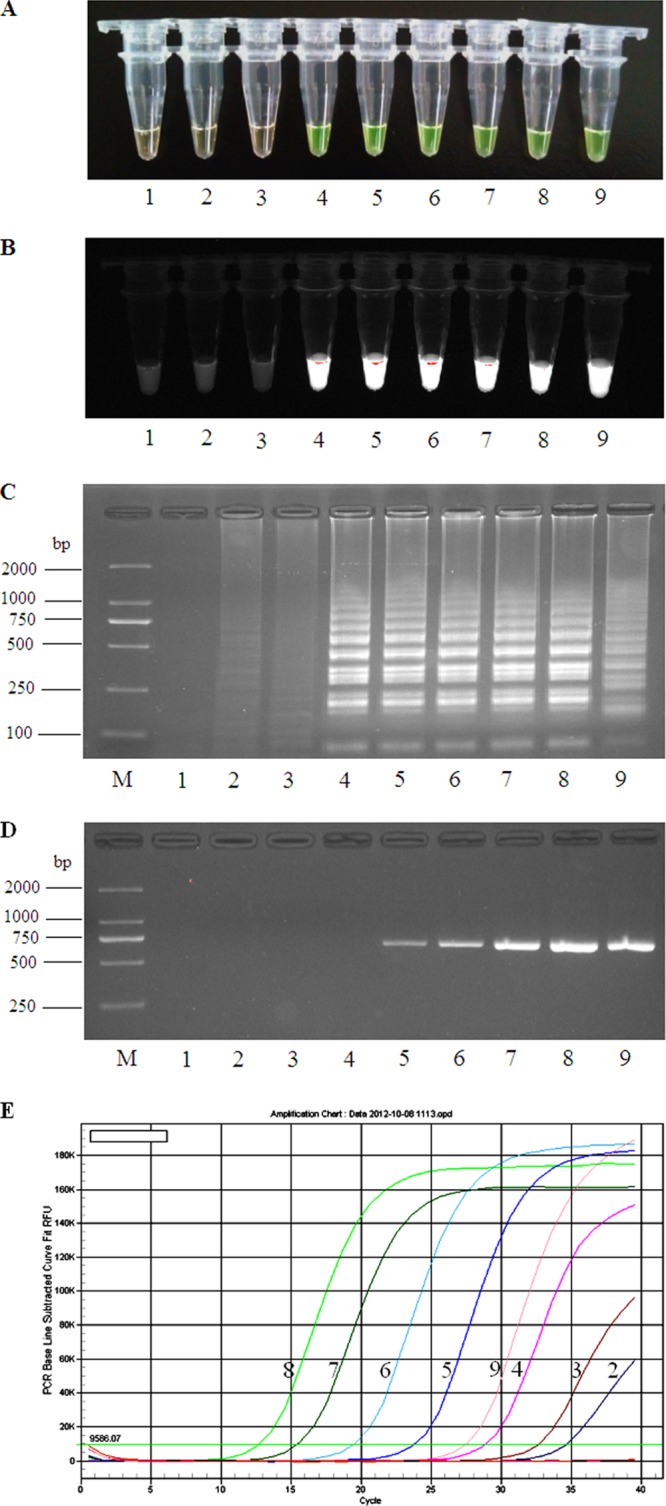
Detection sensitivity of RT-LAMP, RT-PCR, and real-time RT-PCR for New Bunyavirus. RT-LAMP products from virus serial dilutions were visualized by adding FDR dye under visible light (A), UV light (B), and by agarose gel electrophoresis (C). RT-PCR products were evaluated by agarose gel electrophoresis (D) and real-time RT-PCR amplification (E). Lane 1, negative control; lanes 2 to 8, NBV samples from 101 to 107 TCID50/ml serial dilutions; lane 9, positive control; lane M, DL2000 molecular size marker.
Virus isolation.
A total of 138 acute-phase serum samples from SFTS patients and 40 serum samples from HFRS patients were inoculated onto Vero E6 cells. At 2 to 4 days after inoculation, Vero E6 cells showed rounded refractile changes in many of the cell culture flasks, but CPE did not progress in the initial cultures. Slight virus-induced cellular changes were observed at 24 h in subsequent passages. After 3 cell culture passages in Vero E6 cells, 81 virus strains were isolated from the acute-phase sera of SFTS patients, and all were confirmed by real-time RT-PCR. The NBV-positive isolation rate was 58.7% for SFTS samples. No NBV strains were isolated from serum samples from HFRS patients.
Molecular detection of New Bunyavirus in clinical samples.
Of the 138 samples from clinical SFTS patients, 113 (82%) were positive by real-time RT-PCR, 110 (79.71%) were positive by conventional RT-PCR, and 112 (81%) were positive by RT-LAMP. All 3 molecular assays were 100% specific when tested using serum samples from 40 confirmed HFRS patients (Table 2). However, the clinical definition of SFTS was intended to have high sensitivity but not necessarily high specificity, and culture was expected to have high specificity but not necessarily high sensitivity. Thus, RT-LAMP and conventional RT-PCR assays were tested in parallel on the 138 acute-phase serum specimens from SFTS patients as well as 40 acute-phase sera from patients with HFRS and compared to real-time RT-PCR as the diagnostic gold standard. The overall agreement among the 3 molecular assays was high at 97% (P > 0.05).
TABLE 2.
Comparison of detection of New Bunyavirus from clinical specimens by RT-LAMP, RT-PCR, and real-time RT-PCR assaya
| Serum group | Result | No. of samples with indicated result by: |
|||
|---|---|---|---|---|---|
| RT-LAMP | RT-PCR | Real-time RT-PCR | New Bunyavirus isolation | ||
| SFTS patients | Positive | 112 | 110 | 113 | 81 |
| Negative | 26 | 28 | 25 | 57 | |
| Total | 138 | 138 | 138 | 138 | |
| HFRS patients | Positive | 0 | 0 | 0 | 0 |
| Negative | 40 | 40 | 40 | 40 | |
| Total | 40 | 40 | 40 | 40 | |
The chi-square test for the comparison of the four groups resulted in χ2 of 27.6591 and P < 0.0001. In comparison with the real-time RT-PCR assay, the RT-LAMP exhibited a sensitivity of 99% and a specificity of 100%.
None of the conventional RT-PCR-positive samples was negative by RT-LAMP or real-time RT-PCR, while 2 samples that were negative by conventional RT-PCR were positive by RT-LAMP. One sample found positive by real-time RT-PCR was negative with RT-LAMP; no sample negative by real-time RT-PCR tested positive by RT-LAMP assay. The 3 discrepant samples were verified as NBV infection by virus culture and IFA (1, 13). Compared with real-time RT-PCR, the sensitivity of RT-LAMP was 99% (95% confidence interval [CI], 95 to 100%) and specificity was 100% (95% CI, 84 to 100%). Similarly, compared to real time RT-PCR, the gold standard, conventional RT-PCR sensitivity and specificity were 98% (95% CI, 93 to 100%) and 100% (95% CI, 84 to 100%), respectively. As anticipated, among those samples with positive virus cultures (as gold standard), the sensitivity of RT-LAMP was high at 99% (95% CI, 96 to 100%); however, if a positive real-time RT-PCR or RT-LAMP result was considered the gold standard, culture was only 71.7% and 74.6% sensitive, respectively.
Of 138 suspected SFTS samples, 32 were negative by both culture and molecular tests. In our prior studies (1), only 8.4% of clinically suspected SFTS patients were proven to have HGA. If this rate were applied to this cohort, that would represent about 11 to 12 patients likely to have HGA. However, none of the acute-phase sera from any of the 138 patients contained Anaplasma phagocytophilum antibodies, including those that were culture and molecular test negative for NBV. While not definitive, since tests were conducted on acute-phase samples that could lack antibody, these data provided at least indirect evidence that the RT-LAMP assay for NBV did not separately amplify A. phagocytophilum bacterial RNA that could be present in the serum and that it is specific.
DISCUSSION
Severe fever with thrombocytopenia syndrome is a serious infection, with a 12% case fatality rate documented in 6 rural provinces in central and northeast China (14, 15). It is caused by a novel bunyavirus, a type of single-stranded RNA virus and a new member of Bunyaviridae family belonging to the genus Phlebovirus and called New Bunyavirus, SFTSV, or HYSV. Owing to the high case fatality rate and its occurrence in clustered outbreaks related to tick activity, early and rapid identification is needed to prescribe appropriate supportive care and to implement public health measures. Thus, we and others have developed diagnostic methods, including molecular tools, most of which are amenable only to large, high-technology laboratories (1–4). The development of isothermal LAMP assays offers promise for a molecular diagnostic method that could be more generally available to laboratories lacking these capabilities.
In this study, an RT-LAMP assay for the detection of NBV had an analytical sensitivity limit of 103 TCID50/ml of NBV in culture supernatants, which is less than that of real-time RT-PCR and greater than that for conventional RT-PCR. However, when applied to clinical samples, the differences in analytical sensitivity were not reflected in different clinical sensitivities, as equivalence was demonstrated for all 3 molecular diagnostic methods. The RT-LAMP method also offered excellent analytical specificity because of the lack of cross-reactivity with other viruses, and it was 100% specific in a comparison with the gold standard, the real-time RT-PCR method, and likely did not erroneously classify patients with HGA as SFTS. Similar RT-LAMP techniques have proved valuable for rapid detection of other bunyaviruses, including Rift Valley fever virus (RVFV) and Crimean Congo hemorrhagic fever virus (CCHFV) (16, 17); thus, it can be anticipated that RT-LAMP for NBV could be applied as a useful clinical diagnostic tool.
In the past 2 years, virus isolation, IFA, and nucleic acid amplification methods, such as RT-PCR, nested RT-PCR, and real-time RT-PCR, have been developed for identification of NBV (1, 4, 13). Virus isolation is very time-consuming, difficult, and requires expensive instruments or special techniques (18). IFA requires large amounts of virus, skilled technicians, has a subjective interpretation, and is also time-consuming (19). While RT-PCR, nested RT-PCR, and real-time RT-PCR can be completed in 2 to 4 h (20, 21), these techniques require elaborate methods for the detection of amplified products and/or sophisticated instruments. In contrast, the RT-LAMP assay is isothermal at a single temperature of 60 to 65°C for 40 to 60 min and does not require sophisticated instruments or well-trained technicians (16, 22). More importantly, the results of the RT-LAMP assay can be read by visual inspection alone for prospective detection of virus in clinical situations. The facts that neither sensitivity nor specificity suffers for clinical samples from patients with suspected SFTS and that the other standard molecular assays are technically demanding, are costly, and require expensive and sophisticated equipment make RT-LAMP an attractive alternative for clinical laboratories or settings where resources are constrained.
A similar RT-LAMP assay for detection of NBV (SFTSV) was also recently reported, with similar high sensitivity and specificity (9). However, there were fewer patient samples examined, and it was unclear what diagnostic criteria were used to define an SFTSV-positive patient, making difficult the interpretation of the overall clinical sensitivity compared to the assay developed here. The advantages of the current study is that the patients were prospectively enrolled into the study and that state-of-the-art diagnostics, including culture, RT-PCR, and real-time RT-PCR assays, were considered definitive diagnostic criteria, assuring an effective analysis of assay performance.
In summary, RT-LAMP is a simple, rapid, inexpensive, specific, and sensitive genetic testing technology. If optimized further, it could play an important role in the diagnosis, prevention, and control of infectious disease.
ACKNOWLEDGMENTS
This work was supported by the Henan Medical Science Project (200702016), Science and Technology Bureau of Henan Province (122102310268), and China—Australia Health and HIV/AIDS Facility (EID35).
We declare that we have no potential conflicts of interest relevant to this article.
Footnotes
Published ahead of print 4 December 2013
REFERENCES
- 1.Xu BL, Liu LC, Huang XY, Ma H, Zhang Y, Du YH, Wang PZ, Tang XY, Wang HF, Kang K, Zhang SQ, Zhao GH, Wu WL, Yang YH, Chen HM, Mu F, Chen WJ. 2001. Metagenomic analysis of fever, thrombocytopenia and leukopenia syndrome (FTLS) in Henan Province, China: discovery of a new bunyavirus. Plos Pathog. 7(11):e1002369. 10.1371/journal.ppat.1002369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 364:1523–1532. 10.1056/NEJMoa1010095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang YZ, Zhou DJ, Xiong Y, Chen XP, He YW, Sun Q, Yu B, Li J, Dai YA, Tian JH, Qin XC, Jin D, Cui Z, Luo XL, Li W, Lu S, Wang W, Peng JS, Guo WP, Li MH, Li ZJ, Zhang S, Chen C, Wang Y, de Jong MD, Xu J. 2011. Hemorrhagic fever caused by a novel tick-borne Bunyavirus in Huaiyangshan, China. Zhonghua Liu Bing Xue Za Zhi 32:209–220. (In Chinese with English summary.) 10.3760/cma.j.issn.0254-6450.2011.08.024 [DOI] [PubMed] [Google Scholar]
- 4.Sun YL, Liang MF, Qu J, Jin C, Zhang QF, Li JD, Jiang XL, Wang Q, Lu J, Gu W, Zhang S, Li C, Wang XJ, Zhan FX, Yao WQ, Bi ZQ, Wang SW, Li DX. 2012. Early diagnosis of novel SFTS bunyavirus infection by quantitative real-time RT-PCR assay. J. Clin. Virol. 53:48–53. 10.1016/j.jcv.2011.09.031 [DOI] [PubMed] [Google Scholar]
- 5.Mori Y, Notomi T. 2009. Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Infect. Chemother. 15:62–69. 10.1007/s10156-009-0669-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang T, Liu J, Deng YQ, Xu LJ, Li XF, Han JF, Cao RY, Qin ED, Qin CF. 2011. Development and evaluation of a reverse transcription-loop-mediated isothermal amplification assay for rapid detection of enterovirus 71. J. Clin. Microbiol. 49:870–874. 10.1128/JCM.02045-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parida M, Shukla J, Sharma S, Ranghia Santhosh S, Ravi V, Mani R, Thomas M, Khare S, Rai A, Kant Ratho R, Pujari S, Mishra B, Lakshmana Rao PV, Vijayaraghavan R. 2011. Development and evaluation of reverse transcription loop-mediated isothermal amplification assay for rapid and real-time detection of the swine-origin influenza A H1N1 virus. J. Mol. Diagn. 13:100–107. 10.1016/j.jmoldx.2010.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poon LL, Wong BW, Chan KH, Ng SS, Yuen KY, Guan Y, Peiris JS. 2005. Evaluation of real-time reverse transcriptase PCR and real-time loop-mediated amplification assays for severe acute respiratory syndrome coronavirus detection. J. Clin. Microbiol. 43:3457–3459. 10.1128/JCM.43.7.3457-3459.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang G, Li B, Liu L, Huang W, Zhang W, Liu Y. 2012. Development and evaluation of a reverse transcription loop-mediated isothermal amplification assay for rapid detection of a new SFTS bunyavirus. Arch. Virol. 157:1779–1783. 10.1007/s00705-012-1348-1 [DOI] [PubMed] [Google Scholar]
- 10.Nemoto M, Imagawa H, Tsujimura K, Yamanaka T, Kondo T, Matsumura T. 2010. Detection of equine rotavirus by reverse transcription loop-mediated isothermal amplification (RT-LAMP). J. Vet. Med. Sci. 72:823–826 http://dx.doi.org/10.1292/jvms.09-0446 [DOI] [PubMed] [Google Scholar]
- 11.Du YH, Huang XY, Deng WB, Ma HX, Ma H, Man RQ, Kang K, Chen HM, Liu GH, Xu BL. 2012. Culture, isolation and identification of new bunyavirus in African green monkey kidney (Vero) cells. Zhonghua Yu Fang Yi Xue Za Zhi 46:169–172. (In Chinese with English summary.) 10.3760/cma.j.issn.0253-9624.2012.02.016 [DOI] [PubMed] [Google Scholar]
- 12.Li C, Liu F, Liang MF, Zhang QF, Wang XF, Wang T, Li JD, Li DX. 2010. Hantavirus-like particles generated in CHO cells induce specific immune responses in C57BL/6 mice. Vaccine 28:4294–4300. 10.1016/j.vaccine.2010.04.025 [DOI] [PubMed] [Google Scholar]
- 13.Huang XY, Du YH, Li XL, Ma H, Man RQ, Kang K, Tang XY, Chen HM, Liu GH, Xu BL. 2012. Establishment of indirect immunofluorescence assay (IFA) for detection of IgG antibody against new bunyavirus. Zhonghua Yu Fang Yi Xue Za Zhi 46:165–168 (In Chinese with English summary.) 10.3760/cma.j.issn.0253-9624.2012.02.015 [DOI] [PubMed] [Google Scholar]
- 14.Zhao L, Zhai S, Wen H, Cui F, Chi Y, Wang L, Xue F, Wang Q, Wang Z, Zhang S, Song Y, Du J, Yu XJ. 2012. Severe fever with thrombocytopenia syndrome virus, Shandong Province, China. Emerg. Infect. Dis. 18:963–965. 10.3201/eid1806.111345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gai ZT, Zhang Y, Liang MF, Jin C, Zhang S, Zhu CB, Li C, Li XY, Zhang QF, Bian PF, Zhang LH, Wang B, Zhou N, Liu JX, Song XG, Xu A, Bi ZQ, Chen SJ, Li DX. 2012. Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J. Infect. Dis. 206:1095–1102. 10.1093/infdis/jis472 [DOI] [PubMed] [Google Scholar]
- 16.Le Roux CA, Kubo T, Grobbelaar AA, van Vuren PJ, Weyer J, Nel LH, Swanepoel R, Morita K, Paweska JT. 2009. Development and evaluation of a real-time reverse transcription-loop-mediated isothermal amplification assay for rapid detection of Rift Valley fever virus in clinical specimens. J. Clin. Microbiol. 47:645–651. 10.1128/JCM.01412-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osman HA, Eltom KH, Musa NO, Bilal NM, Elbashir MI, Aradaib IE. 2013. Development and evaluation of loop-mediated isothermal amplification assay for detection of Crimean Congo hemorrhagic fever virus in Sudan. J. Virol. Methods 190:4–10. 10.1016/j.jviromet.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 18.López Roa P, Catalán P, Giannella M, Garcias de Viedma D, Sandonis V, Bouza E. 2011. Comparison of real-time RT-PCR, shell vial culture, and conventional cell culture for the detection of the pandemic influenza A (H1N1) in hospitalized patients. Diagn. Microbiol. Infect. Dis. 69:428–431. 10.1016/j.diagnmicrbio.2010.11.007 [DOI] [PubMed] [Google Scholar]
- 19.Yang DK, Shin EK, Oh YI, Lee KW, Lee CS, Kim SY, Lee JA, Song JY. 2012. Comparison of four diagnostic methods for detecting rabies viruses circulating in Korea. J. Vet. Sci. 13:43–48. 10.4142/jvs.2012.13.1.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam WY, Leung TF, Lee N, Cheung JL, Yeung AC, Ho YI, Chan RC, Fung KS, Barr IG, Hui DS, Sung JJ, Chan PK. 2010. Development and comparison of molecular assays for the rapid detection of the pandemic influenza A (H1N1) 2009 virus. J. Med. Virol. 82:675–683. 10.1002/jmv.21725 [DOI] [PubMed] [Google Scholar]
- 21.Reddy V, Ravi V, Desai A, Parida M, Powers AM, Johnson BW. 2012. Utility of IgM ELISA, TaqMan real-time PCR, reverse transcription PCR, and RT-LAMP assay for the diagnosis of Chikungunya fever. J. Med. Virol. 84:1771–1778. 10.1002/jmv.23406 [DOI] [PubMed] [Google Scholar]
- 22.Shi W, Li K, Ji Y, Jiang Q, Shi M, Mi Z. 2011. Development and evaluation of reverse transcription-loop-mediated isothermal amplification assay for rapid detection of enterovirus 71. BMC Infect. Dis. 11:197. 10.1186/1471-2334-11-197 [DOI] [PMC free article] [PubMed] [Google Scholar]


