Abstract
We report a fatal case of Schizophyllum commune empyema thoracis with cross-reactive cryptococcal antigenemia. In vitro testing confirmed the ability of the fungus to cause a positive cryptococcal antigen latex agglutination system (CALAS) test result. Such a result may lead to delay in diagnosis and treatment, as most strains of S. commune are resistant to fluconazole.
CASE REPORT
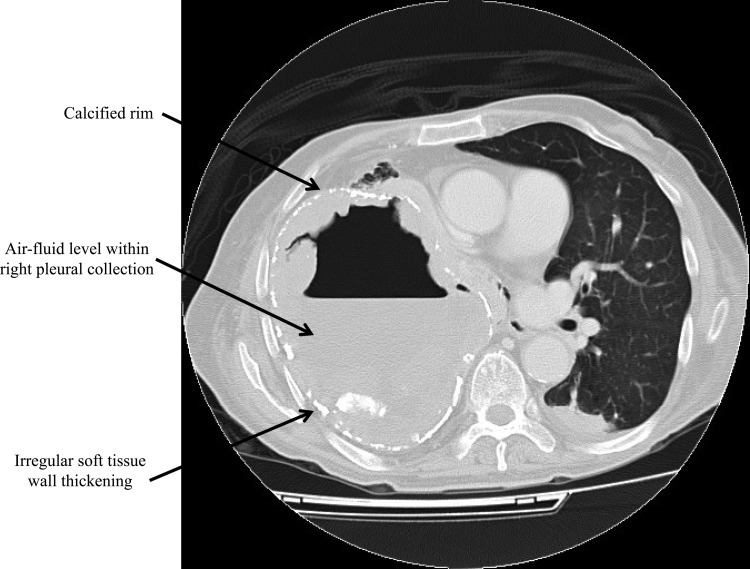
A 78-year-old Chinese man was admitted for fever, productive cough, and dyspnea for 2 months. His medical history included pulmonary tuberculosis 40 years earlier, chronic obstructive pulmonary disease, hypertension, congestive heart failure, and gout. On admission, he had a temperature of 38.5°C, a saturation level of oxygen in hemoglobin (SaO2) of 95% with 2 liters/min supplementary oxygen, and stony dullness on percussion and reduced breath sound over the right chest. Blood tests showed leukocytosis with neutrophilia and lymphopenia (white cell count, 21.47 × 109/liter [normal range, 3.70 to 9.30 × 109/liter]; neutrophil count, 20.20 × 109/liter [normal range, 1.80 to 6.20 × 109/liter]; lymphocyte count, 0.64 × 109/liter [normal range, 1.00 to 3.20 × 109/liter]), raised C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) levels (CRP, 59.8 mg/liter [normal value, <8.0 mg/liter]; ESR, 132 mm/h [normal value, <15 mm/h]), and normal liver and renal function tests. A computerized tomography scan of the thorax showed a large right pleural collection with peripheral irregular soft-tissue densities and intermittent calcified rim, air-fluid level within the collection, fibrosis of the right upper lobe, collapsed right middle and lower lobes, and bilateral emphysematous changes (Fig. 1). Gram-stained smear of sputum sample showed numerous white cells, but culture for bacteria revealed only scanty amount of commensals. Ziehl-Neelsen-stained smears of multiple sputum samples were negative for acid-fast bacilli. Blood culture yielded no growth. The patient was treated with oral levofloxacin (500 mg daily) with partial improvement in symptoms and moderate resolution of leukocytosis and elevated CRP and ESR levels initially. However, he developed worsening fever and dyspnea after 8 weeks of levofloxacin and was referred to our hospital for further management.
FIG 1.
Axial contrast-enhanced computerized tomography scan of the thorax at level of the pulmonary trunk displayed at the lung window showing a large right pleural collection with peripheral irregular soft-tissue densities, intermittent calcified rim, and air-fluid level within the collection (arrows).
The results of further microbiological investigations for chronic cavitary pneumonia, including assays of serum cryptococcal antigen (cryptococcal antigen latex agglutination system [CALAS]; Meridian Bioscience, Inc.), Aspergillus galactomannan (Platelia Aspergillus enzyme immunoassay [EIA]; Bio-Rad Laboratories, Inc.), and Penicillium marneffei immunofluorescent antibody (1), were negative except for the serum CALAS test result, which was positive at a dilution titer of up to 1:32. Results of immunological workups, including a combined HIV antigen/antibody test and anti-gamma interferon autoantibody test, for underlying immunodeficiencies associated with cryptococcosis were negative (2–4). The patient's antimicrobial regimen was changed to intravenous meropenem administered at 1 g every 8 h and oral fluconazole at 400 mg daily, but the clinical response was suboptimal after 2 weeks of therapy. Therefore, ultrasonography-guided drainage of the right pleural collection was performed. The pH of the pleural fluid was 7.0, and the white cell and red cell counts were 75,200/mm3 with 96% polymorphs and 29,600/mm3, respectively. The pleural-fluid-to-serum ratios of total protein and lactate dehydrogenase were 45:55 and 12,000:278, respectively. The Gram-stained smear of the pleural fluid showed abundant white cells without any visible organism. PCR for Mycobacterium tuberculosis was negative. Culture of the pleural fluid was negative for bacteria, yeasts, and acid-fast bacilli but yielded a mold after 96 h. His antimicrobial therapy was changed to intravenous voriconazole at 6 mg/kg of body weight every 12 h for the first day followed by 4 mg/kg every 12 h. As he was not considered to be fit for surgical decortications of the lung, multiple image-guided drainages were performed instead and yielded the same mold. However, despite repeated drainage and voriconazole, his condition continued to deteriorate and he died 4 weeks after admission. No postmortem examination was performed.
Phenotypic characterization.
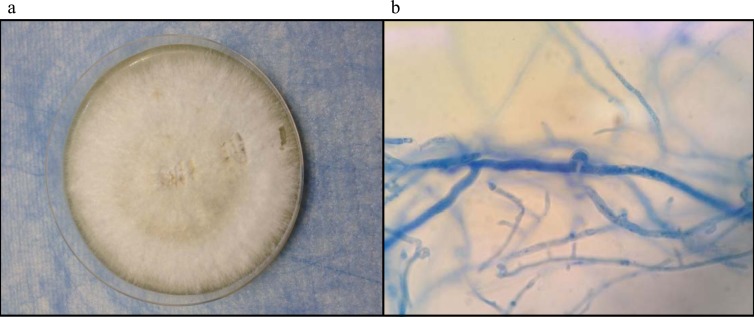
The pleural fluid was inoculated onto Sabouraud dextrose agar (SDA) (Sigma-Aldrich) for fungal culture and incubated at room temperature and 37°C. White, woolly colonies with a yellow-brown reverse were seen after 96 h of incubation at room temperature and 37°C (Fig. 2a). Lactophenol cotton blue mount of the mold colonies showed hyaline, septate, and nondichotomously branching hyphae with clamp connections and spicules suggestive of basidiomycetes (Fig. 2b). Basidiocarps were observed after 4 weeks of incubation with intermittent sunlight exposure. Lactophenol cotton blue mount from the basidiocarps showed basiodiospores.
FIG 2.
(a) White, woolly colonies of Schizophyllum commune in Sabouraud dextrose agar after incubation of the patients' pleural fluid samples at 37°C and room temperature for 7 days. (b) Lactophenol cotton blue mount of the colonies showing hyaline, septate, and nondichotomously branching hyphae with clamp connections and spicules.
Detection of cross-reactivity with CALAS test.
One isolate each of Cryptococcus neoformans (clinical isolate), Candida albicans (ATCC 90028), and Trichosporon sp. (clinical isolate) and the case isolate were inoculated into brain heart infusion (BHI) broth. The broth cultures and another BHI broth without fungus inoculation used as a negative control were incubated at 37°C for 48 h and then autoclaved at 121°C for 15 min. A 25-μl volume of a 0.5 McFarland standard of each broth was used to perform CALAS testing according to the manufacturer's instructions. The C. neoformans isolate, the Trichosporon sp. isolate, and the case isolate showed positive results at dilution titers of up to 1:4,096, 1:256, and 1:64, respectively. C. albicans and the BHI broth without fungus inoculation showed negative results.
ITS1-5.8S-ITS2 rRNA gene cluster sequencing and phylogenetic characterization.
PCR amplification and DNA sequencing of the internal transcribed spacer (ITS) ITS1-5.8S-ITS2 rRNA gene cluster of the case isolate were performed as we previously described with slight modifications using ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′ and ITS-4 (5′-TCCTCCGCTTATTGATATGC-3′) (Gibco BRL, Rockville, MD) as the PCR and sequencing primers (5–8). PCR analysis of the ITS region showed a band at about 600 bp. The sequence of the PCR product was compared with sequences of closely related species listed in the GenBank database by multiple-sequence alignment using ClustalX 1.83. The ITS sequence of the case isolate was identical to that of S. commune (GenBank accession no. JX848644.1). Comparison with sequences of validly published Schizophyllum species revealed differences in 7 bases (1.1%) relative to the sequence of S. radiatum (GenBank accession no. AY571060.1), 29 bases (4.7%) relative to that of S. fasciatum (GenBank accession no. L43385.1), and 33 bases (5.4%) relative to that of S. umbrinum (GenBank accession no. AF249391.1).
Partial large-subunit (LSU) rRNA gene sequencing and phylogenetic characterization.
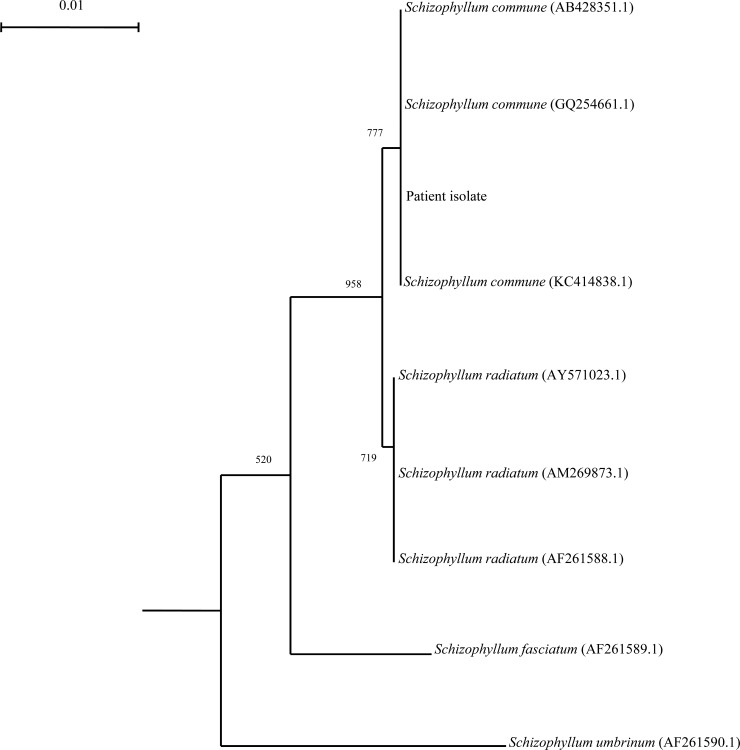
PCR amplification and DNA sequencing of the partial LSU rRNA gene of the case isolate was performed by amplifying a 475-bp fragment of the LSU gene of Schizophyllum sp. using conserved primers LPW24262 (5′-AGTTGTAATTTAGAGAAGCGTTA-3′) and LPW24263 (5′-AGCATCCTAAGCACGAACGT-3′) (Sigma-Proligo, Singapore) designed by multiple alignments of the nucleotide sequences of available LSU rRNA genes of a known Schizophyllum sp. PCR analysis of the LSU gene showed a band at about 500 bp. The sequence of the PCR product was compared with sequences of closely related species listed in the GenBank database by multiple-sequence alignment using ClustalX 1.83. Phylogenetic relationships were determined using the neighbor-joining method (Fig. 3). A total of 475 nucleotide positions were used. The LSU gene sequence of the case isolate was identical to that of S. commune (GenBank accession no. KC414838.1). Comparison with sequences of other Schizophyllum sp. revealed differences in one base (0.2%) relative to the sequence of S. radiatum (GenBank accession no. AM269873.1), 9 bases (1.9%) relative to that of S. fasciatum (GenBank accession no. AF261589.1), and 15 bases (3.2%) relative to that of S. umbrinum (GenBank accession no. AF261590.1).
FIG 3.
Phylogenetic tree showing the relationships of the case isolate to related species. The tree was inferred from LSU data by the neighbor-joining method and rooted using the LSU rRNA gene sequence of Rhodotorula mucilaginosa (GenBank accession number KC494739.1). Bootstrap values were calculated from 1,000 trees. The scale bar indicates the estimated number of substitutions per 100 bases. Names and accession numbers are given as cited in the GenBank database.
Nucleotide sequence accession numbers.
The ITS sequence and the partial LSU gene sequence of the case isolate have been deposited in GenBank under accession numbers KF679517 and KF679518.
S. commune is a wood-degrading filamentous basidiomycete with a worldwide distribution that is best known for its major role as a genetically tractable model for studying mushroom development (9). It belongs to the phylum Basidiomycota, subphylum Agaricomycotina, class Agaricomycetes, subclass Agaricomycetidae, order Agaricales, family Schizophyllaceae. It is most commonly found in fallen branches and timber of deciduous trees and other decaying organic matters in the environment. Phenotypically, S. commune is characterized by its macroscopic appearance as rapid growing, densely wooly white mold colonies with a yellow-brown reverse, production of a tart and disagreeable smell, formation of basidiocarp after incubation with intermittent sunlight exposure, and microscopic features that include septate, branched, hyaline hyphae with clamp connections, spicules, and/or basidiospores. Unfortunately, its definitive identification is often problematic in the clinical microbiology laboratory because its distinctive phenotypic features may be absent in primary cultures incubated for short periods of time or without periodic light exposure and in monokaryotic isolates which do not exhibit spicules or clamp connections (10–12). In recent years, accurate identification has been increasingly achieved by molecular methods using nucleotide sequencing of the ITS, the LSU rRNA gene, and/or the 18S rRNA gene (10, 13, 14). Our case isolate was unambiguously identified as S. commune by its characteristic phenotypic features and phylogenetically by ITS and partial LSU gene sequencing.
Human infection by S. commune is uncommon, although recent reports suggested that the incidence might be underestimated because of difficulties encountered in laboratory identification (12). Since the first report of human S. commune infection manifesting as onychomycosis in 1950 (15), approximately 70 more cases have been described in different parts of the world, especially Japan, over the past 60 years (12). The clinical syndromes of human infection by S. commune can be broadly classified into five major groups: (i) asymptomatic colonization in diseased lungs (16); (ii) superficial mycoses such as onychomycosis (15); (iii) sinopulmonary infections with or without involvement of contiguous anatomical structures, including fungal bronchopneumonia (17), pulmonary mycetoma (18), pulmonary nodules (19), bronchial mucoid impaction (20), bronchogenic cyst infection (11), and maxillary sinusitis with orbital involvement (21); (iv) disseminated or extrapulmonary invasive infections such as brain abscess and palatal ulceration, especially in immunocompromised hosts (22, 23); and (v) hypersensitivity disorders such as chronic eosinophilic pneumonia (24), asthma (25), allergic bronchopulmonary mycosis (12, 26), and allergic sinusitis (27–29). Sinopulmonary diseases are the most common manifestation and account for over 90% of all the cases, suggesting that the most common route of acquisition is inhalation. Local and systemic risk factors for invasive sinopulmonary S. commune infection include fibrocavities from past tuberculosis, chronic lung disease, diabetes mellitus, and corticosteroid therapy (11). Our patient, who had tuberculosis in the past and chronic obstructive pulmonary disease, likely developed a fibrocavity resulting from past tuberculosis which was then infected by S. commune and evolved into empyema thoracis. Unexpectedly, the patient had a positive serum cryptococcal antigen titer of 1:32, which prompted the clinicians to commence fluconazole treatment for the possibility of pulmonary cryptococcosis. We excluded coinfection with C. neoformans or other pathogens that might show cross-reactivity with the cryptococcal antigen test such as Trichosporon beigelli and Pseudallescheria sp. by culture of the pleural collection aspirate, sputum, and blood (30, 31). We confirmed the in vitro ability of S. commune to induce a positive CALAS test result. Therefore, we believe that S. commune infection should be included as a possible cause for a cross-reactive serum cryptococcal antigen test result. As in our patient, the result might lead to delayed diagnosis and delayed appropriate antifungal treatment. The diagnosis of S. commune empyema thoracis was established only after drainage of the pleural collection after 2 weeks of ineffective treatment with fluconazole. Notably, fluconazole has a higher geometric mean MIC for S. commune than amphotericin B, itraconazole, voriconazole, isavuconazole, and flucytosine (10). Although antifungal treatment for S. commune infection is not standardized, most patients who recovered required treatment with antifungals other than fluconazole or combinational treatment with or without fluconazole (10).
ACKNOWLEDGMENTS
We thank Kathy C. K. Wong of the Department of Radiology, The University of Hong Kong, for her facilitation of the study.
This work is partly supported by the donation of Larry Chi-Kin Yung, the University Development Fund, and the Committee for Research and Conference Grant, The University of Hong Kong.
Footnotes
Published ahead of print 13 November 2013
REFERENCES
- 1.Yuen KY, Wong SS, Tsang DN, Chau PY. 1994. Serodiagnosis of Penicillium marneffei infection. Lancet 344:444–445. 10.1016/S0140-6736(94)91771-X [DOI] [PubMed] [Google Scholar]
- 2.Tang BS, Chan JF, Chen M, Tsang OT, Mok MY, Lai RW, Lee R, Que TL, Tse H, Li IW, To KK, Cheng VC, Chan EY, Zheng B, Yuen KY. 2010. Disseminated penicilliosis, recurrent bacteremic nontyphoidal salmonellosis, and burkholderiosis associated with acquired immunodeficiency due to autoantibody against gamma interferon. Clin. Vaccine Immunol. 17:1132–1138. 10.1128/CVI.00053-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, Kirk JL, Jutivorakool K, Zaman R, Ding L, Hsu AP, Patel SY, Olivier KN, Lulitanond V, Mootsikapun P, Anunnatsiri S, Angkasekwinai N, Sathapatayavongs B, Hsueh PR, Shieh CC, Brown MR, Thongnoppakhun W, Claypool R, Sampaio EP, Thepthai C, Waywa D, Dacombe C, Reizes Y, Zelazny AM, Saleeb P, Rosen LB, Mo A, Iadarola M, Holland SM. 2012. Adult-onset immunodeficiency in Thailand and Taiwan. N. Engl. J. Med. 367:725–734. 10.1056/NEJMoa1111160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan JF, Trendell-Smith NJ, Chan JC, Hung IF, Tang BS, Cheng VC, Yeung CK, Yuen KY. 2013. Reactive and infective dermatoses associated with adult-onset immunodeficiency due to anti-interferon-gamma autoantibody: Sweet's syndrome and beyond. Dermatology 226:157–166. 10.1159/000347112 [DOI] [PubMed] [Google Scholar]
- 5.To KK, Lau SK, Wu AK, Lee RA, Ngan AH, Tsang CC, Ling IW, Yuen KY, Woo PC. 2012. Phaeoacremonium parasiticum invasive infections and airway colonization characterized by agar block smear and ITS and beta-tubulin gene sequencing. Diagn. Microbiol. Infect. Dis. 74:190–197. 10.1016/j.diagmicrobio.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 6.Cheng VC, Chan JF, Ngan AH, To KK, Leung SY, Tsoi HW, Yam WC, Tai JW, Wong SS, Tse H, Li IW, Lau SK, Woo PC, Leung AY, Lie AK, Liang RH, Que TL, Ho PL, Yuen KY. 2009. Outbreak of intestinal infection due to Rhizopus microsporus. J. Clin. Microbiol. 47:2834–2843. 10.1128/JCM.00908-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo PC, Leung SY, To KK, Chan JF, Ngan AH, Cheng VC, Lau SK, Yuen KY. 2010. Internal transcribed spacer region sequence heterogeneity in Rhizopus microsporus: implications for molecular diagnosis in clinical microbiology laboratories. J. Clin. Microbiol. 48:208–214. 10.1128/JCM.01750-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo PC, Ngan AH, Tsang CC, Ling IW, Chan JF, Leung SY, Yuen KY, Lau SK. 2013. Clinical spectrum of exophiala infections and a novel Exophiala species, Exophiala hongkongensis. J. Clin. Microbiol. 51:260–267. 10.1128/JCM.02336-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohm RA, de Jong JF, Lugones LG, Aerts A, Kothe E, Stajich JE, de Vries RP, Record E, Levasseur A, Baker SE, Bartholomew KA, Coutinho PM, Erdmann S, Fowler TJ, Gathman AC, Lombard V, Henrissat B, Knabe N, Kues U, Lilly WW, Lindquist E, Lucas S, Magnuson JK, Piumi F, Raudaskoski M, Salamov A, Schmutz J, Schwarze FW, vanKuyk PA, Horton JS, Grigoriev IV, Wosten HA. 2010. Genome sequence of the model mushroom Schizophyllum commune. Nat. Biotechnol. 28:957–963. 10.1038/nbt.1643 [DOI] [PubMed] [Google Scholar]
- 10.Chowdhary A, Kathuria S, Singh PK, Agarwal K, Gaur SN, Roy P, Randhawa HS, Meis JF. 2013. Molecular characterization and in vitro antifungal susceptibility profile of Schizophyllum commune, an emerging basidiomycete in bronchopulmonary mycoses. Antimicrob. Agents Chemother. 57:2845–2848. 10.1128/AAC.02619-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulajic N, Cvijanovic V, Vukojevic J, Tomic D, Johnson E. 2006. Schizophyllum commune associated with bronchogenous cyst. Mycoses 49:343–345. 10.1111/j.1439-0507.2006.01247.x [DOI] [PubMed] [Google Scholar]
- 12.Chowdhary A, Randhawa HS, Gaur SN, Agarwal K, Kathuria S, Roy P, Klaassen CH, Meis JF. 2013. Schizophyllum commune as an emerging fungal pathogen: a review and report of two cases. Mycoses 56:1–10. 10.1111/j.1439-0507.2012.02190.x [DOI] [PubMed] [Google Scholar]
- 13.Singh PK, Kathuria S, Agarwal K, Gaur SN, Meis JF, Chowdhary A. 31 July 2013. Clinical significance and molecular characterization of nonsporulating molds isolated from the respiratory tracts of bronchopulmonary mycosis patients with special reference to basidiomycetes. J. Clin. Microbiol. 10.1128/JCM.01486-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baron O, Cassaing S, Percodani J, Berry A, Linas MD, Fabre R, Serrano E, Magnaval JF. 2006. Nucleotide sequencing for diagnosis of sinusal infection by Schizophyllum commune, an uncommon pathogenic fungus. J. Clin. Microbiol. 44:3042–3043. 10.1128/JCM.00211-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kligman AM. 1950. A basidiomycete probably causing onychomycosis. J. Invest. Dermatol. 14:67–70, illust [DOI] [PubMed] [Google Scholar]
- 16.Iizasa T, Kamei K, Chiyo M, Suzuki M, Baba M, Toyosaki T, Hiroshima K, Ohwada H, Kanno S, Nishimura K, Fujisawa T. 2001. Colonization with Schizophyllum commune of localized honeycomb lung with mucus. Respiration 68:201–203. 10.1159/000050493 [DOI] [PubMed] [Google Scholar]
- 17.Tullio V, Mandras N, Banche G, Allizond V, Gaido E, Roana J, Cuffini AM, Carlone NA. 2008. Schizophyllum commune: an unusual of agent bronchopneumonia in an immunocompromised patient. Med. Mycol. 46:735–738. 10.1080/13693780802256091 [DOI] [PubMed] [Google Scholar]
- 18.Sigler L, de la Maza LM, Tan G, Egger KN, Sherburne RK. 1995. Diagnostic difficulties caused by a nonclamped Schizophyllum commune isolate in a case of fungus ball of the lung. J. Clin. Microbiol. 33:1979–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roan JN, Hsieh HY, Tsai HW, Wu CJ, Hsu CH, Wu SY, Yang YJ, Chang TC. 2009. Pulmonary nodules caused by Schizophyllum commune after cardiac transplantation. J. Infect. 58:164–167. 10.1016/j.jinf.2008.11.012 [DOI] [PubMed] [Google Scholar]
- 20.Amitani R, Nishimura K, Niimi A, Kobayashi H, Nawada R, Murayama T, Taguchi H, Kuze F. 1996. Bronchial mucoid impaction due to the monokaryotic mycelium of Schizophyllum commune. Clin. Infect. Dis. 22:146–148. 10.1093/clinids/22.1.146 [DOI] [PubMed] [Google Scholar]
- 21.Sa HS, Ko KS, Woo KI, Peck KR, Kim YD. 2012. A case of sino-orbital infection caused by the Schizophyllum commune. Diagn. Microbiol. Infect. Dis. 73:376–377. 10.1016/j.diagmicrobio.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 22.Rihs JD, Padhye AA, Good CB. 1996. Brain abscess caused by Schizophyllum commune: an emerging basidiomycete pathogen. J. Clin. Microbiol. 34:1628–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Restrepo A, Greer DL, Robledo M, Osorio O, Mondragon H. 1973. Ulceration of the palate caused by a basidiomycete Schizophyllum commune. Sabouraudia 11:201–204. 10.1080/00362177385190431 [DOI] [PubMed] [Google Scholar]
- 24.Kawayama T, Fujiki R, Rikimaru T, Aizawa H. 2003. Chronic eosinophilic pneumonia associated with Schizophyllum commune. Respirology 8:529–531. 10.1046/j.1440-1843.2003.00504.x [DOI] [PubMed] [Google Scholar]
- 25.Ogawa H, Fujimura M, Takeuchi Y, Makimura K. 2011. Two cases of Schizophyllum asthma: is this a new clinical entity or a precursor of ABPM? Pulm. Pharmacol. Ther. 24:559–562. 10.1016/j.pupt.2011.04.030 [DOI] [PubMed] [Google Scholar]
- 26.Kamei K, Unno H, Nagao K, Kuriyama T, Nishimura K, Miyaji M. 1994. Allergic bronchopulmonary mycosis caused by the basidiomycetous fungus Schizophyllum commune. Clin. Infect. Dis. 18:305–309. 10.1093/clinids/18.3.305 [DOI] [PubMed] [Google Scholar]
- 27.Won EJ, Shin JH, Lim SC, Shin MG, Suh SP, Ryang DW. 2012. Molecular identification of Schizophyllum commune as a cause of allergic fungal sinusitis. Ann. Lab. Med. 32:375–379. 10.3343/alm.2012.32.5.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed MK, Ishino T, Takeno S, Hirakawa K. 2009. Bilateral allergic fungal rhinosinusitis caused by Schizophillum commune and Aspergillus niger. A case report. Rhinology 47:217–221 [PubMed] [Google Scholar]
- 29.Clark S, Campbell CK, Sandison A, Choa DI. 1996. Schizophyllum commune: an unusual isolate from a patient with allergic fungal sinusitis. J. Infect. 32:147–150. 10.1016/S0163-4453(96)91436-X [DOI] [PubMed] [Google Scholar]
- 30.McManus EJ, Jones JM. 1985. Detection of a Trichosporon beigelii antigen cross-reactive with Cryptococcus neoformans capsular polysaccharide in serum from a patient with disseminated Trichosporon infection. J. Clin. Microbiol. 21:681–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rüchel R. 1994. False-positive reaction of a Cryptococcus antigen test owing to Pseudallescheria mycosis. Mycoses 37:69. [DOI] [PubMed] [Google Scholar]