Abstract
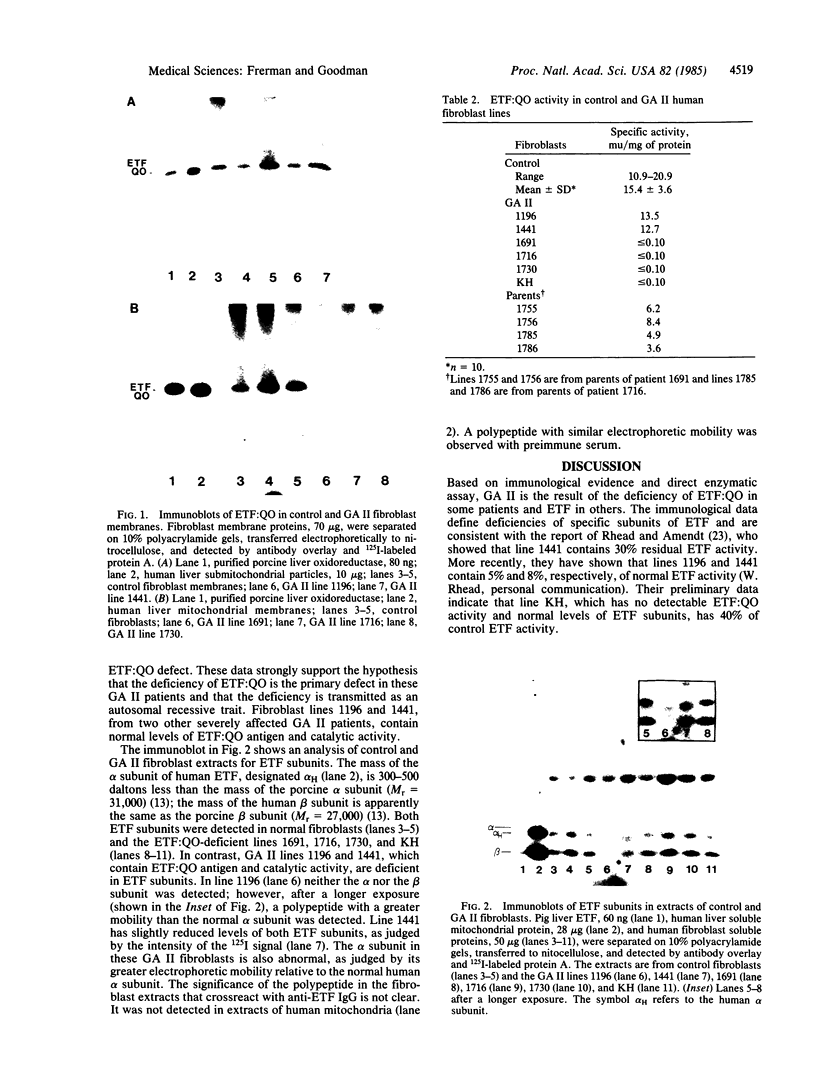
Glutaric acidemia type II (GA II) is a human genetic disorder. It has been suggested that the primary defect in this disorder is a deficiency of a protein involved in electron transport between the acyl-CoA dehydrogenases and the bc1 complex of the mitochondrial respiratory chain. Antisera were raised to purified porcine electron transfer flavoprotein (ETF) and electron transfer flavoprotein:ubiquinone oxidoreductase (ETF:QO). The antisera were used to detect the two electron transferases in control and GA II fibroblasts by immunoblotting. Fibroblasts from three unrelated GA II patients were deficient in immunologically detectable ETF:QO and extracts from these three fibroblast lines contained no detectable ETF:QO catalytic activity. Fibroblasts from parents of two of these patients had ETF:QO activity intermediate between activities in control fibroblasts and fibroblasts from the patients. These data indicate that the primary defect in these patients is a deficiency of ETF:QO and that the mode of transmission of the gene is autosomal recessive. Fibroblasts from two other patients with severe GA II had normal levels of ETF-QO activity and antigen but were deficient in immunoreactive ETF. These findings show that GA II results from a deficiency of ETF in some patients and ETF:QO in others. In addition, these investigations provide strong evidence for the specificity and physiological function of the iron-sulfur flavoprotein ETF:QO.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckmann J. D., Frerman F. E. The effects of pH, ionic strength, and chemical modifications on the reaction of electron transfer flavoprotein with an acyl coenzyme A dehydrogenase. J Biol Chem. 1983 Jun 25;258(12):7563–7569. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- CRANE F. L., BEINERT H. On the mechanism of dehydrogenation of fatty acyl derivatives of coenzyme A. II. The electron-transferring flavoprotein. J Biol Chem. 1956 Feb;218(2):717–731. [PubMed] [Google Scholar]
- Coude F. X., Ogier H., Charpentier C., Thomassin G., Checoury A., Amedee-Manesme O., Saudubray J. M., Frezal J. Neonatal glutaric aciduria type II: an X-linked recessive inherited disorder. Hum Genet. 1981;59(3):263–265. doi: 10.1007/BF00283677. [DOI] [PubMed] [Google Scholar]
- Flatmark T., Ruzicka F. J., Beinert H. The pattern of iron--sulfur centers in brown adipose tissue mitochondria: preponderance of ETF dehydrogenase and invariance with the thermogenic state. FEBS Lett. 1976 Mar 15;63(1):51–55. doi: 10.1016/0014-5793(76)80192-5. [DOI] [PubMed] [Google Scholar]
- Frerman F. E., Sabran J. L., Taylor J. L., Grossberg S. E. Leucine catabolism during the differentiation of 3T3-L1 cells. Expression of a mitochondrial enzyme system. J Biol Chem. 1983 Jun 10;258(11):7087–7093. [PubMed] [Google Scholar]
- Goodman S. I., Frerman F. E. Glutaric acidaemia type II (multiple acyl-CoA dehydrogenation deficiency). J Inherit Metab Dis. 1984;7 (Suppl 1):33–37. doi: 10.1007/BF03047371. [DOI] [PubMed] [Google Scholar]
- Goodman S. I., McCabe E. R., Fennessey P. V., Mace J. W. Multiple acyl-CoA dehydrogenase deficiency (glutaric aciduria type II) with transient hypersarcosinemia and sarcosinuria; possible inherited deficiency of an electron transfer flavoprotein. Pediatr Res. 1980 Jan;14(1):12–17. doi: 10.1203/00006450-198001000-00004. [DOI] [PubMed] [Google Scholar]
- Goodman S. I., Reale M., Berlow S. Glutaric acidemia type II: a form with deleterious intrauterine effects. J Pediatr. 1983 Mar;102(3):411–413. doi: 10.1016/s0022-3476(83)80665-9. [DOI] [PubMed] [Google Scholar]
- Goodman S. I., Stene D. O., McCabe E. R., Norenberg M. D., Shikes R. H., Stumpf D. A., Blackburn G. K. Glutaric acidemia type II: clinical, biochemical, and morphologic considerations. J Pediatr. 1982 Jun;100(6):946–950. doi: 10.1016/s0022-3476(82)80525-8. [DOI] [PubMed] [Google Scholar]
- Gregersen N., Kølvraa S., Rasmussen K., Christensen E., Brandt N. J., Ebbesen F., Hansen F. H. Biochemical studies in a patient with defects in the metabolism of acyl-CoA and sarcosine: another possible case of glutaric aciduria type II. J Inherit Metab Dis. 1980;3(3):67–72. doi: 10.1007/BF02312527. [DOI] [PubMed] [Google Scholar]
- HOSKINS D. D., MACKENZIE C. G. Solubilization and electron transfer flavoprtein requirement of mitochondrial sarcosine dehydrogenase and dimethylglycine dehydrogenase. J Biol Chem. 1961 Jan;236:177–183. [PubMed] [Google Scholar]
- Husain M., Steenkamp D. J. Electron transfer flavoprotein from pig liver mitochondria. A simple purification and re-evaluation of some of the molecular properties. Biochem J. 1983 Feb 1;209(2):541–545. doi: 10.1042/bj2090541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Dabrowski C., Tanaka K. Separation and properties of five distinct acyl-CoA dehydrogenases from rat liver mitochondria. Identification of a new 2-methyl branched chain acyl-CoA dehydrogenase. J Biol Chem. 1983 Jan 25;258(2):1066–1076. [PubMed] [Google Scholar]
- Inui K., Emmett M., Wenger D. A. Immunological evidence for deficiency in an activator protein for sulfatide sulfatase in a variant form of metachromatic leukodystrophy. Proc Natl Acad Sci U S A. 1983 May;80(10):3074–3077. doi: 10.1073/pnas.80.10.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKean M. C., Beckmann J. D., Frerman F. E. Subunit structure of electron transfer flavoprotein. J Biol Chem. 1983 Feb 10;258(3):1866–1870. [PubMed] [Google Scholar]
- McKean M. C., Frerman F. E., Mielke D. M. General acyl-CoA dehydrogenase from pig liver. Kinetic and binding studies. J Biol Chem. 1979 Apr 25;254(8):2730–2735. [PubMed] [Google Scholar]
- Niederwieser A., Steinmann B., Exner U., Neuheiser F., Redweik U., Wang M., Rampini S., Wendel U. Multiple acyl-Co A dehydrogenation deficiency (MADD) in a boy with nonketotic hypoglycemia, hepatomegaly, muscle hypotonia and cardiomyopathy. Detection of N-isovalerylglutamic acid and its monoamide. Helv Paediatr Acta. 1983 Mar;38(1):9–26. [PubMed] [Google Scholar]
- Orsi B. A., Tipton K. F. Kinetic analysis of progress curves. Methods Enzymol. 1979;63:159–183. doi: 10.1016/0076-6879(79)63010-0. [DOI] [PubMed] [Google Scholar]
- Przyrembel H., Wendel U., Becker K., Bremer H. J., Bruinvis L., Ketting D., Wadman S. K. Glutaric aciduria type II: report on a previously undescribed metabolic disorder. Clin Chim Acta. 1976 Jan 16;66(2):227–239. doi: 10.1016/0009-8981(76)90060-7. [DOI] [PubMed] [Google Scholar]
- Rhead W. J., Amendt B. A. Electron-transferring flavoprotein deficiency in the multiple acyl-CoA dehydrogenation disorders, glutaric aciduria type II and ethylmalonic--adipic aciduria. J Inherit Metab Dis. 1984;7 (Suppl 2):99–100. doi: 10.1007/978-94-009-5612-4_24. [DOI] [PubMed] [Google Scholar]
- Rhead W., Mantagos S., Tanaka K. Glutaric aciduria type II: in vitro studies on substrate oxidation, acyl-CoA dehydrogenases, and electron-transferring flavoprotein in cultured skin fibroblasts. Pediatr Res. 1980 Dec;14(12):1339–1342. doi: 10.1203/00006450-198012000-00013. [DOI] [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H. A new iron-sulfur flavoprotein of the respiratory chain. A component of the fatty acid beta oxidation pathway. J Biol Chem. 1977 Dec 10;252(23):8440–8445. [PubMed] [Google Scholar]
- Sweetman L., Nyhan W. L., Tauner D. A., Merritt T. A., Singh M. Glutaric aciduria Type II. J Pediatr. 1980 Jun;96(6):1020–1026. doi: 10.1016/s0022-3476(80)80629-9. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]