Abstract
Rapid influenza diagnostic tests (RIDTs) are commonly used by clinicians to guide patient management. Data on sensitivities among hospitalized patients are limited. Here, we evaluated the clinical and virologic factors affecting the sensitivities of 2 commercially available RIDTs (BinaxNOW Influenza A&B and QuickVue Influenza A+B) on nasopharyngeal aspirate (NPA) specimens collected from elderly patients and young children hospitalized for influenza. Influenza cases and age-matched negative controls were prospectively enrolled during the 2011-2012 influenza season in Hong Kong. NPA specimens were collected at presentation before antiviral treatment. Real-time reverse transcription-PCR (RT-PCR) results were used as references for the sensitivity analyses. One hundred patients (57 influenza cases and 43 controls) were studied. Both RIDTs had 100% specificities. The sensitivities of the BinaxNOW Influenza A&B and QuickVue Influenza A+B tests were 70% and 82%, respectively. For both tests, the sensitivities were lower in cases with presentation times beyond 2 days of illness onset than for those within this time (50 to 71% versus 85 to 91%, respectively). There were trends toward lower sensitivities for influenza B than for influenza A (66 to 81% versus 76 to 84%, respectively), among young children than among the elderly patients (63 to 78% versus 80 to 88%, respectively), and among cases with pneumonia than those without pneumonia (75% versus 82 to 94%, respectively). The sensitivities of the RIDTs decreased with reduced NPA viral RNA levels (5.6 to 15.0% reduction per 1-log decrease), which declined progressively after illness onset (Spearman's rho, −0.47 [P < 0.05] and −0.66 [P < 0.001] for influenza A and B, respectively). Collectively, late presentation, a low NPA viral load, and probably lower respiratory manifestation are factors associated with reduced sensitivities of RIDTs for diagnosing influenza in hospitalized patients. A negative RIDT result should be interpreted with caution.
INTRODUCTION
Seasonal influenza A and B viruses can cause severe respiratory tract infections, leading to hospitalizations and deaths among young children, elderly individuals, and those with underlying conditions (1–4). Emerging evidence suggests that patients hospitalized for severe influenza may benefit from early antiviral treatment, and this is recommended for all such patients according to the current clinical practice guidelines (5–8). A timely diagnosis of influenza is therefore essential. Reverse transcription-PCR (RT-PCR) is the most sensitive approach, but this assay may not be readily available in many health care settings. Instead, antigen-based rapid influenza diagnostic tests (RIDTs) are frequently used for case identification, especially in ambulatory care settings, because of their rapid turnaround time of around 15 min (9). Studies have shown that these tests have relatively low sensitivities when applied to outpatients (10 to 50%) because the quality of the specimens is often suboptimal, and the viral loads in such patients with mild infections are usually low (9). Data on the diagnostic performance of RIDTs among sicker, hospitalized patients are, however, limited. In this study, we evaluated clinical and virologic factors affecting the clinical sensitivities of 2 commercially available RIDTs (BinaxNOW Influenza A&B and QuickVue Influenza A+B) applied on nasopharyngeal aspirate (NPA) specimens collected from elderly patients and young children, the 2 age groups associated with the highest hospitalization rates of influenza in our settings (1).
MATERIALS AND METHODS
Patients and specimens.
The clinical management and diagnosis of patients hospitalized for influenza in our settings have been described previously (10–12). In brief, patients presenting with symptoms of acute respiratory tract infections were admitted based on clinical evaluations regardless of perceived etiology. NPA specimens were collected immediately after admission and before antiviral treatment. In our routine practice, NPA specimens were tested for a panel of common respiratory viruses, including influenza A and B viruses, parainfluenza 1, 2, and 3 viruses, respiratory syncytial viruses, and adenoviruses by immunofluorescence assays (IFA) because of their reasonably fast turnaround time (within hours) and moderately high sensitivity for diagnosis. Influenza virus cultures were also performed in parallel. Influenza A virus subtyping was performed by the regional WHO National Influenza Centre (Centre for Health Protection, Hong Kong). During the study period (December 2011 to March 2012, the seasonal peak in Hong Kong), cases and controls were enrolled on a fixed day each week. Freshly collected NPA specimens which tested positive for influenza A or B by routine IFA were age stratified into 2 groups (<5 years and ≥75 years) and then randomly selected for this study. Age-matched hospitalized patients with influenza-like illnesses who tested negative for influenza by IFA were randomly selected as controls. All the NPA specimens collected were immediately subjected to additional tests, including (i) quantitative one-step RT-PCR assays and (ii) antigen detection by 2 RIDTs, as described below. Ethical approval for conducting this study was granted by the institutional review board (Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee).
Routine influenza virus detection by direct immunofluorescence antigen tests and virus cultures.
Direct immunofluorescence antigen tests on NPA specimens was performed using a Light Diagnostics Influenza A & B DFA kit (Millipore, Billerica, MA). Influenza virus isolation was performed with standard tube cultures using Madin-Darby canine kidney cells. Cell cultures were monitored daily for evidence of cytopathic effects up to 10 days after inoculation.
Influenza NPA viral RNA level quantification by one-step RT-PCR assays.
Influenza viral RNA levels in NPA specimens were quantified by virus type-specific, probe-based, one-step RT-PCR assays targeting the viral matrix gene. Briefly, viral RNA was first extracted from residual NPA specimens using a PureLink Viral RNA/DNA minikit (Life Technologies, Carlsbad, CA). Viral RNA quantitative detection was then performed using a SuperScript III Platinum One-Step qRT-PCR kit (Life Technologies) on a StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA). For influenza A, primers and probe sequences as well as the thermal cycling profile from our previous study were adopted (13). For influenza B, the forward primer 5′-GAC GTC CAA AAR CTG GCA GAA-3′, the reverse primer 5′-TTG CCC CAA GRG ATC TCA-3′, and the hydrolysis probe 5′-FAM-TGC AAA GCA ACA TTG GA-MGB-3′ were used; the thermal cycling profile was 52°C for 30 min and 95°C for 2 min, followed by 40 cycles of 95°C for 15 s and 56°C for 1 min. A cycle threshold (CT) value for each NPA specimen was determined. Inferences of copy numbers were made with reference to serial dilutions of known amounts of plasmid standards containing target amplicons. The lower limits of detection for influenza A and B viral RNA were 250 copies/ml and 200 copies/ml of viral transport medium, respectively. RT-PCR positivity was used as a reference for comparing the sensitivity of other diagnostic methods.
Influenza virus detection by RIDTs.
Two commercially available RIDTs were evaluated: the BinaxNOW Influenza A&B test (Alere, Waltham, MA) and the QuickVue Influenza A+B test (Quidel, San Diego, CA). Residual NPA specimens in viral transport medium were immediately tested by a qualified technician in the research laboratory in parallel as per the manufacturers' instructions. Briefly, for the BinaxNOW Influenza A&B test, specimens were spotted onto the sample pads of the test devices with the transfer pipettes provided, and the results were read after an incubation of 15 min. For the QuickVue Influenza A+B test, specimens were mixed with extraction reagent in the extraction tubes provided. Test strips were then placed into extraction tubes, and the results were read after an incubation period of 10 min.
Statistical analysis.
The clinical sensitivities and specificities of RIDTs were calculated using RT-PCR assays as references. The chi-square test or Fisher's exact test was used for categorical comparisons, where appropriate. Correlations of the NPA viral RNA levels with the time intervals from illness onset were calculated using the Spearman rank correlation coefficient test. Univariate comparisons of NPA viral RNA levels with RIDT results (true positivity versus false negativity) were performed using the Mann-Whitney U tests. Cumulative probit regression analysis was used to estimate the effects of viral RNA level changes on the sensitivities of RIDTs. Statistical analyses were performed using SPSS Statistics, version 20 (IBM, Armonk, NY), or Prism 5.04 (GraphPad, La Jolla, CA), where appropriate. Two-tailed P values of <0.05 were considered statistically significant.
RESULTS
Patient descriptions.
Demographic, clinical, and virological features of the study population, which comprised 57 influenza cases and 43 controls, are summarized in Table 1. There were 32 young children (aged <5 years) and 25 elderly patients (aged ≥75 years). The male-to-female ratio was 1:1. Influenza A and B were diagnosed in 25 (44%) and 32 (56%), respectively, of the total 57 influenza cases. All influenza A cases but one were caused by virus subtype H3N2. The median time interval from illness onset to specimen collection was 2 days (interquartile range [IQR], 1 to 4 days). Notably, elderly cases presented earlier than young children (median, 1 [IQR, 1 to 2] versus 3.5 [2 to 6] days; P < 0.0001). A majority (80%) of elderly cases reported at least 1 underlying comorbid illness, whereas none was reported from young children. Pneumonia confirmed by chest X ray was observed in 8 (32%) elderly cases. Overall, patients in the control group had demographic and baseline characteristics comparable to those in patients with influenza.
TABLE 1.
Demographic, clinical, and virologic characteristics of the study population
| Characteristic | Values for young children (aged <5 yr)a |
Values for elderly patients (aged ≥75 yr) |
||||
|---|---|---|---|---|---|---|
| Case (n = 32) | Control (n = 18) | P value | Case (n = 25) | Control (n = 25) | P valuea | |
| Age (mean ± SD) (yr) | 3.0 ± 0.9 | 3.0 ± 1.0 | NS | 83.6 ± 6.3 | 82.5 ± 6.2 | NS |
| Male gender (n [%]) | 15 (47) | 7 (39) | NS | 12 (48) | 13 (52) | NS |
| Illness onset-to-presentation interval (median [IQR]) (days) | 3.5 (2.0–6.0) | 3.0 (1.5–4.0) | NS | 1.0 (1.0–2.0) | 1.0 (1.0–2.5) | NS |
| Virus subtype (n [%]) | ||||||
| Influenza A | 13 (41) | 12 (48) | ||||
| H1N1 | 1 (3) | 0 (0) | ||||
| H3N2 | 12 (38) | 12 (48) | ||||
| Influenza B | 19 (59) | 13 (52) | ||||
| Comorbidity (n [%]) | 0 (0) | 0 (0) | 20 (80) | 17 (68) | NS | |
| Pneumonia (n [%]) | 0 (0) | 3 (17) | <0.05 | 8 (32) | 10 (40) | NS |
Cases and controls were defined using RT-PCR assays as references. There were 7 influenza cases of young children who initially tested negative by immunofluorescence assay and later positive by RT-PCR assays for influenza B. The estimated sensitivity of the immunofluorescence assay was 88% for all influenza cases (with reference to RT-PCR assay). NS, not significant.
Diagnostic performance of RIDTs.
The diagnostic performances of the 2 RIDTs are summarized in Table 2. With RT-PCR positivity as a reference, both tests had 100% specificities. No false positivity was observed in the control group. The overall sensitivities of the BinaxNOW Influenza A&B and QuickVue Influenza A+B tests were 70% and 82%, respectively. For both tests, the sensitivities were lower in cases with a presentation time beyond 2 days of illness onset than in those presenting within that period (BinaxNOW Influenza A&B, 50% versus 85%, P < 0.01; QuickVue Influenza A+B, 71% versus 91%, P < 0.1). There were trends toward lower sensitivities for influenza B than for influenza A (BinaxNOW Influenza A&B, 66% versus 76%; QuickVue Influenza A+B, 81% versus 84%), among young children than the among elderly (63% versus 80% and 78% versus 88%) and among cases with pneumonia than those without pneumonia (75% versus 82% and 75% versus 94%). However, these trends were not statistically significant.
TABLE 2.
Clinical sensitivity of the two RIDTs and virus culture grouped by age group, virus type, clinical severity, and time of presentation
| Characteristic | No. of specimens | Sensitivity (%) usinga: |
||
|---|---|---|---|---|
| BinaxNOW Influenza A&B | QuickVue Influenza A+B | Virus culture | ||
| Virus type | ||||
| Influenza A | 25 | 76 | 84 | 80 |
| Influenza B | 32 | 66 | 81 | 88 |
| Age group | ||||
| Young children (<5 yr) | 32 | 63 | 78 | 81 |
| Elderly patients (≥75 yr) | 25 | 80 | 88 | 88 |
| Presence of pneumoniab | ||||
| Yes | 8 | 75 | 75 | 75 |
| No | 17 | 82 | 94 | 94 |
| Time of presentation | ||||
| ≤2 days | 33 | 85c | 91d | 94e |
| >2 days | 24 | 50 | 71 | 71 |
The sensitivity was calculated with reference to quantitative RT-PCR assays. Both RIDTs had 100% specificity.
The diagnosis was based on both clinical and radiological assessments. Only elderly cases were included for analysis because no young children had influenza-associated pneumonia in this cohort.
P < 0.01; chi-square or Fisher's exact tests, where appropriate.
P < 0.1.
P < 0.05.
NPA viral RNA levels and RIDT diagnostic performance.
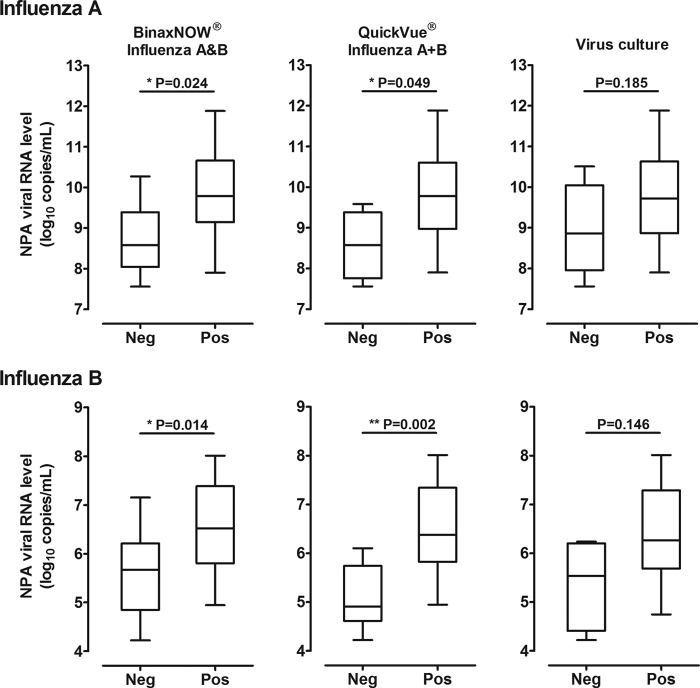
For both influenza virus types, the median (IQR) NPA viral RNA levels of elderly cases on admission were higher than those of young children (influenza A, 10.4 [9.0 to 11.2] versus 9.1 [8.4 to 9.8] copies/ml, P < 0.05; influenza B, 7.3 [6.1 to 7.5] versus 5.9 [5.0 to 6.2] copies/ml, P < 0.001). However, there were negative correlations between the viral RNA levels and times elapsed from onset (influenza A, rho = −0.471, P < 0.05; influenza B, rho = −0.655, P < 0.001) (Fig. 1). Samples with false-negative RIDT results had significantly lower viral RNA levels (by ∼1 log10 for both virus types) compared to those with true-positive results (Fig. 2). False-negative virus isolation results were similarly associated with lower viral RNA levels. The sensitivities of the 2 RIDTs declined progressively with decreases in viral RNA levels, as indicated by increasing CT values for the quantitative one-step RT-PCR assays (Table 3). Based on cumulative probit regression analysis, it was estimated that a 1 log10 decrease in the viral RNA concentration would result in 5.6% and 8.9% decreases in the sensitivities of the BinaxNOW Influenza A&B and QuickVue Influenza A+B tests for influenza A, respectively; for influenza B there were 7.9% and 15.0% decreases in sensitivities, respectively.
FIG 1.
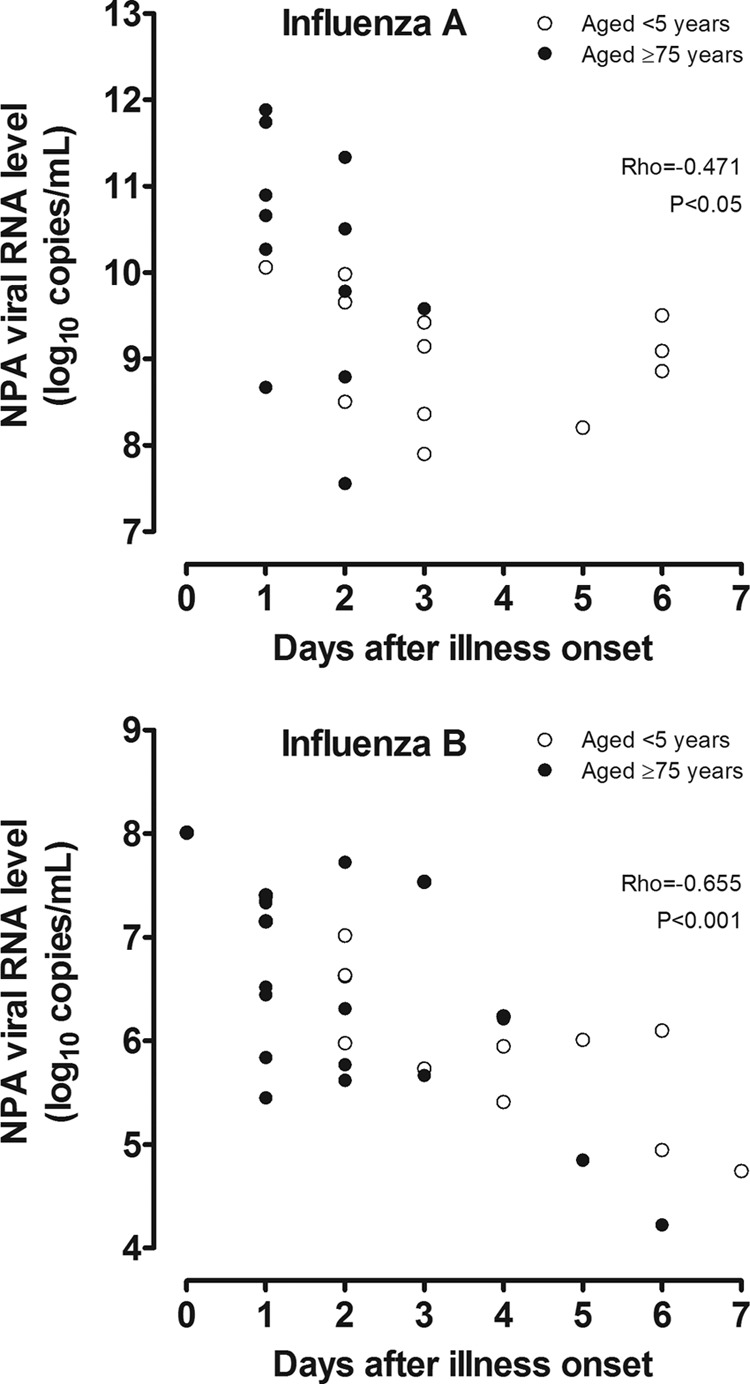
Nasopharyngeal aspirate (NPA) viral RNA levels of influenza A (upper panel) and influenza B (lower panel) as determined by virus type-specific quantitative one-step reverse transcription-PCR assays, shown according to the time from illness onset. For clarity, only specimens collected within 7 days after illness onset are shown.
FIG 2.
Box plots of nasopharyngeal aspirate (NPA) viral RNA levels of influenza A (upper panel) and influenza B (lower panel), shown according to false-negative (Neg) and true-positive (Pos) RIDT results. Similar plots of virus cultures are provided for reference.
TABLE 3.
Clinical sensitivities of the two RIDTs and virus cultures shown according to cycle threshold values of the quantitative one-step RT-PCR assays and the corresponding nasopharyngeal viral RNA levels
| CT valuea | Viral RNA level (log10 copies/ml) | No. of specimens | Sensitivity (%) of: |
||
|---|---|---|---|---|---|
| BinaxNOW Influenza A&B | QuickVue Influenza A+B | Virus culture | |||
| Influenza A | |||||
| <20 | >9.8 | 10 | 90 | 100 | 90 |
| 20–25 | 8.3–9.8 | 12 | 75 | 75 | 75 |
| >25 | <8.3 | 3 | 33 | 67 | 67 |
| Influenza B | |||||
| <25 | >6.8 | 9 | 89 | 100 | 100 |
| 25–30 | 5.5–6.8 | 16 | 63 | 88 | 88 |
| >30 | <5.5 | 7 | 43 | 43 | 71 |
CT, cycle threshold.
DISCUSSION
Our results indicate that although highly specific, RIDTs are only moderately sensitive for diagnosing seasonal influenza among hospitalized elderly patients and young children. The overall sensitivities of the QuickVue Influenza A+B and BinaxNOW Influenza A&B tests were 82% and 71%, respectively. The findings agree with those of other studies on these RIDTs using NPA specimens, which contain more epithelial cells, as specimens for testing (pooled sensitivities of 19.5 to 85.7% for the QuickVue Influenza A+B test [14–17] and 21.6 to 71.0% for the BinaxNOW Influenza A&B test [14, 18–20]). Notably, the sensitivity revealed in our population of hospitalized patients is among the highest reported for RIDTs.
We have identified several factors that may affect the sensitivities of RIDTs, which include the time elapsed from illness onset, the viral RNA level at presentation, the virus type, and possibly the predominant site of clinical involvement. Our observation that RIDTs have lower sensitivities for influenza B is consistent with a recent meta-analysis of 159 studies (9). Although RIDTs have been reported to perform better among young children (presumably related to the higher viral RNA levels among young children) (9), our data suggest that the sensitivity may not be lower in hospitalized elderly patients. This can be addressed, at least in part, by our findings. We found a significant negative correlation between time elapsed from illness onset and NPA viral RNA level, which explained the much reduced clinical sensitivity (>20%) among patients who presented after 2 days. Notably, this also suggests that the time of specimen collection is another important confounding factor for the clinical sensitivity of RIDTs and may partially explain the relatively higher sensitivity of the RIDTs in the elderly patients as they presented overall 2 days earlier than the young children in our study. The observation that the sensitivity of RIDTs tended to be lower in cases with pneumonia has serious clinical implications and should warrant further study. There are increasing reports of false-negative test results for upper respiratory samples in influenza patients who developed pneumonia (21), even with the use of PCR assays. Because of a relatively low level of viral shedding in the upper respiratory tract, obtaining a lower respiratory tract sample (e.g., sputum, tracheal aspirate, or bronchoalveolar lavage specimens) for testing is generally advisable (5, 22). Notably, our study provides useful data to show that the sensitivities of RIDTs for seasonal influenza virus infections are largely determined by the virus concentrations present in the specimens. Compared with samples with true-positive results, those with false-negative RIDT results had 10-fold lower NPA viral RNA levels. We estimate that the test sensitivity will be reduced by approximately 10% per 1 log decrease in the NPA viral RNA level. Our study supports the current view that a negative RIDT result does not necessarily exclude influenza, even among hospitalized patients with NPA specimens submitted for testing and must be interpreted with great caution (9, 22).
Our study has several limitations. The sample size was relatively small, and we only included two available RIDTs for study. Although the use of the less-sensitive IFA for case identification might tend to select cases with higher viral levels, highly sensitive RT-PCR assays were performed on both case and control groups and were used as the references for calculating RIDTs sensitivities. Since influenza B and A(H3N2) were the predominant circulating viruses during the 2011-2012 winter season (see http://www.chp.gov.hk/files/pdf/fluexpress_week26_7_4_2013_eng.pdf), we were unable to study the A(H1N1) 2009 virus, which is already known to be associated with a low RIDT sensitivity. We did not assess the effect of vaccination as data on vaccination status were incomplete in this cohort. However, the influenza vaccination rates of the age groups studied here in the Hong Kong population is generally low (30 to 40%) (10, 12).
In conclusion, we found that RIDTs have only moderate sensitivities for diagnosing influenza in hospitalized patients. Late presentation, low NPA viral RNA levels, and probably clinical manifestations of the lower respiratory tract are factors associated with the reduced sensitivities. In settings where these tests are used, clinicians should be aware of the limitations and pitfalls.
ACKNOWLEDGMENTS
This principal investigator-initiated study was jointly supported by a research grant from F. Hoffmann-La Roche Ltd., an internal research fund from the Department of Medicine and Therapeutics of the Chinese University of Hong Kong, and the Area of Excellence Scheme of the University Grants Committee of the HKSAR Government (grant AoE/M-12/06).
N. Lee has received honoraria for consultancy work from GlaxoSmithKline and conference support from Sanofi-Aventis Hong Kong Ltd., MSD (Asia) Ltd., and Pfizer Hong Kong. P.K.S. Chan has received consultancy fees and research funding from F. Hoffmann-La Roche and has received honoraria and conference support from GlaxoSmithKline. P.K.S. Chan has served as an advisory board member for GlaxoSmithKline.
Footnotes
Published ahead of print 27 November 2013
REFERENCES
- 1.Chan PK, Chan MC, Cheung JL, Lee N, Leung TF, Yeung AC, Wong MC, Ngai KL, Nelson EA, Hui DS. 2013. Influenza B lineage circulation and hospitalization rates in a subtropical city, Hong Kong, 2000-2010. Clin. Infect. Dis. 56:677–684. 10.1093/cid/cis885 [DOI] [PubMed] [Google Scholar]
- 2.Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, Simmerman JM, Gordon A, Sato M, Howie S, Krishnan A, Ope M, Lindblade KA, Carosone-Link P, Lucero M, Ochieng W, Kamimoto L, Dueger E, Bhat N, Vong S, Theodoratou E, Chittaganpitch M, Chimah O, Balmaseda A, Buchy P, Harris E, Evans V, Katayose M, Gaur B, O'Callaghan-Gordo C, Goswami D, Arvelo W, Venter M, Briese T, Tokarz R, Widdowson MA, Mounts AW, Breiman RF, Feikin DR, Klugman KP, Olsen SJ, Gessner BD, Wright PF, Rudan I, Broor S, Simoes EA, Campbell H. 2011. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 378:1917–1930. 10.1016/S0140-6736(11)61051-9 [DOI] [PubMed] [Google Scholar]
- 3.He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, Wang J, Chen CS, Chen J, Wildman RP, Klag MJ, Whelton PK. 2005. Major causes of death among men and women in China. N. Engl. J. Med. 353:1124–1134. 10.1056/NEJMsa050467 [DOI] [PubMed] [Google Scholar]
- 4.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. 2004. Influenza-associated hospitalizations in the United States. JAMA 292:1333–1340. 10.1001/jama.292.11.1333 [DOI] [PubMed] [Google Scholar]
- 5.Lee N, Ison MG. 2012. Diagnosis, management and outcomes of adults hospitalized with influenza. Antivir. Ther. 17:143–157. 10.3851/IMP2059 [DOI] [PubMed] [Google Scholar]
- 6.Hsu J, Santesso N, Mustafa R, Brozek J, Chen YL, Hopkins JP, Cheung A, Hovhannisyan G, Ivanova L, Flottorp SA, Saeterdal I, Wong AD, Tian J, Uyeki TM, Akl EA, Alonso-Coello P, Smaill F, Schunemann HJ. 2012. Antivirals for treatment of influenza: a systematic review and meta-analysis of observational studies. Ann. Intern. Med. 156:512–524. 10.7326/0003-4819-156-7-201204030-00411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayden FG, Pavia AT. 2006. Antiviral management of seasonal and pandemic influenza. J. Infect. Dis. 194(Suppl. 2):S119–S126. 10.1086/507552 [DOI] [PubMed] [Google Scholar]
- 8.Harper SA, Bradley JS, Englund JA, File TM, Gravenstein S, Hayden FG, McGeer AJ, Neuzil KM, Pavia AT, Tapper ML, Uyeki TM, Zimmerman RK. 2009. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1003–1032. 10.1086/598513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chartrand C, Leeflang MM, Minion J, Brewer T, Pai M. 2012. Accuracy of rapid influenza diagnostic tests: a meta-analysis. Ann. Intern. Med. 156:500–511. 10.7326/0003-4819-156-7-201204030-00403 [DOI] [PubMed] [Google Scholar]
- 10.Lee N, Chan PK, Choi KW, Lui G, Wong B, Cockram CS, Hui DS, Lai R, Tang JW, Sung JJ. 2007. Factors associated with early hospital discharge of adult influenza patients. Antivir. Ther. 12:501–508 [PubMed] [Google Scholar]
- 11.Lee N, Chan PK, Hui DS, Rainer TH, Wong E, Choi KW, Lui GC, Wong BC, Wong RY, Lam WY, Chu IM, Lai RW, Cockram CS, Sung JJ. 2009. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J. Infect. Dis. 200:492–500. 10.1086/600383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee N, Choi KW, Chan PK, Hui DS, Lui GC, Wong BC, Wong RY, Sin WY, Hui WM, Ngai KL, Cockram CS, Lai RW, Sung JJ. 2010. Outcomes of adults hospitalised with severe influenza. Thorax 65:510–515. 10.1136/thx.2009.130799 [DOI] [PubMed] [Google Scholar]
- 13.Chan MC, Lee N, Ngai KL, Wong BC, Lee MK, Choi KW, Lai RW, Chan PK. 2013. A “pre-seasonal” hospital outbreak of influenza pneumonia caused by the drift variant A/Victoria/361/2011-like H3N2 viruses, Hong Kong, 2011. J. Clin. Virol. 56:219–225. 10.1016/j.jcv.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 14.Chan KH, Chan KM, Ho YL, Lam YP, Tong HL, Poon LL, Cowling BJ, Peiris JS. 2012. Quantitative analysis of four rapid antigen assays for detection of pandemic H1N1 2009 compared with seasonal H1N1 and H3N2 influenza A viruses on nasopharyngeal aspirates from patients with influenza. J. Virol. Methods 186:184–188. 10.1016/j.jviromet.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 15.Harnden A, Brueggemann A, Shepperd S, White J, Hayward AC, Zambon M, Crook D, Mant D. 2003. Near patient testing for influenza in children in primary care: comparison with laboratory test. BMJ 326:480. 10.1136/bmj.326.7387.480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rouleau I, Charest H, Douville-Fradet M, Skowronski DM, De Serres G. 2009. Field performance of a rapid diagnostic test for influenza in an ambulatory setting. J. Clin. Microbiol. 47:2699–2703. 10.1128/JCM.00762-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruest A, Michaud S, Deslandes S, Frost EH. 2003. Comparison of the Directigen flu A+B test, the QuickVue influenza test, and clinical case definition to viral culture and reverse transcription-PCR for rapid diagnosis of influenza virus infection. J. Clin. Microbiol. 41:3487–3493. 10.1128/JCM.41.8.3487-3493.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diederen BM, Veenendaal D, Jansen R, Herpers BL, Ligtvoet EE, Ijzerman EP. 2010. Rapid antigen test for pandemic (H1N1) 2009 virus. Emerg. Infect. Dis. 16:897–898; author reply 898. 10.3201/eid1605.091574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuenzalida L, Blanco S, Prat C, Vivancos M, Dominguez MJ, Modol JM, Rodrigo C, Ausina V. 2010. Utility of the rapid antigen detection BinaxNOW Influenza A&B test for detection of novel influenza A (H1N1) virus. Clin. Microbiol. Infect. 16:1574–1576. 10.1111/j.1469-0691.2010.03160.x [DOI] [PubMed] [Google Scholar]
- 20.Nilsson AC, Alemo B, Bjorkman P, Dillner L, Melhus A, Nilsson B, Widell A. 2008. Around-the-clock, rapid diagnosis of influenza by means of membrane chromatography antigen testing confirmed by polymerase chain reaction. Infect. Control Hosp. Epidemiol. 29:177–179. 10.1086/526446 [DOI] [PubMed] [Google Scholar]
- 21.Lee N, Chan PK, Wong CK, Wong KT, Choi KW, Joynt GM, Lam P, Chan MC, Wong BC, Lui GC, Sin WW, Wong RY, Lam WY, Yeung AC, Leung TF, So HY, Yu AW, Sung JJ, Hui DS. 2011. Viral clearance and inflammatory response patterns in adults hospitalized for pandemic 2009 influenza A(H1N1) virus pneumonia. Antivir. Ther. 16:237–247. 10.3851/IMP1722 [DOI] [PubMed] [Google Scholar]
- 22.Self WH, McNaughton CD, Grijalva CG, Zhu Y, Chappell JD, Williams JV, Talbot HK, Shay DK, Griffin MR. 2012. Diagnostic performance of the BinaxNow Influenza A&B rapid antigen test in ED patients. Am. J. Emerg. Med. 30:1955–1961. 10.1016/j.ajem.2012.04.018. [DOI] [PubMed] [Google Scholar]