Abstract
Small-colony variants (SCVs) of bacteria are associated with recurrent and persistent infections. We describe for the first time SCVs of Streptococcus tigurinus in a patient with a prosthetic joint infection. S. tigurinus is a novel pathogen of the Streptococcus mitis group and causes invasive infections. We sought to characterize S. tigurinus SCVs using experimental methods and find possible genetic explanations for their phenotypes. The S. tigurinus SCVs were compared with the wild-type (WT) isolate using phenotypic methods, including growth under different conditions, autolysis, and visualization of the cell ultrastructure by use of transmission electron microscopy (TEM). Furthermore, comparative genome analyses were performed. The S. tigurinus SCVs displayed reduced growth compared to the WT and showed either a very stable or a fluctuating SCV phenotype. TEM analyses revealed major alterations in cell separation and morphological abnormalities, which were partially explained by impaired autolytic behavior. Intriguingly, the SCVs were more resistant to induced autolysis. Whole-genome sequencing revealed mutations in the genes involved in general cell metabolism, cell division, stringent response, and virulence. Clinically, the patient recovered after a 2-stage exchange of the prosthesis. Comparative whole-genome sequencing in clinical strains is a useful tool for identifying novel genetic signatures leading to the most persistent bacterial forms. The detection of viridans streptococcal SCVs is challenging in a clinical laboratory due to the small colony size. Thus, it is of major clinical importance for microbiologists and clinicians to be aware of viridans streptococcal SCVs, such as those of S. tigurinus, which lead to difficult-to-treat infections.
INTRODUCTION
Small-colony variants (SCVs) of bacteria are frequently associated with foreign-body material, such as cardiac devices (1, 2) and prosthetic joints (3), and cause recurrent and persistent infections, which makes definite eradication very difficult (3–5). SCVs are characterized by reduced growth, small colony size, and atypical colony morphology, and they are often linked to a deficiency in electron transport or thymidine biosynthesis resulting in auxotrophy for hemin, menadione, or thymidine (1, 6). The morphological and biochemical characteristics of SCVs have been extensively studied in staphylococci (1). However, SCVs are found in various genera and species, e.g., enterococci (7), Escherichia coli (8), and Pseudomonas aeruginosa (1).
Viridans streptococci are usually commensals of the oral cavity, but when entering the bloodstream, they can cause severe invasive infections (9). To date, the prevalence of SCVs in viridans streptococci has been rather unknown. A few reports described Streptococcus pneumoniae mucoid variants and SCVs in biofilms (10, 11). Considering the small colony size of viridans streptococci, the SCV phenotype might be easily overlooked due to overgrowth by the wild-type (WT) phenotype, when present. However, the accurate detection of these variants has a major clinical impact on patient management as well as antibiotic therapy.
We present for the first time a clinical case of a prosthetic joint infection (PJI) caused by SCVs of Streptococcus tigurinus, a novel species belonging to the Streptococcus mitis group. S. tigurinus causes severe invasive infections, such as infective endocarditis, spondylodiscitis, and meningitis (12, 13), and it is highly virulent in experimental animal models (14). S. tigurinus forms alpha-hemolytic, smooth, white-to-grayish colonies with a diameter of 0.5 to 1 mm after incubation at 37°C with CO2 for 24 h on sheep blood agar (13). The accurate identification of S. tigurinus by conventional phenotypic methods is limited because of the morphological resemblance to its closest related species, i.e., S. mitis, Streptococcus oralis, S. pneumoniae, Streptococcus pseudopneumoniae, and Streptococcus infantis. However, analyses of the 5′ end of the 16S rRNA gene allow for accurate identification of S. tigurinus, since a significant sequence demarcation to the most closely related species was demonstrated previously (13).
We characterized the SCV phenotype of S. tigurinus by experimental methods and applied whole-genome comparison to unravel the genetic changes associated with this most adapted and persistent form of S. tigurinus.
MATERIALS AND METHODS
Bacterial strains.
The clinical S. tigurinus SCV strains 2425 and 2426 were compared with their parental strain S. tigurinus 1366. As a reference strain, the type strain S. tigurinus AZ_3aT (CCOS 600; Culture Collection of Switzerland, Wädenswil, Switzerland) was included. The strains were taken from −80°C and grown on Columbia agar plates containing 5% defibrinated sheep blood (bioMérieux, Marcy l'Etoile, France) (COS) at 37°C with CO2 for 24 h.
Analysis of 16S rRNA gene.
An 800-bp fragment of the 16S rRNA gene was obtained as described previously (12). A 16S rRNA gene BLAST analysis was performed using the SmartGene software (SmartGene, Zug, Switzerland).
Antibiotic susceptibility testing.
MICs were determined using Etest strips (AB bioMérieux). Susceptibility testing was performed on Mueller-Hinton agar supplemented with 5% sheep blood, using overnight cultures at a 0.5 McFarland standard, followed by incubation at 35 ± 2°C with 5% CO2 for 20 to 24 h. In addition, the MICs were read at 48 h to take into account the slow growth of the SCVs. Interpretation was done according to the CLSI 2012 guidelines, if available (15).
Effects of serial passages and auxotrophic testing.
At least 8 passages were performed on COS by picking single colonies for incubation at 37°C with CO2 for 24 h. Auxotrophy for hemin, thymidine, and menadione was tested by the disk diffusion method. Commercially available standard disks of hemin (X-factor) were used (Sigma-Aldrich, Buchs, Switzerland), and blank disks were impregnated with 15 μl of menadione at 10 μg/ml, 25 μg/ml, and 125 μg/ml and of thymidine (Sigma-Aldrich) at 100 μg/ml, respectively. To determine auxotrophy, 0.5 McFarland standards of overnight cultures were swabbed on Mueller-Hinton agar, and the disks were placed on the agar surface. The isolate was considered an auxotroph if it showed normal-sized colonies or increased growth surrounding the disks compared to the periphery after 24 h to 48 h of incubation at 37°C with CO2 (16). Staphylococcus aureus MS17 with a hemB mutation (17) was used as a hemin auxotroph control, and the S. aureus strain A22616/3 (18) was used as a menadione auxotroph control. The experiments were repeated twice independently.
Growth curves.
Bacterial growth was monitored in brain heart infusion (BHI) broth (Becton, Dickinson, Germany) at 37°C using a microplate spectrophotometer (PowerWave XS; Bio-Tek, Winooski, VT, USA). Overnight cultures were diluted and adjusted to the same optical density at 600 nm (OD600), and 100 μl was transferred into a clear 96-well flat-bottom plate (BD Biosciences, Franklin Lakes, NJ, USA). To ensure homogeneous turbidity, the plate was shaken every 20 min for 10 s, and the optical density was measured hourly. The experiment was performed with six technical and three biological replicates.
Transmission electron microscopy.
The bacterial strains were grown until the exponential phase and centrifuged for 5 min at 5,000 rpm. Transmission electron microscopy (TEM) was performed by the Center of Microscopy and Image Analysis, University of Zurich, Zurich, Switzerland.
Autolysis experiments.
The bacterial strains were grown to exponential phase, harvested by centrifugation, and washed with phosphate-buffered saline (PBS) (pH 7.4). The OD600 was adjusted to 0.3 in 0.01 M sodium phosphate buffer (pH 7), and the cultures were split. Added to the culture was 0.01% Triton X-100 (TX) (Sigma-Aldrich) or an equal amount of phosphate buffer. Autolysis was monitored every hour at 37°C using a microplate spectrophotometer, with vigorous shaking every 20 min for 10 s. The experiment was performed with three technical and three biological replicates. Statistical analysis was done by Student's t test.
Whole-genome and comparative genomic analyses.
Purified bacterial genomic DNA was obtained from colonies on COS following cell disruption in lysis medium containing 150 U of mutanolysin, 500 U of achromopeptidase, and 40,000 U of lysozyme (Sigma-Aldrich) for 1 h. After lysis, the DNA was purified using a DNeasy kit (Qiagen AG, Hombrechtikon, Switzerland), according to the manufacturer's recommendations. Genomic DNA was subjected to whole-genome shotgun sequencing using a HiSeq 2000 system (Illumina, Inc.). A comparison of the genome content of S. tigurinus AZ_3aT and S. tigurinus 1366 has been described (19). Following fragmentation, end reparation, and sample tagging, the sequencer produced 1.27 and 0.85 million of reads for S. tigurinus 2425 and 2426, respectively.
Nucleotide sequence accession numbers.
This whole-genome shotgun project was deposited at DDBJ/EMBL/GenBank under the accession numbers ASWZ00000000 and ASXA00000000 for S. tigurinus SCV strains 2425 and 2426, respectively. The versions described in this paper are accession numbers ASWZ00000000.1 and ASXA00000000.1. Partial 16S rRNA gene sequences of the S. tigurinus strains 1366, 2425, and 2426 were deposited in GenBank under accession numbers KC598122, KC598123, and KC598124, respectively.
RESULTS
Case report.
One year after a cemented total knee arthroplasty (in which the cement contained gentamicin), an 83-year-old woman complained of gradually increasing pain leading to limited joint motion. On examination, the knee was hot and swollen with moderate effusion. No sinus tract was observed. Loosening of the tibial prosthesis was evident by conventional X-ray, and a PJI was suspected. Aspiration of the knee revealed purulent joint fluid with a leukocyte count of 17,700 cells/μl, with 94% neutrophils. Viridans streptococci grew in the culture obtained from the aspirate, displaying a normal phenotype (i.e., that of parental strain 1366), and it was subsequently identified as S. tigurinus by 16S rRNA gene analyses. The strain was susceptible to penicillin (MIC, 0.012 μg/ml). The dental status of the patient was checked, but no possible bacterial entry source was detected. A transesophageal echocardiogram showed no cardiac vegetation and there were no signs of respiratory infection. No antibiotic treatment had been started.
The patient was referred to a 2-stage exchange of a total knee prosthesis 4 weeks later. During surgery, pus surrounding the prosthesis was observed. Open debridement was performed and a cement spacer was implanted. Six biopsy samples from periprosthetic tissue were obtained before the intravenous administration of amoxicillin-clavulanate. In 5 out of 6 samples, viridans streptococci exhibiting slow growth and small and pinpointed colonies were cultured (SCV strains 2425 and 2426). They were confirmed to be S. tigurinus by 16S rRNA gene analyses, as they showed identical sequences to the rapidly growing parental strain 1366. Histopathologic examination showed signs of chronic inflammation of the synovial tissue. A late PJI was diagnosed according to the standard criteria (20). Penicillin was administered intravenously for 3 weeks until reimplantation. Three biopsy specimens from the periprosthetic tissue taken during reimplantation revealed no bacterial growth. Oral amoxicillin was administered for a total duration of 6 months. At the last follow-up examination 16 weeks after reimplantation, the patient showed no clinical or laboratory findings suggestive of infection.
Antibiotic susceptibility testing.
SCVs are frequently selected by antibiotic pressure (1). We did not observe any antibiotic susceptibility differences in the S. tigurinus SCVs compared to the parental strain. All strains displayed full susceptibility to penicillin (MIC range, 0.012 to 0.047 μg/ml), cefoxitin (MIC range, 1.0 to 2.0 μg/ml), ceftriaxone (MIC range, 0.032 to 0.125 μg/ml), and trimethoprim-sulfamethoxazole (MIC range, 1.9 to 7.6 μg/ml), and no high-level gentamicin resistance was observed (MIC range, 12 to 24 μg/ml). Similar results (≤1 dilution difference) were obtained when the MICs were reread at 48 h.
Colony morphology, effect of serial passages, and auxotrophic testing.
S. tigurinus 1366 and the AZ_3aT (data not shown) displayed normal colony morphologies, with a diameter of 0.4 to 0.5 mm (Fig. 1A). The SCV strain 2425 showed very small pinpoint colonies, with a diameter of 0.03 to 0.05 mm (Fig. 1B), whereas SCV strain 2426 produced colonies of various sizes, from very small to almost normal size, with a diameter ranging from 0.03 to 0.3 mm (Fig. 1C). Compared to the WT strains, alpha-hemolysis of the SCV strains was reduced.
FIG 1.
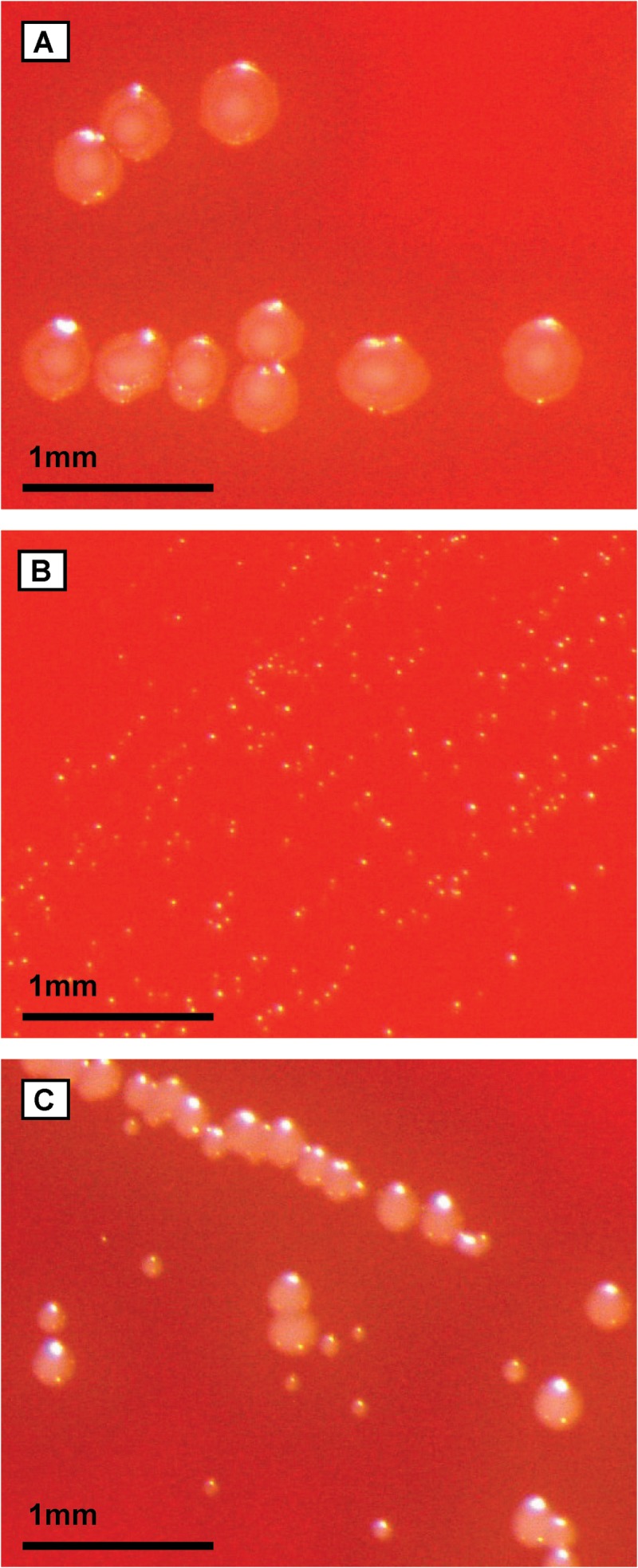
Colony morphologies of the S. tigurinus parental strain 1366 (A) and SCV strains 2425 (B) and 2426 (C) on sheep blood agar after 24 h of incubation.
The S. tigurinus SCV strains showed different phenotypes after serial passages. Strain 2425 retained a very stable SCV phenotype for >8 passages. Conversely, strain 2426 was unstable and showed a “fluctuating phenotype,” a phenomenon previously observed in S. aureus SCVs (2): following the initial reversion after 1 or 2 passages to their normal size, further passage of the normal colonies resulted in the reappearance of the SCV phenotype. Interestingly, for both S. tigurinus SCV strains, no auxotrophy for hemin, menadione, or thymidine was detected, even under prolonged incubation of 72 h (data not shown), suggesting that their SCV phenotypes were caused neither by a deficiency in electron transport nor in thymidine synthesis.
Growth curves.
In general, SCVs are characterized by slow growth (1); thus, we monitored the growth of S. tigurinus strains. The SCV strain 2425 displayed a slightly slower exponential growth and reached a lower final OD600, whereas SCV strain 2426 showed a considerably reduced exponential growth but reached similar OD600 levels after 12 h in comparison to the parental strain 1366 (Fig. 2). The S. tigurinus WT strains seem to have characteristic growth profiles, as strain 1366 grew faster in the exponential phase but reached the stationary phase earlier and at a lower OD600 than AZ_3aT (Fig. 2).
FIG 2.
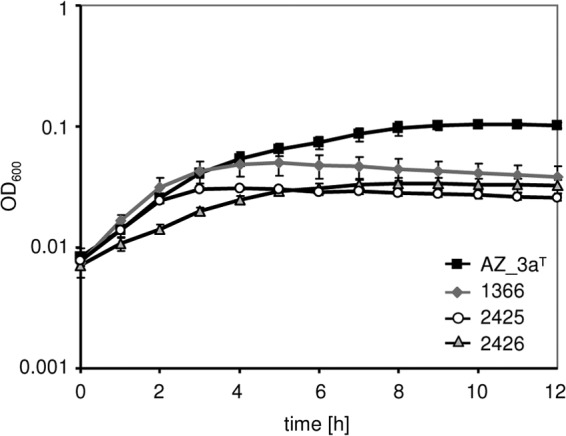
Growth of S. tigurinus strains AZ_3aT, 1366, 2425, and 2426 in BHI broth at 37°C. The mean values of three independent experiments performed in six replicates are shown. The error bars are standard deviations.
TEM analyses.
In addition to deficient growth, SCVs have been shown to display an increased cell size (6). The ultrastructure of S. tigurinus strains was therefore determined to assess whether cell division differed between the WT and SCV strains. S. tigurinus AZ_3aT (data not shown) and the parental strain 1366 displayed a normal phenotype, exhibiting regular cell separation with single cross walls (Fig. 3A and D). In contrast, the cells of SCVs were heterogeneous, frequently enlarged, and displayed aberrant cell separation with multiple cross walls, resulting in the formation of clustered cells (Fig. 3B, C, and F). Additionally, the SCV cells demonstrated aberrant morphological characteristics, such as mesosome-like structures (Fig. 3E and F).
FIG 3.
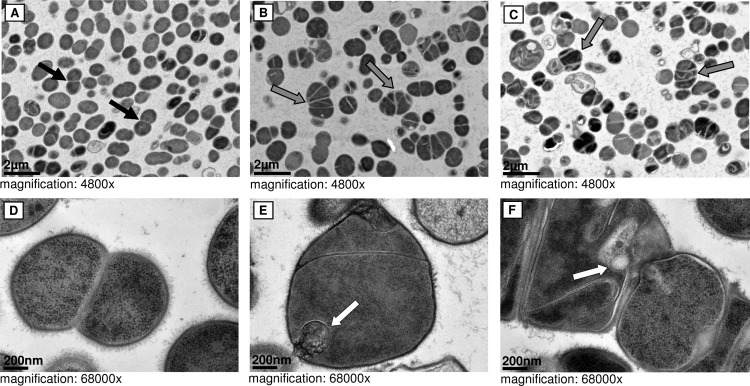
Cell morphology. TEM pictures of the S. tigurinus parental strain 1366 (A and D) and SCV strains 2425 (B and E) and 2426 (C and F). Black arrows, regular cell separation; gray arrows, atypical and enlarged SCV cells with multiple cross walls; white arrows, irregularly shaped cells with mesosome-like structures.
Autolysis experiments.
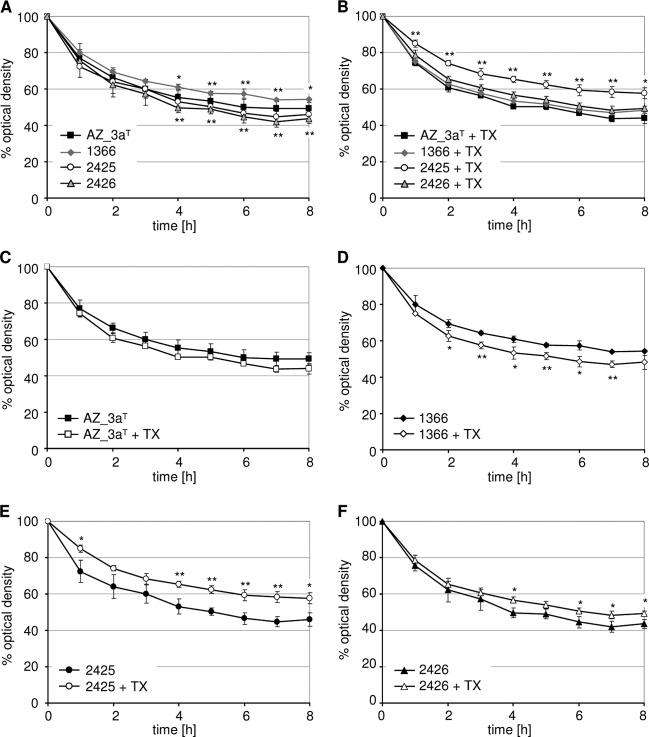
To assess whether the impaired cell separation was due to aberrant autolytic behavior, both spontaneous and induced autolytic conditions were tested. A considerable difference was observed in the extent of spontaneous autolysis between the WT strains AZ_3aT and 1366 (Fig. 4A), indicating that WT S. tigurinus strains can differ in several characteristics, including growth (see above) and autolysis. Spontaneous autolysis of both SCVs was significantly increased compared to the parental strain but in the range of the type strain AZ_3aT (Fig. 4A). Triton X-100-induced autolysis was significantly reduced in the SCV strain 2425 compared to the parental and the type strain (Fig. 4B), whereas the SCV strain 2426 showed comparable levels of autolysis to the WT strains. However, both SCV strains showed increased resistance to Triton X-100-induced autolysis, which was contrary to the WT strains, as seen in Fig. 4C to F, which showed strain-specific autolytic patterns.
FIG 4.
Spontaneous (A) and Triton X-100 (TX)-induced (B) autolysis of S. tigurinus strains AZ_3aT (C), 1366 (D), 2425 (E), and 2426 (F). The mean values of three independent experiments performed in triplicate are shown. The error bars are standard deviations. The statistical analyses shown in panels A and B were performed between the parental strain 1366 and the SCVs 2425 (asterisks above curve) and 2426 (asterisks below curve), respectively. *, P < 0.05; **, P < 0.01.
Whole-genome sequencing of S. tigurinus SCVs.
To identify any possible genetic reason for the observed differences between the S. tigurinus SCVs and the parental strain, the whole genomes of S. tigurinus SCV strains 2425 and 2426 were sequenced. De novo assembly was performed using Edena version 3.130110 (21) and resulted in core genomes of 1.87 Mb, with a G+C content of 40.9%, and 1,877 genes encoding a minimum of 1,823 proteins for S. tigurinus SCV strains 2425 and 2426. These two genomes were assembled in 15 and 25 contigs, respectively, and comparison at the single nucleotide polymorphism (SNP) level was performed using the MUMmer software package (22). The analysis revealed that the larger contigs were 850 kbp for strain 2425 and 739 kbp for strain 2426. The overall assembly values were satisfactory (for strain 2425, sum, 1.87 Mbp and N50, 672 kbp; for strain 2426, sum, 1.88 Mbp and N50, 520 kbp). For SCV strains 2425 and 2426, we detected the same number of genes as in the parental strain 1366 (19) and did not find any large deletions. However, several differences were found between strains 1366, 2425, and 2426. Strain 2426 contains two plasmids (contig GenBank accession no. ASXA01000015, 7,309 bp, and ASXA01000016, 2,491 bp) that are absent from the two other strains. After detailed sequence comparisons, 45 mutations were detected in the core genome (Tables 1 to 3), most of them being SNPs. Overall, one-half of the mutations (23/45) were identified in both SCV mutants at the same positions, suggesting that SCV strains 2425 and 2426 might have evolved from a common SCV precursor clone (Table 1). Only 7 mutations were identified in noncoding regions (Tables 1 to 3). Selected genes carrying mutations are described below regarding their possible relevance for the SCV phenotypes.
TABLE 1.
Mutations and their locations identified in S. tigurinus SCV strains 2425 and 2426 relative to parental strain 1366
| Strain 1366 contig GenBank accession no. | Position | Mutation | Effect | Locus tag(s) | Gene product(s) |
|---|---|---|---|---|---|
| AORX01000001 | 20263 to 23836 | Inversiona | Unknown | H353_00090 and H353_00095 | Serine hydroxymethyltransferase and predicted ATPase |
| AORX01000001 | 27927 | G→A | Synonymous | H353_00130 | Hypothetical protein |
| AORX01000001 | 64202 | •→T | Frameshift | H353_00315 | Homoserine kinase ThrB |
| AORX01000001 | 138651 | A→C | 104 F→L | H353_00685 | Amino acid ABC transporter ATP-binding protein |
| AORX01000001 | 159403 | T→C | Synonymous | H353_00795 | Uracil phosphoribosyltransferase (Upp) |
| AORX01000001 | 210055 | C→T | 162 A→V | H353_01045 | Pyridine nucleotide-disulfide oxidoreductase |
| AORX01000001 | 494595 | C→A | 98 C→F | H353_02420 | Acetyltransferase, GNAT family protein |
| AORX01000001 | 615189 | T→C | Intergenic | ||
| AORX01000001 | 665652 | G→A | 102 R→STOP | H353_03270 | Zn2+-responsive transcriptional repressor AdcR |
| AORX01000001 | 754073 | A→C | 85 S→R | H353_03690 | β-Lactamase |
| AORX01000001 | 758016 | C→T | 364 A→V | H353_03705 | Cell division protein FtsH |
| AORX01000002 | 34504 | C→T | Intergenic | ||
| AORX01000002 | 92064 | C→T | 61 S→F | H353_04258 | 4′-Phosphopantetheinyl transferase AcpS |
| AORX01000002 | 235844 | A→T | 58 I→F | H353_04923 | Hypothetical protein |
| AORX01000002 | 454207 | A→C | 182 D→A | H353_06053 | F0F1- ATP synthase alpha subunit |
| AORX01000002 | 501697 | C→G | 530 S→STOP | H353_06288 | Phosphoenolpyruvate-protein phosphotransferase |
| AORX01000002 | 610271 | •→T | Frameshift | H353_06833 | ATPase component of ABC transporter |
| AORX01000002 | 670375 | T→C | Synonymous | H353_07143 | 3-Isopropylmalate dehydrogenase |
| AORX01000002 | 708850 | A→• | Intergenic | ||
| AORX01000003 | 16071 | •→T | Frameshift | H353_07674 | Glutamine synthetase |
| AORX01000003 | 55915 | C→T | Synonymous | H353_07859 | Hypothetical protein |
| AORX01000003 | 75333 | G→A | 243 A→V | H353_07954 | Peptide ABC transporter permease |
| AORX01000005 | 31194 | A→G | 297 R→G | H353_08704 | Maltodextrin ABC transporter permease |
Full 3.5-kbp inversion; both H353_00090 and H353_00095 are complete, and the next gene (H353_00100) is annotated as tyrosine recombinase. •, deleted position.
TABLE 3.
Mutations and their locations identified only in S. tigurinus SCV strain 2426 relative to parental strain 1366
| Strain 1366 contig accession no. | Position | Mutationa | Effect | Locus tag | Gene product |
|---|---|---|---|---|---|
| AORX01000001 | 159120 | C→G | 26 R→P | H353_00790 | ATP-dependent Clp protease ClpP, proteolytic subunit |
| AORX01000001 | 217597 | A→C | 25 S→R | H353_01080 | Transporter, major facilitator family protein |
| AORX01000001 | 272919 | G→A | 182 M→I | H353_01325 | Manganese ABC transporter permease |
| AORX01000001 | 323922 | G→T | 424 T→K | H353_01545 | Hypothetical protein-type I RM system |
| AORX01000001 | 475131 | •→“CTCA” | Frameshift | H353_02310 | Hypothetical protein |
| AORX01000001 | 686770 | G→A | 158 L→F | H353_03365 | ATP-dependent Clp protease ClpP, ATP-binding subunit |
| AORX01000001 | 717575 | C→T | 53 A→T | H353_03520 | 30S ribosomal protein S2 RpsB |
| AORX01000002 | 63127 | G→T | 132 G→V | H353_04073 | 7,8-Dihydro-8-oxoguanine triphosphatase MutT |
| AORX01000002 | 180239 | G→T | 292 D→Y | H353_04668 | Endoglucanase |
| AORX01000004 | 21522 | G→T | 280 G→V | H353_08358 | Phosphoribosylformylglycinamidine synthase |
| AORX01000005 | 48072 | C→T | 31 G→E | H353_08809 | Hypothetical protein |
| AORX01000007 | 38264 | A→G | Intergenic |
•, deleted position.
Mutated genes in S. tigurinus SCVs are involved in stringent response and virulence.
Almost all genes of S. tigurinus SCVs found to be altered in their nucleotide sequence (Tables 1 to 3) have been identified as differentially regulated either in S. aureus SCVs (23) or under conditions inducing a stringent response (24). Alterations in the stringent response that confer growth defects were previously described for S. aureus SCVs (25). thrB (which encodes the enzyme homoserine kinase) (Table 1) was found to be changed in S. aureus SCVs and under stringent response conditions (23, 24). Homoserine kinase is a key element in threonine metabolism, a pathway that contributes to virulence in different microorganisms (26–28). The 7,8-dihydro-8-oxoguanine triphosphatase MutT of the Nudix superfamily is downregulated under stringent response conditions (Table 3) (24). MutT is proposed to protect cells from mutagenic nucleotides (29). Two other genes more directly related to virulence expression were identified in our study: the gene encoding the Zn2+-responsive transcriptional repressor AdcR was found to be truncated in both SCVs (Table 1). In Streptococcus suis, deletion of the adcR gene leads to a growth defect (30); thus, truncation of this regulator might have contributed to the SCV phenotypes of S. tigurinus strains 2425 and 2426. Furthermore, Zn2+, being an important trace metal ion, has been shown to regulate the expression of several virulence genes in streptococci (31); AdcR truncation might therefore have played a role in causing the infection reported here. The second factor likely affecting virulence, the iron uptake ABC transporter ATP-binding protein (Table 2), was found to be mutated in SCV strain 2425. It is reported to be involved in various stress responses and is essential for the expression of virulence in animal models of acute pneumonia caused by S. pneumoniae (32).
TABLE 2.
Mutations and their locations identified only in S. tigurinus SCV strain 2425 relative to parental strain 1366
| Strain 1366 contig GenBank accession no. | Position | Mutationa | Effect | Locus tag | Gene product |
|---|---|---|---|---|---|
| AORX01000001 | 189337 | G→C | 171 S→T | H353_00940 | Ribosomal small subunit pseudouridine synthase A |
| AORX01000001 | 192466 | A→G | 292 E→G | H353_00965 | N-Acetylmuramoyl-l-alanine amidase (Atl) |
| AORX01000001 | 238421 | •→T | Intergenic | ||
| AORX01000001 | 575645 | •→A | Intergenic | ||
| AORX01000001 | 682287 | G→A | Synonymous | H353_03350 | Hypothetical protein |
| AORX01000001 | 758211 | C→T | 429 A→V | H353_03705 | Cell division protein FtsH |
| AORX01000002 | 169627 | •→“TATA” | Intergenic | ||
| AORX01000002 | 258156 | G→T | 22 S→I | H353_05018 | Glycyl-tRNA ligase beta subunit (GlyS) |
| AORX01000003 | 31503 | A→T | 18 D→V | H353_07734 | Iron uptake ABC transporter ATP-binding protein |
| AORX01000007 | 22400 to 22392 | “TGTGATGAG”→ • | 73 “CDE”→• | H353_09253 | Hypothetical protein |
•, deleted position.
Mutated genes in S. tigurinus SCVs affecting cell metabolism.
For both S. tigurinus 2425 and 2426, we identified a mutation in the acpS gene encoding the 4′-phosphopantetheinyl transferase AcpS, which is an important enzyme in the type II fatty acid biosynthesis pathway (Table 1). Fatty acids are essential components of bacterial membrane lipids and lipopolysaccharides (33).
SCV mutants carrying a defect in the electron transport system have a reduced membrane potential, which is the driving force for the ATP synthetic machinery, and therefore, they produce less ATP (1). Reduced amounts of ATP, which furnishes energy for numerous biological processes, are assumed to contribute to the growth defect in these SCVs. Interestingly, we have identified a mutation in the atpA gene, which encodes the FoF1-ATP synthase alpha subunit (Table 1). Yet, whether the mutation we found in the S. tigurinus SCV strains contributes to the observed changes in growth remains to be determined.
Mutated genes in S. tigurinus SCVs affecting autolysis and cell division.
In S. tigurinus SCV 2425, we found a mutation of the autolytic N-acetylmuramoyl-l-alanine amidase, Atl (Table 2) (34). Autolysins are peptidoglycan hydrolases that play important roles in cellular processes, such as lysis of the bacterial septum after cell division, cell wall growth, cell wall turnover, and recycling of muropeptides (34). Since strain 2425 did not show a generally decreased level of autolysis, further work is required to determine how the mutation might have contributed to a higher resistance only to Triton X-100-induced autolysis.
Other mutations affecting cell division were found in the cell division protein FtsH of both S. tigurinus SCVs (Tables 1 and 2); however, the importance of these SNPs remains to be assessed in future experiments.
DISCUSSION
We present here the first clinical case of S. tigurinus SCVs in a patient with PJI. Although PJIs caused by viridans streptococci are less frequently encountered and are associated with a better outcome than those caused by other microorganisms (35, 36), caution must be exercised not only in view of the pathogenic potential of species, such as S. tigurinus, but also because the development of SCVs may lead to difficult-to-treat infections. By applying the newest technologies, i.e., comparative complete genome analyses, we found possible genetic reasons for the SCV phenotype observed in S. tigurinus. A number of SNPs were identified in the S. tigurinus SCV strains evolving from an infective S. tigurinus parental strain in genes likely to be associated with cell division and growth, autolytic behavior, metabolic key reactions, virulence, and stringent response.
The S. tigurinus SCVs showed typical SCV characteristics, such as slow growth, whereas other phenotypic traits frequently found in SCVs were missing. We did not observe any auxotrophy nor did we detect any difference in the antimicrobial susceptibility patterns of the SCVs compared to those of their parental strain. No long-term antibiotic therapy and thus no antibiotic pressures were documented in our patient, which might explain the selection of such bacterial forms. Nevertheless, the cement of the primary arthroplasty contained gentamicin, which can select for SCVs in staphylococci (37). Serial passages revealed different S. tigurinus SCV phenotypes: strain 2425 retained a very stable SCV phenotype, whereas strain 2426 showed a fluctuating phenotype, with reversion to normal-sized colonies and back to SCVs after further passages. The simplest explanation for this difference between the two SCVs might be the presence of an SNP in the mutT gene. The deletion of mutT has previously been shown to increase mutation rates in E. coli (38). Furthermore, higher mutation rates due to oxidative stress, producing damaged nucleotides that are mutagenic and normally degraded by MutT, have been associated with the emergence of SCVs in S. pneumoniae (11). However, it remains to be confirmed whether the specific SNP found in the SCV strain 2426 increases the mutation rate.
To the best of our knowledge, only a few SCVs have been analyzed by whole-genome sequencing to date, and a maximum of 4 mutations were found in these cases (25, 39). The S. tigurinus SCVs carry relatively high numbers of SNPs: 33 in strain 2425 and 35 in strain 2426. It is possible that numerous mutations usually accumulate in clinical SCVs, a phenomenon that has possibly not yet been reported due to the high costs and limited availability of whole-genome sequencing approaches. Should the SNP in the mutT gene in SCV strain 2426 prove to increase mutation rates, one might speculate that a common ancestor of the SCVs 2425 and 2426 existed that had a mutator phenotype. This would explain the numerous mutations and why approximately 50% of the mutations were found in both S. tigurinus SCV strains. In that assumptive model, further SNPs were individually accumulated by the two SCVs before mutT changed back to the WT allele in strain 2425.
Alterations in the cell wall structure leading to abnormal bacterial growth and irregular cell shapes were previously described for S. oralis, a species closely related to S. tigurinus. Horne et al. (40) observed typical SCV cell morphologies, such as heterogeneous cell sizes, irregular septa, cell clusters, and slow growth in S. oralis bacteria with choline-deprived wall teichoic acids. We found abnormal cell division and autolytic behavior in S. tigurinus SCVs, possibly influenced by alterations of the cell envelope components and autolysins. For instance, we detected a mutation in the acpS gene in both S. tigurinus SCVs. AcpS not only affects the biosynthesis of fatty acids but also of the d-alanylated lipoteichoic acids (LTAs) of bacterial cell walls (41). Modulations in the d-alanyl content of the cell wall directly influence the autolytic mechanism (41). We hypothesize that AcpS function was affected by the mutation, leading to deficiencies of d-alanylated LTAs, which are inhibitors of cell autolysis (42). In agreement with these findings, we observed a significant increase in spontaneous autolysis of the S. tigurinus SCVs. In contrast, Triton X-100-induced autolysis was reduced. Triton X-100 is known to be a potent inducer of cell autolysis (43); therefore, one would expect increased autolysis. The opposite autolytic behavior caused by the presence of Triton X-100 in the S. tigurinus SCVs must be due to additional alterations in the cell envelope properties or autolysin regulation compared to the autolytic behavior that might be caused by the mutated acpS and might not be reflected at the genome level.
Comparative genomic analysis is a useful tool to enlighten putative pathogenic mechanisms in clinical strains. Future investigations will assess the correlation between the mutations found and the SCV phenotype in S. tigurinus. The accumulation of these mutations is probably not the result of random events but rather is from the emergence of adapted variants under selective antibiotic pressure conditions that survive in a hostile environment. Clinicians and microbiologists should be aware of this most adapted and persistent form of S. tigurinus leading to difficult-to-treat infections, as it has a major clinical impact on appropriate patient management.
ACKNOWLEDGMENTS
The study was supported by the University of Zurich and the Gottfried und Julia Bangerter-Rhyner-Stiftung to C. Quiblier.
We thank the laboratory technicians for their dedicated help and A. Kaech and U. Luethy from the Center for Microscopy and Image Analysis, University of Zurich, for the TEM analysis. We also thank C. von Eiff, Institute of Medical Microbiology, University Hospital Münster, Germany, for kindly providing the menadione control strain.
Footnotes
Published ahead of print 27 November 2013
REFERENCES
- 1.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4:295–305. 10.1038/nrmicro1384 [DOI] [PubMed] [Google Scholar]
- 2.Maduka-Ezeh A, Seville MT, Kusne S, Vikram HR, Blair JE, Greenwood-Quaintance K, Arabia F, Patel R. 2012. Thymidine auxotrophic Staphylococcus aureus small-colony variant endocarditis and left ventricular assist device infection. J. Clin. Microbiol. 50:1102–1105. 10.1128/JCM.01170-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sendi P, Rohrbach M, Graber P, Frei R, Ochsner PE, Zimmerli W. 2006. Staphylococcus aureus small colony variants in prosthetic joint infection. Clin. Infect. Dis. 43:961–967. 10.1086/507633 [DOI] [PubMed] [Google Scholar]
- 4.Vaudaux P, Kelley WL, Lew DP. 2006. Staphylococcus aureus small colony variants: difficult to diagnose and difficult to treat. Clin. Infect. Dis. 43:968–970. 10.1086/507643 [DOI] [PubMed] [Google Scholar]
- 5.von Eiff C, Becker K, Metze D, Lubritz G, Hockmann J, Schwarz T, Peters G. 2001. Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with Darier's disease. Clin. Infect. Dis. 32:1643–1647. 10.1086/320519 [DOI] [PubMed] [Google Scholar]
- 6.Kahl BC, Belling G, Reichelt R, Herrmann M, Proctor RA, Peters G. 2003. Thymidine-dependent small-colony variants of Staphylococcus aureus exhibit gross morphological and ultrastructural changes consistent with impaired cell separation. J. Clin. Microbiol. 41:410–413. 10.1128/JCM.41.1.410-413.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groebner S, Beck J, Schaller M, Autenrieth IB, Schulte B. 2012. Characterization of an Enterococcus faecium small-colony variant isolated from blood culture. Int. J. Med. Microbiol. 302:40–44. 10.1016/j.ijmm.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 8.Sendi P, Frei R, Maurer TB, Trampuz A, Zimmerli W, Graber P. 2010. Escherichia coli variants in periprosthetic joint infection: diagnostic challenges with sessile bacteria and sonication. J. Clin. Microbiol. 48:1720–1725. 10.1128/JCM.01562-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spellerberg B, Brandt C. 2011. Streptococcus, p 331–349 In Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW. (ed), Manual of clinical microbiology, 10th ed, vol 1 ASM Press, Washington, DC [Google Scholar]
- 10.Allegrucci M, Sauer K. 2007. Characterization of colony morphology variants isolated from Streptococcus pneumoniae biofilms. J. Bacteriol. 189:2030–2038. 10.1128/JB.01369-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allegrucci M, Sauer K. 2008. Formation of Streptococcus pneumoniae non-phase-variable colony variants is due to increased mutation frequency present under biofilm growth conditions. J. Bacteriol. 190:6330–6339. 10.1128/JB.00707-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zbinden A, Mueller NJ, Tarr PE, Eich G, Schulthess B, Bahlmann AS, Keller PM, Bloemberg GV. 2012. Streptococcus tigurinus, a novel member of the Streptococcus mitis group, causes invasive infections. J. Clin. Microbiol. 50:2969–2973. 10.1128/JCM.00849-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zbinden A, Mueller NJ, Tarr PE, Spröer C, Keller PM, Bloemberg GV. 2012. Streptococcus tigurinus sp. nov., isolated from blood of patients with endocarditis, meningitis and spondylodiscitis. Int. J. Syst. Evol. Microbiol. 62:2941–2945. 10.1099/ijs.0.038299-0 [DOI] [PubMed] [Google Scholar]
- 14.Veloso TR, Zbinden A, Andreoni F, Giddey M, Vouillamoz J, Moreillon P, Zinkernagel AS, Entenza JM. 2013. Streptococcus tigurinus is highly virulent in a rat model of experimental endocarditis. Int. J. Med. Microbiol. 303:498–504. 10.1016/j.ijmm.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute 2012. Performance standards for antimicrobial susceptibility testing; 22nd informational supplement. CLSI M100-S22 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 16.Kahl B, Herrmann M, Everding AS, Koch HG, Becker K, Harms E, Proctor RA, Peters G. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 177:1023–1029. 10.1086/515238 [DOI] [PubMed] [Google Scholar]
- 17.Senn MM, Bischoff M, von Eiff C, Berger-Bächi B. 2005. σB activity in a Staphylococcus aureus hemB mutant. J. Bacteriol. 187:7397–7406. 10.1128/JB.187.21.7397-7406.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lannergård J, von Eiff C, Sander G, Cordes T, Seggewiss J, Peters G, Proctor RA, Becker K, Hughes D. 2008. Identification of the genetic basis for clinical menadione-auxotrophic small-colony variant isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 52:4017–4022. 10.1128/AAC.00668-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gizard Y, Zbinden A, Schrenzel J, François P. 2013. Whole-genome sequences of Streptococcus tigurinus type strain AZ_3a and S. tigurinus 1366, a strain causing prosthetic joint infection. Genome Announc. 1(2):e00210-12. 10.1128/genomeA.00210-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR, Infectious Diseases Society of America 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 56:1–10. 10.1093/cid/cis803 [DOI] [PubMed] [Google Scholar]
- 21.Hernandez D, François P, Farinelli L, Østerås M, Schrenzel J. 2008. De novo bacterial genome sequencing: millions of very short reads assembled on a desktop computer. Genome Res. 18:802–809. 10.1101/gr.072033.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. 2004. Versatile and open software for comparing large genomes. Genome Biol. 5:R12. 10.1186/gb-2004-5-2-r12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seggewiss J, Becker K, Kotte O, Eisenacher M, Yazdi MR, Fischer A, McNamara P, Al Laham N, Proctor R, Peters G, Heinemann M, von Eiff C. 2006. Reporter metabolite analysis of transcriptional profiles of a Staphylococcus aureus strain with normal phenotype and its isogenic hemB mutant displaying the small-colony-variant phenotype. J. Bacteriol. 188:7765–7777. 10.1128/JB.00774-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson KL, Roberts C, Disz T, Vonstein V, Hwang K, Overbeek R, Olson PD, Projan SJ, Dunman PM. 2006. Characterization of the Staphylococcus aureus heat shock, cold shock, stringent, and SOS responses and their effects on log-phase mRNA turnover. J. Bacteriol. 188:6739–6756. 10.1128/JB.00609-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao W, Chua K, Davies JK, Newton HJ, Seemann T, Harrison PF, Holmes NE, Rhee HW, Hong JI, Hartland EL, Stinear TP, Howden BP. 2010. Two novel point mutations in clinical Staphylococcus aureus reduce linezolid susceptibility and switch on the stringent response to promote persistent infection. PLoS Pathog. 6:e1000944. 10.1371/journal.ppat.1000944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paik S, Senty L, Das S, Noe JC, Munro CL, Kitten T. 2005. Identification of virulence determinants for endocarditis in Streptococcus sanguinis by signature-tagged mutagenesis. Infect. Immun. 73:6064–6074. 10.1128/IAI.73.9.6064-6074.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau GW, Haataja S, Lonetto M, Kensit SE, Marra A, Bryant AP, McDevitt D, Morrison DA, Holden DW. 2001. A functional genomic analysis of type 3 Streptococcus pneumoniae virulence. Mol. Microbiol. 40:555–571. 10.1046/j.1365-2958.2001.02335.x [DOI] [PubMed] [Google Scholar]
- 28.Coulter SN, Schwan WR, Ng EY, Langhorne MH, Ritchie HD, Westbrock-Wadman S, Hufnagle WO, Folger KR, Bayer AS, Stover CK. 1998. Staphylococcus aureus genetic loci impacting growth and survival in multiple infection environments. Mol. Microbiol. 30:393–404. 10.1046/j.1365-2958.1998.01075.x [DOI] [PubMed] [Google Scholar]
- 29.Mildvan AS, Xia Z, Azurmendi HF, Saraswat V, Legler PM, Massiah MA, Gabelli SB, Bianchet MA, Kang LW, Amzel LM. 2005. Structures and mechanisms of Nudix hydrolases. Arch. Biochem. Biophys. 433:129–143. 10.1016/j.abb.2004.08.017 [DOI] [PubMed] [Google Scholar]
- 30.Aranda J, Garrido ME, Fittipaldi N, Cortés P, Llagostera M, Gottschalk M, Barbé J. 2010. The cation-uptake regulators AdcR and Fur are necessary for full virulence of Streptococcus suis. Vet. Microbiol. 144:246–249. 10.1016/j.vetmic.2009.12.037 [DOI] [PubMed] [Google Scholar]
- 31.Shafeeq S, Kloosterman TG, Kuipers OP. 2011. Transcriptional response of Streptococcus pneumoniae to Zn2+ limitation and the repressor/activator function of AdcR. Metallomics 3:609–618. 10.1039/c1mt00030f [DOI] [PubMed] [Google Scholar]
- 32.Brown JS, Gilliland SM, Holden DW. 2001. A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol. Microbiol. 40:572–585. 10.1046/j.1365-2958.2001.02414.x [DOI] [PubMed] [Google Scholar]
- 33.McAllister KA, Peery RB, Meier TI, Fischl AS, Zhao G. 2000. Biochemical and molecular analyses of the Streptococcus pneumoniae acyl carrier protein synthase, an enzyme essential for fatty acid biosynthesis. J. Biol. Chem. 275:30864–30872. 10.1074/jbc.M004475200 [DOI] [PubMed] [Google Scholar]
- 34.Antignac A, Sieradzki K, Tomasz A. 2007. Perturbation of cell wall synthesis suppresses autolysis in Staphylococcus aureus: evidence for coregulation of cell wall synthetic and hydrolytic enzymes. J. Bacteriol. 189:7573–7580. 10.1128/JB.01048-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meehan AM, Osmon DR, Duffy MC, Hanssen AD, Keating MR. 2003. Outcome of penicillin-susceptible streptococcal prosthetic joint infection treated with debridement and retention of the prosthesis. Clin. Infect. Dis. 36:845–849. 10.1086/368182 [DOI] [PubMed] [Google Scholar]
- 36.Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N. Engl. J. Med. 351:1645–1654. 10.1056/NEJMra040181 [DOI] [PubMed] [Google Scholar]
- 37.von Eiff C, Bettin D, Proctor RA, Rolauffs B, Lindner N, Winkelmann W, Peters G. 1997. Recovery of small colony variants of Staphylococcus aureus following gentamicin bead placement for osteomyelitis. Clin. Infect. Dis. 25:1250–1251. 10.1086/516962 [DOI] [PubMed] [Google Scholar]
- 38.Yamada M, Shimizu M, Katafuchi A, Grúz P, Fujii S, Usui Y, Fuchs RP, Nohmi T. 2012. Escherichia coli DNA polymerase III is responsible for the high level of spontaneous mutations in mutT strains. Mol. Microbiol. 86:1364–1375. 10.1111/mmi.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei Q, Tarighi S, Dötsch A, Haussler S, Müsken M, Wright VJ, Cámara M, Williams P, Haenen S, Boerjan B, Bogaerts A, Vierstraete E, Verleyen P, Schoofs L, Willaert R, De Groote VN, Michiels J, Vercammen K, Crabbé A, Cornelis P. 2011. Phenotypic and genome-wide analysis of an antibiotic-resistant small colony variant (SCV) of Pseudomonas aeruginosa. PLoS One 6:e29276. 10.1371/journal.pone.0029276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horne DS, Tomasz A. 1993. Possible role of a choline-containing teichoic acid in the maintenance of normal cell shape and physiology in Streptococcus oralis. J. Bacteriol. 175:1717–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.May JJ, Finking R, Wiegeshoff F, Weber TT, Bandur N, Koert U, Marahiel MA. 2005. Inhibition of the d-alanine:d-alanyl carrier protein ligase from Bacillus subtilis increases the bacterium's susceptibility to antibiotics that target the cell wall. FEBS J. 272:2993–3003. 10.1111/j.1742-4658.2005.04700.x [DOI] [PubMed] [Google Scholar]
- 42.Cleveland RF, Wicken AJ, Daneo-Moore L, Shockman GD. 1976. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J. Bacteriol. 126:192–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raychaudhuri D, Chatterjee AN. 1985. Use of resistant mutants to study the interaction of Triton X-100 with Staphylococcus aureus. J. Bacteriol. 164:1337–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]