Abstract
The Darwin Prospective Melioidosis Study has documented 785 melioidosis cases over 23 years. Recurrent melioidosis occurred in 39/679 (5.7%) patients surviving initial infection; 29 patients suffered relapse of the original infection, and 10 presented with a new Burkholderia pseudomallei infection. With improved therapy, relapse has become rare in recent years.
TEXT
Burkholderia pseudomallei, the causative agent of melioidosis, is endemic to the environment of Southeast Asia and northern Australia. Almost all B. pseudomallei infections are caused by environmental exposure to contaminated water or soils, with the commonest route being skin inoculation or, less frequently, inhalation or ingestion (1). Melioidosis can present as an acute, subacute, chronic, or recurrent disease and is fatal in between 10 and 50% of cases, depending on geographical region (2). Melioidosis treatment is protracted, and recurrent disease is a well-recognized concern, being documented for 13 to 23% of patients in Thailand (3–7) and in 6% of patients in Australia (3, 8, 9). Recurrent melioidosis can result either from relapse due to failure to clear an infection or from reinfection with a new B. pseudomallei strain.
Since 1 October 1989, the ongoing Darwin Prospective Melioidosis Study has documented all melioidosis cases in the tropical north of the Northern Territory of Australia, with the vast majority of cases managed at Royal Darwin Hospital in the capital city of Darwin (12.5° S). In the 23 years until 30 September 2012, there were 785 cases of culture-confirmed melioidosis, with 106 (13.5%) dying from their initial infection. Thirty-nine (5.7%) of the 679 survivors have subsequently presented with recurrent melioidosis (Table 1). We define recurrent melioidosis as culture-confirmed melioidosis occurring in a patient who re-presents following the due date for completion of their planned antibiotic therapy (10). Therapy consists of a minimum 2 weeks of intravenous antibiotics followed by a minimum 3 months of oral eradication therapy (2). Patients re-presenting during this period of therapy are considered to have recrudescent rather than recurrent melioidosis and have been excluded from our analysis. To classify recurrent melioidosis cases as either relapse or reinfection, both the initial and subsequent B. pseudomallei isolates were subjected to multilocus sequence typing (MLST) (11). Consecutive isolates were available for all but three cases (Table 1). This study was approved by the Human Research Ethics Committee of the Northern Territory Department of Health and the Menzies School of Health Research (HREC 02/38).
TABLE 1.
Relapse and reinfection cases from the Darwin Prospective Melioidosis Study, 1 October 1989 through 30 September 2012a
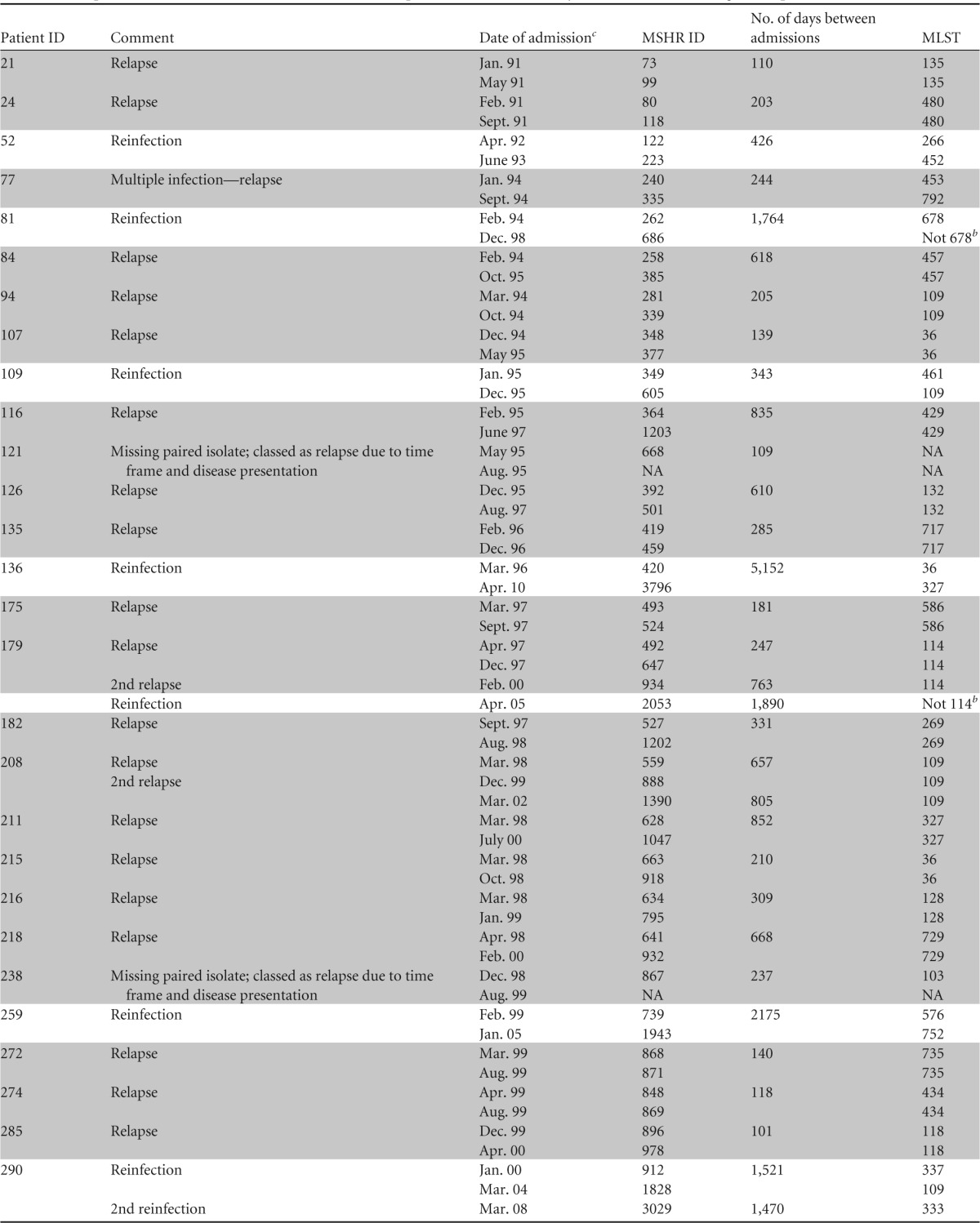
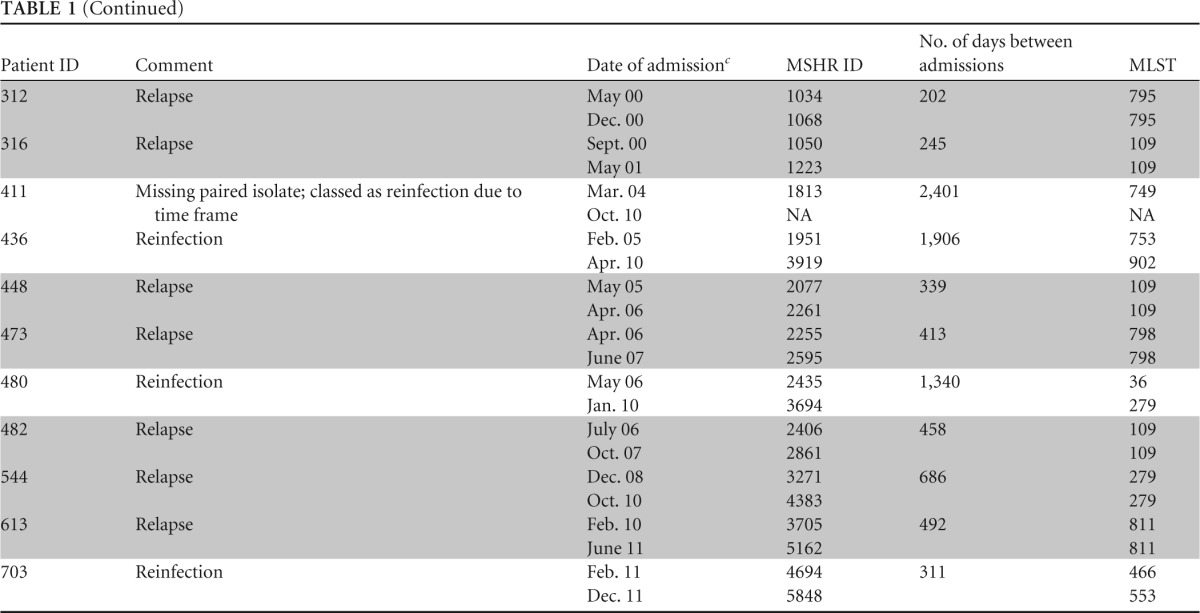
Abbreviations: MSHR, Menzies School of Health Research; MLST, multilocus sequence typing; NA, not applicable. Relapse melioidosis cases are shaded for clarity.
Sequence type differed from that of original strain by at least two MLST loci.
Month and last two digits of year.
Based on MLST and clinical factors, 29 (74%) of 39 recurrent melioidosis cases were attributed to relapse. Of these 29, 26 had identical sequence types (STs) for initial and recurrent isolates. Paired isolates were not available for 2 of the 29 cases (identification numbers [IDs] 121 and 238), but their time course and clinical history supported relapse (Table 1). An additional patient (ID 77), previously reported by Haase and coworkers, had discordant strains according to MLST but was thought to have relapsed from an initial infection with multiple strains (9). Of the 29 relapse cases, two patients relapsed twice, with one of these patients then presenting a third time with a fatal new infection with a different B. pseudomallei ST (i.e., reinfection after two relapses). Recurrent melioidosis in the remaining 10 patients (26%) was attributed to reinfection. Of these, 9 had discordant STs between initial and recurrent isolates. Paired isolates were not available for the remaining patient (ID 411), but the protracted time interval (6.6 years) between infections strongly supported reinfection.
The median time from first to second admission for relapse cases was 285 days (9.4 months), with an interquartile range of 416 days (∼14 months) (range, 3.3 to 28 months). For reinfection cases, the median time between admissions was 1,643 days (∼54 months), with an interquartile range of 1,158 days (∼38 months) (range, 10.2 to 169.4 months). We constructed a Kaplan-Meier time-to-event curve analysis using the software program Stata version 12 (Stata Corporation, Texas) of relapse versus reinfection melioidosis patients, which demonstrated that reinfection cases show increased time between disease presentations compared with relapse patients (Fig. 1). Previous studies have reported median time spans for relapsed melioidosis of between 6 and 8 months, although it should be noted that studies conducted in Thailand have included some patients with very early relapse while still on therapy, which we would define as recrudescent melioidosis (3, 8). Despite the difference in case definitions, the increased time span for reinfection compared with relapse cases seen in our study is consistent with findings from Thailand (3), indicating that time is an important determinant of the nature of recurrent melioidosis.
FIG 1.
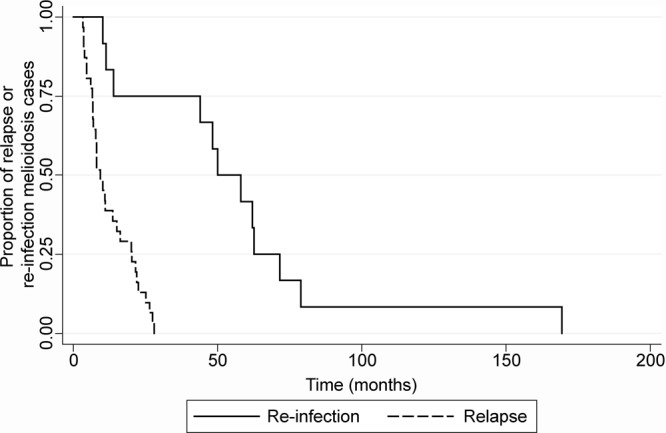
Kaplan-Meier time-to-event curve analysis of relapse versus reinfection melioidosis patients within the Darwin Prospective Melioidosis Study. Thirty-nine episodes of recurrent melioidosis occurred over the time frame of this study; 29 episodes were due to relapse of infection, and 10 were attributed to a new infection with B. pseudomallei. Patients presenting with reinfection are likely to have an increased time between disease presentations compared with that for relapse patients.
Over the past decade, we have increasingly seen a reversal of attribution for recurrent melioidosis from predominantly relapse to predominantly reinfection. Of 375 melioidosis patients admitted prior to 30 September 2003, 24 (6.4%) have subsequently relapsed, in comparison to only 5 of 410 patients (1.2%) admitted from 1 October 2003 to 30 September 2012 (Fisher's exact test, P = <0.001). The observed decline of relapsed melioidosis is most likely due to improved use of efficacious antimicrobials, most notably a lengthened intravenous treatment phase for complex cases. Indeed, almost half of our melioidosis patients now receive at least 4 weeks of intravenous ceftazidime and/or meropenem for the primary treatment phase, with antibiotic choice and duration based on disease presentation and severity (2). For the last 3 years of our study (until 30 September 2012), we have treated 252 melioidosis patients, of which 29 cases (11.5%) were fatal. To date there has been only one episode of relapse in these 223 survivors. These data suggest that current antibiotic regimens are now truly eradicating B. pseudomallei infection in patients with melioidosis.
We recognize that a limitation of our study is the assumption that in all but one instance, individual infections are not caused by multiple B. pseudomallei strains. It is also possible that patients can be reinfected with a B. pseudomallei strain with an ST identical to that of their primary isolate, resulting in misattribution to relapse. However, the diversity of STs observed in the Northern Territory (12) and the increasing rarity of relapse cases seen in our study support the notion that reinfection with an identical ST would be an infrequent occurrence. More highly resolving molecular fingerprinting methods, such as whole-genome sequencing, would be required to differentiate such scenarios. Finally, we acknowledge that relapse may still occur in melioidosis patients diagnosed toward the end of our study, although it is now more than 16 months since the last case was admitted (June 2012).
Collectively, our data show that recurrent melioidosis in northern Australia is in decline and is now due predominantly to reinfection with a new strain of B. pseudomallei rather than to relapse with the original strain. The decreased rate of relapse cases within the Darwin Prospective Melioidosis Study over recent years can be attributed to improved antibiotic therapy and in particular prolongation of the intravenous phase.
ACKNOWLEDGMENTS
This work was supported by project grants from the Australian National Health and Medical Research Council.
We thank our clinical and laboratory colleagues for their ongoing support for this study and Brian Spratt and Daniel Godoy at Imperial College, London, for support with MLST.
Footnotes
Published ahead of print 27 November 2013
REFERENCES
- 1.Cheng AC, Currie BJ. 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 18:383–416. 10.1128/CMR.18.2.383-416.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga WJ, Currie BJ, Peacock SJ. 2012. Melioidosis. N. Engl. J. Med. 367:1035–1044. 10.1056/NEJMra1204699 [DOI] [PubMed] [Google Scholar]
- 3.Limmathurotsakul D, Chaowagul W, Chantratita N, Wuthiekanun V, Biaklang M, Tumapa S, White NJ, Day NP, Peacock SJ. 2008. A simple scoring system to differentiate between relapse and re-infection in patients with recurrent melioidosis. PLoS Negl. Trop. Dis. 2:e327. 10.1371/journal.pntd.0000327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maharjan B, Chantratita N, Vesaratchavest M, Cheng A, Wuthiekanun V, Chierakul W, Chaowagul W, Day NP, Peacock SJ. 2005. Recurrent melioidosis in patients in northeast Thailand is frequently due to reinfection rather than relapse. J. Clin. Microbiol. 43:6032–6034. 10.1128/JCM.43.12.6032-6034.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaowagul W, Suputtamongkol Y, Dance DA, Rajchanuvong A, Pattara-arechachai J, White NJ. 1993. Relapse in melioidosis: incidence and risk factors. J. Infect. Dis. 168:1181–1185. 10.1093/infdis/168.5.1181 [DOI] [PubMed] [Google Scholar]
- 6.Limmathurotsakul D, Chaowagul W, Chierakul W, Stepniewska K, Maharjan B, Wuthiekanun V, White NJ, Day NP, Peacock SJ. 2006. Risk factors for recurrent melioidosis in northeast Thailand. Clin. Infect. Dis. 43:979–986. 10.1086/507632 [DOI] [PubMed] [Google Scholar]
- 7.Limmathurotsakul D, Chaowagul W, Day NP, Peacock SJ. 2009. Patterns of organ involvement in recurrent melioidosis. Am. J. Trop. Med. Hyg. 81:335–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Currie BJ, Ward L, Cheng AC. 2010. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl. Trop. Dis. 4:e900. 10.1371/journal.pntd.0000900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haase A, Melder A, Smith-Vaughan H, Kemp D, Currie B. 1995. RAPD analysis of isolates of Burkholderia pseudomallei from patients with recurrent melioidosis. Epidemiol. Infect. 115:115–121. 10.1017/S0950268800058179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Currie BJ, Fisher DA, Anstey NM, Jacups SP. 2000. Melioidosis: acute and chronic disease, relapse and re-activation. Trans. R. Soc. Trop. Med. Hyg. 94:301–304. 10.1016/S0035-9203(00)90333-X [DOI] [PubMed] [Google Scholar]
- 11.Godoy D, Randle G, Simpson AJ, Aanensen DM, Pitt TL, Kinoshita R, Spratt BG. 2003. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J. Clin. Microbiol. 41:2068–2079. 10.1128/JCM.41.5.2068-2079.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng AC, Ward L, Godoy D, Norton R, Mayo M, Gal D, Spratt BG, Currie BJ. 2008. Genetic diversity of Burkholderia pseudomallei isolates in Australia. J. Clin. Microbiol. 46:249–254. 10.1128/JCM.01725-07 [DOI] [PMC free article] [PubMed] [Google Scholar]


