LETTER
Our laboratory in The Gambia, West Africa, performs Koga agar (1.5% bacteriological agar, 0.5% sodium chloride, 0.5% meat extract, 0.1% bacteriological peptone) culture for larvae of strongyle nematodes on human fecal samples for which parasitological investigation has been requested. We have recovered free-living amoebae (FLA) from human fecal specimens on two occasions over a period of 9 months (representing 130 individual fecal cultures) on this agar (Fig. 1). The amoebae in both cases were identified as Hartmannella species based upon the morphology of trophozoites and cysts in agar culture (Fig. 2) and their inability to enflagellate in distilled water after 8 h of incubation at 37°C. No other types of FLA have been recovered. Due to resource constraints, sequencing of the partial 18S rRNA gene and internal transcribed spacer (ITS) region could not be performed, and this identification must remain presumptive. Specimens were collected into sterile containers, and Koga culture plates were sterile and sealed with Parafilm prior to incubation at 30°C for 5 days; therefore, environmental contamination with FLA was very unlikely. Repeat specimens could not be obtained from subjects, and so it was not possible to determine if these findings represent transient passage of FLA or true intestinal colonization.
FIG 1.
Tracks of presumptive Hartmannella species on the surface of a Koga agar culture of human feces after 5 days of incubation at 30°C.
FIG 2.
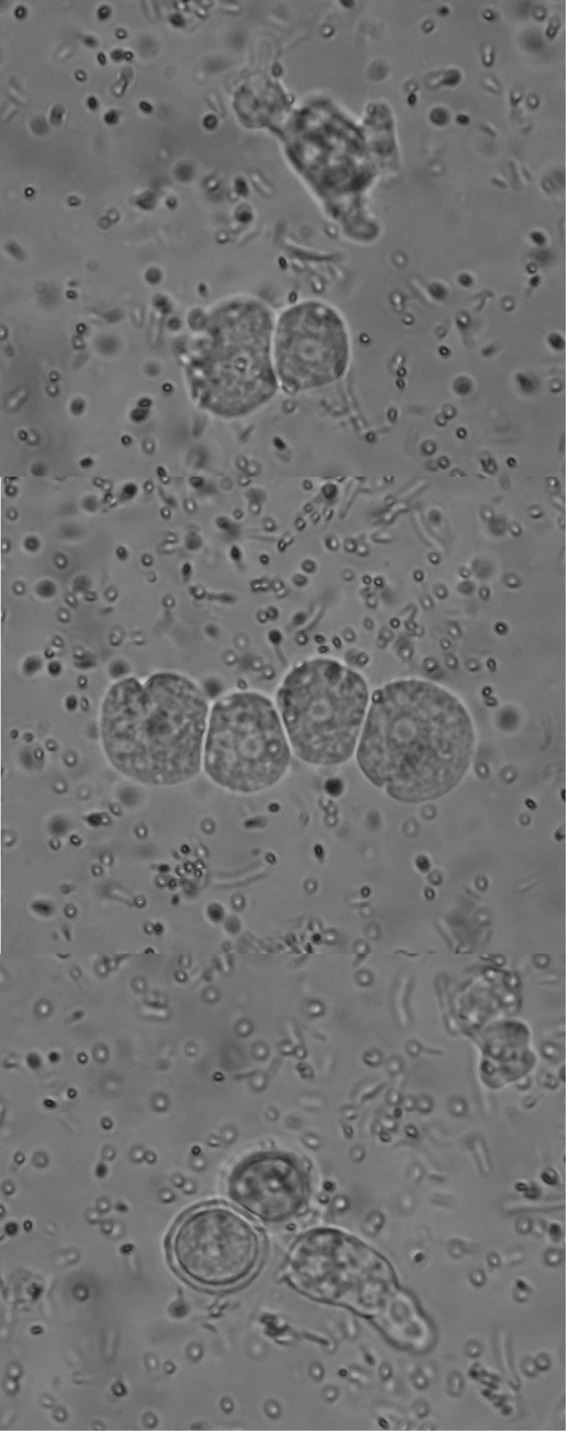
Characteristic morphology of presumptive Hartmannella cysts and trophozoites on agar culture.
It has been postulated that FLA provide a vehicle for bacterial pathogens to gain entry into the human respiratory tract (1). The possibility that free-living amoeba may have a similar role in the introduction of bacterial pathogens into the human intestinal tract (and particularly in defense against stomach acid during transit to the duodenum) should be further considered, given these findings. FLA are potential environmental reservoirs of several important pathogens of the human intestinal tract, including Salmonella spp., Shigella spp., Campylobacter spp., and enterohemorrhagic Escherichia coli (2–5). Specifically, due to the large infective dose of Vibrio cholerae (108 to 109 cells), it has been suggested that Acanthamoebae may act as both environmental hosts and intracellular multipliers of this organism, allowing it to grow sufficiently to be able to cause human infection (6).
Human respiratory tract (7, 8) and urinary tract (9) colonization with Acanthamoeba spp. may occur. Recovery of FLA, including Hartmannella spp. from the intestinal tracts of rodents (10), reptiles (11), and fish and birds (12), has been previously reported. Only one previous reference in the literature describes the recovery of FLA from the human intestinal tract (13).
The potential for transient passage or true colonization of the human intestinal tract with FLA may also provide a novel potential portal of entry into the human body for FLA causing primary amoebic meningitis (PAM) and granulomatous amoebic encephalitis (GAE). These findings may also require a review of safety practices when dealing with such cultures, including the use of biosafety cabinets during manipulation of Koga agar cultures to avoid cultures potentially containing Naegleria fowleri being inappropriately manipulated on a benchtop in the laboratory.
In summary, we report the recovery of FLA in Koga agar culture of feces from two separate individuals in West Africa. It is unknown if this represents transient passage or true intestinal colonization. These findings demonstrate an important novel mechanism of entry for bacterial intestinal pathogens and possibly pathogenic FLA themselves into the human body. They also raise the need for awareness of the potential for FLA to be recovered from Koga agar culture in order to ensure the health and safety of laboratory staff performing such cultures.
ACKNOWLEDGMENTS
This research is jointly funded by the United Kingdom Medical Research Council (MRC; grant MC-A760-5QX00) and the United Kingdom Department for International Development (DFID) under the MRC/DFID Concordat agreement.
Footnotes
Published ahead of print 13 November 2013
REFERENCES
- 1.Khan NA. 2006. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol. Rev. 30:564–595. 10.1111/j.1574-6976.2006.00023 [DOI] [PubMed] [Google Scholar]
- 2.Wildschutte H, Lawrence JG. 2007. Differential Salmonella survival against communities of intestinal amoebae. Microbiology 153:1781–1789. 10.1099/mic.0.2006/003616-0 [DOI] [PubMed] [Google Scholar]
- 3.Bui XT, Winding A, Qvortrup K, Wolff A, Bang DD, Creuzenet C. 2012. Survival of Campylobacter jejuni in co-culture with Acanthamoeba castellanii: role of amoeba-mediated depletion of dissolved oxygen. Environ. Microbiol. 14:2034–2047. 10.1111/j.1462-2920.2011.02655.x [DOI] [PubMed] [Google Scholar]
- 4.Jeong HJ, Jang ES, Han BI, Lee KH, Ock MS, Kong HH, Chung DI, Seol SY, Cho DT, Yu HS. 2007. Acanthamoeba: could it be an environmental host of Shigella? Exp. Parasitol. 115:181–186. 10.1016/j.exppara.2006.08.002 [DOI] [PubMed] [Google Scholar]
- 5.Chekabab SM, Daigle F, Charette SJ, Dozois CM, Harel J. 2012. Survival of enterohemorrhagic Escherichia coli in the presence of Acanthamoeba castellanii and its dependence on Pho regulon. Microbiologyopen 1:427–437. 10.1002/mbo3.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abd H, Shanan S, Saeed A, Sandström G. 2011. Survival of Vibrio cholerae inside Acanthamoeba and detection of both microorganisms from natural water samples may point out the amoeba as a protozoal host for V. cholerae. J. Bacteriol. Parasitol. S1-003. 10.4172/2155-9597.S1-003 [DOI] [Google Scholar]
- 7.Madrigal Sesma MJ, Santillana López I. 1989. Isolation of free-living amoebas from samples of respiratory origin. Rev. Sanid. Hig. Publica (Madr.) 63:63–72 [PubMed] [Google Scholar]
- 8.Rivera F, Medina F, Ramírez P, Alcocer J, Vilaclara G, Robles E. 1984. Pathogenic and free-living protozoa cultured from the nasopharyngeal and oral regions of dental patients. Environ. Res. 33:428–440. 10.1016/0013-9351(84)90040-9 [DOI] [PubMed] [Google Scholar]
- 9.Santos LC, Oliveira MS, Lobo RD, Higashino HR, Costa SF, van der Heijden IM, Giudice MC, Silva AR, Levin AS. 2009. Acanthamoeba spp. in urine of critically ill patients. Emerg. Infect. Dis. 15:1144–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerdraon G, Simitzis-Le Flohic AM, Couatarmanac'h A. 1986. Amphizoic stage of free amoebae, myth or reality (could oxygen be the limiting factor?). Bull. Soc. Pathol. Exot. Filiales 79:66–73 [PubMed] [Google Scholar]
- 11.Hassl A, Benyr G, Appelt S. 2000. Free-living amoebas as opportunistic intestinal parasites of reptiles. Mitt. Osterr. Ges. Tropenmed. Parasitol. 22:49–54 [Google Scholar]
- 12.Franke ED, Mackiewicz JS. 1982. Isolation of Acanthamoeba and Naegleria from the intestinal contents of freshwater fishes and their potential pathogenicity. J. Parasitol. 68:164–166. 10.2307/3281343 [DOI] [PubMed] [Google Scholar]
- 13.de Moura H, Salazar HC, Fernandes O, Lisboa DC, de Carvalho FG. 1985. Free-living amoeba in the human intestine. Evidences of parasitism. Rev. Inst. Med. Trop. Sao Paulo 27:150–156. 10.1590/S0036-46651985000300007 [DOI] [PubMed] [Google Scholar]



