Abstract
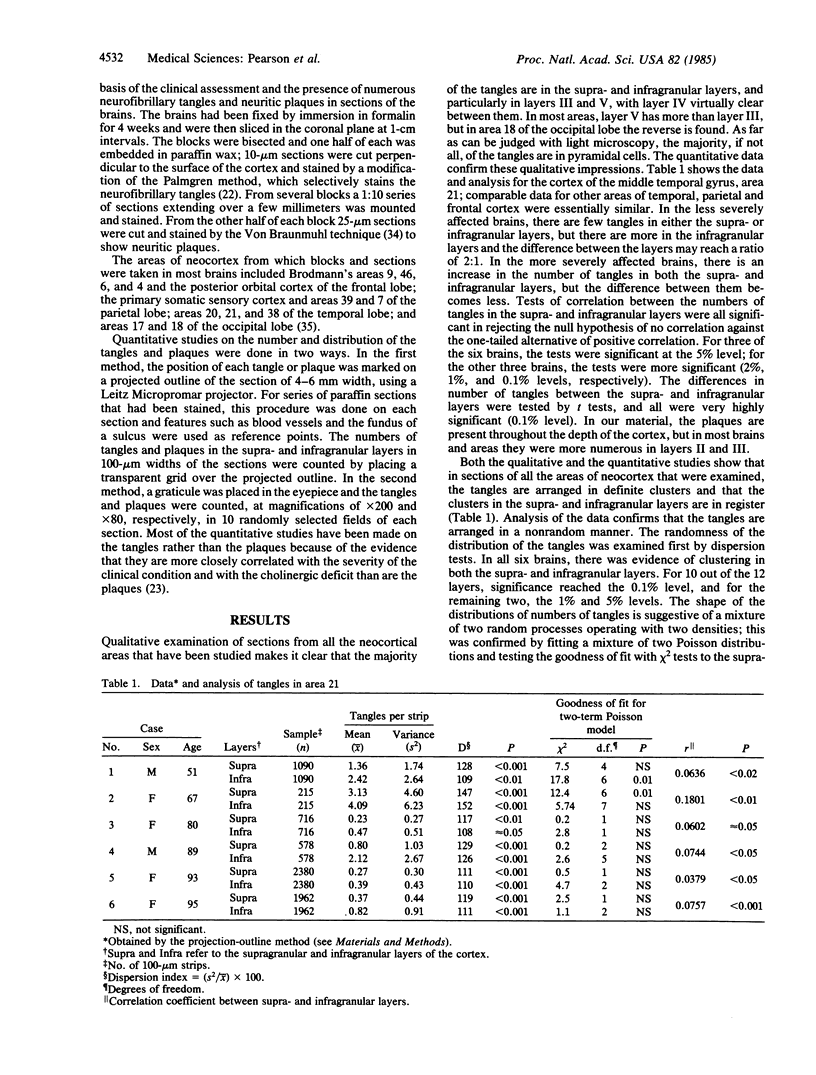
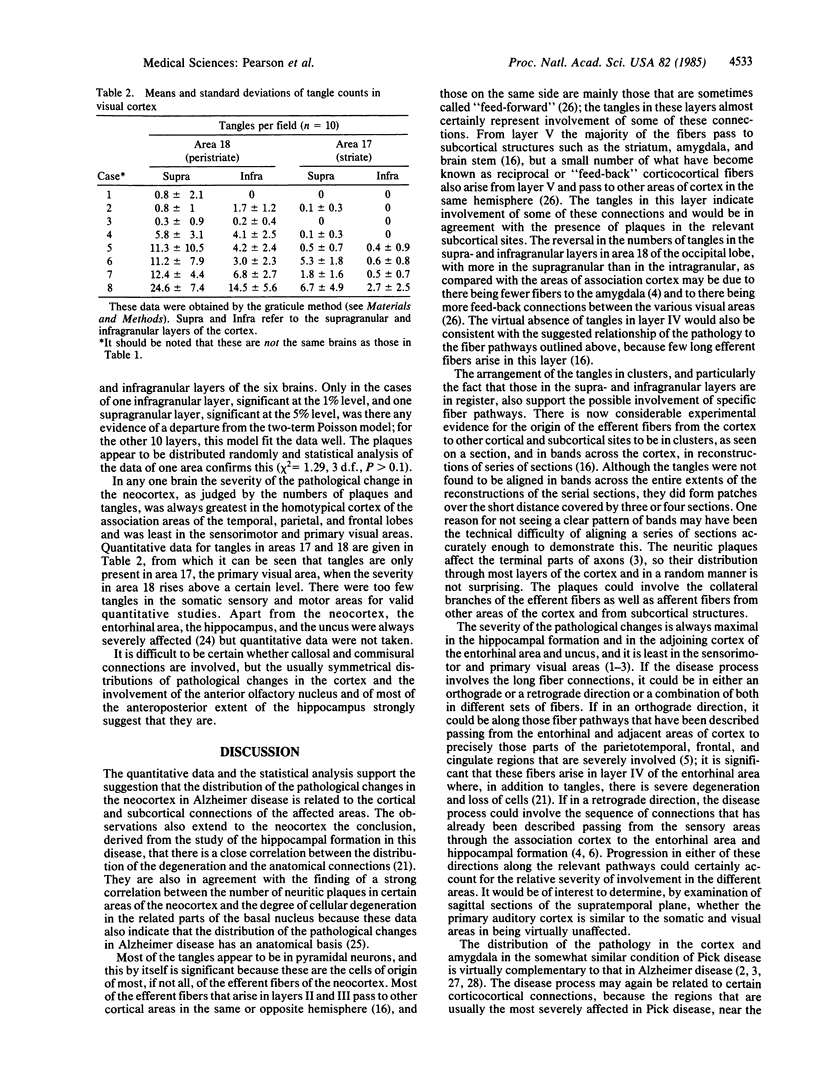
The numbers and distribution of the neurofibrillary tangles and neuritic plaques have been determined in several areas of the neocortex in brains affected by various degrees of severity of Alzheimer disease. The homotypical cortex of the "association" areas of the temporal, parietal, and frontal lobes are severely involved, whereas the motor, somatic sensory, and primary visual areas are virtually unaffected. The neurofibrillary tangles are mainly in the supra- and infragranular layers, particularly in layers III and V. In all areas except area 18 in the occipital lobe, there are approximately twice as many tangles in layer V as in layer III. The tangles are arranged in definite clusters, and those in the supra- and infragranular layers are in register. The neuritic plaques occur in all layers but predominantly affect layers II and III and do not show clustering. These data on the severity of the pathological involvement in different areas of the neocortex and the laminar distribution and the clustering of the tangles support the suggestion that the pathological changes in Alzheimer disease affect regions that are interconnected by well-defined groups of connections and that the disease process may extend along the connecting fibers. The invariable and severe involvement of the olfactory areas of the brain in this disease is in striking contrast to the minimal changes in the somatic sensory and primary visual areas and raises the possibility that the olfactory pathway may be initially involved.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaral D. G., Insausti R., Cowan W. M. The commissural connections of the monkey hippocampal formation. J Comp Neurol. 1984 Apr 10;224(3):307–336. doi: 10.1002/cne.902240302. [DOI] [PubMed] [Google Scholar]
- Amaral D. G., Price J. L. Amygdalo-cortical projections in the monkey (Macaca fascicularis). J Comp Neurol. 1984 Dec 20;230(4):465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Arendt T., Bigl V., Tennstedt A., Arendt A. Neuronal loss in different parts of the nucleus basalis is related to neuritic plaque formation in cortical target areas in Alzheimer's disease. Neuroscience. 1985 Jan;14(1):1–14. doi: 10.1016/0306-4522(85)90160-5. [DOI] [PubMed] [Google Scholar]
- Brun A., Gustafson L. Distribution of cerebral degeneration in Alzheimer's disease. A clinico-pathological study. Arch Psychiatr Nervenkr (1970) 1976 Dec 31;223(1):15–33. doi: 10.1007/BF00367450. [DOI] [PubMed] [Google Scholar]
- Brun A., Gustafson L. Limbic lobe involvement in presenile dementia. Arch Psychiatr Nervenkr (1970) 1978 Nov 14;226(2):79–93. doi: 10.1007/BF00345945. [DOI] [PubMed] [Google Scholar]
- Cross R. B. Demonstration of neurofibrillary tangles in paraffin sections: a quick and simple method using a modification of Palmgren's method. Med Lab Sci. 1982 Jan;39(1):67–69. [PubMed] [Google Scholar]
- Cummings J. L., Duchen L. W. Kluver-Bucy syndrome in Pick disease: clinical and pathologic correlations. Neurology. 1981 Nov;31(11):1415–1422. doi: 10.1212/wnl.31.11.1415. [DOI] [PubMed] [Google Scholar]
- DAITZ H. M., POWELL T. P. Studies of the connexions of the fornix system. J Neurol Neurosurg Psychiatry. 1954 Feb;17(1):75–82. doi: 10.1136/jnnp.17.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner F. F., Myers R. E. Distribution of corpus callosum and anterior commissure in cat and raccoon. J Comp Neurol. 1965 Jun;124(3):353–365. doi: 10.1002/cne.901240306. [DOI] [PubMed] [Google Scholar]
- Esiri M. M., Wilcock G. K. The olfactory bulbs in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1984 Jan;47(1):56–60. doi: 10.1136/jnnp.47.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUXE K. EVIDENCE FOR THE EXISTENCE OF MONOAMINE NEURONS IN THE CENTRAL NERVOUS SYSTEM. IV. DISTRIBUTION OF MONOAMINE NERVE TERMINALS IN THE CENTRAL NERVOUS SYSTEM. Acta Physiol Scand Suppl. 1965:SUPPL 247–247:37+. [PubMed] [Google Scholar]
- Goldman-Rakic P. S., Schwartz M. L. Interdigitation of contralateral and ipsilateral columnar projections to frontal association cortex in primates. Science. 1982 May 14;216(4547):755–757. doi: 10.1126/science.6177037. [DOI] [PubMed] [Google Scholar]
- Herzog A. G., Kemper T. L. Amygdaloid changes in aging and dementia. Arch Neurol. 1980 Oct;37(10):625–629. doi: 10.1001/archneur.1980.00500590049006. [DOI] [PubMed] [Google Scholar]
- Herzog A. G., Van Hoesen G. W. Temporal neocortical afferent connections to the amygdala in the rhesus monkey. Brain Res. 1976 Oct 8;115(1):57–69. doi: 10.1016/0006-8993(76)90822-2. [DOI] [PubMed] [Google Scholar]
- Hyman B. T., Van Hoesen G. W., Damasio A. R., Barnes C. L. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984 Sep 14;225(4667):1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Ishii T. Distribution of Alzheimer's neurofibrillary changes in the brain stem and hypothalamus of senile dementia. Acta Neuropathol. 1966 Mar 4;6(2):181–187. doi: 10.1007/BF00686763. [DOI] [PubMed] [Google Scholar]
- Jamada M., Mehraein P. Verteilungsmuster der senilen Veränderungen im Gehirn. Die Beteiligung des limbischen Systems bei hirnatrophischen Prozessen des Seniums und bei Morbus Alzheimer. Arch Psychiatr Nervenkr (1970) 1968;211(3):308–324. doi: 10.1007/BF00340827. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Powell T. P. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;93(4):793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- Kemp J. M., Powell T. P. The cortico-striate projection in the monkey. Brain. 1970;93(3):525–546. doi: 10.1093/brain/93.3.525. [DOI] [PubMed] [Google Scholar]
- Kosel K. C., Van Hoesen G. W., Rosene D. L. Non-hippocampal cortical projections from the entorhinal cortex in the rat and rhesus monkey. Brain Res. 1982 Jul 29;244(2):201–213. doi: 10.1016/0006-8993(82)90079-8. [DOI] [PubMed] [Google Scholar]
- Maunsell J. H., van Essen D. C. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983 Dec;3(12):2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M. M., Mufson E. J., Levey A. I., Wainer B. H. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983 Feb 20;214(2):170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- NAUTA W. J. Fibre degeneration following lesions of the amygdaloid complex in the monkey. J Anat. 1961 Oct;95:515–531. [PMC free article] [PubMed] [Google Scholar]
- Pearson R. C., Gatter K. C., Brodal P., Powell T. P. The projection of the basal nucleus of Meynert upon the neocortex in the monkey. Brain Res. 1983 Jan 17;259(1):132–136. doi: 10.1016/0006-8993(83)91075-2. [DOI] [PubMed] [Google Scholar]
- Powell T. P., Cowan W. M., Raisman G. The central olfactory connexions. J Anat. 1965 Oct;99(Pt 4):791–813. [PMC free article] [PubMed] [Google Scholar]
- Raisman G., Cowan W. M., Powell T. P. An experimental analysis of the efferent projection of the hippocampus. Brain. 1966 Mar;89(1):83–108. doi: 10.1093/brain/89.1.83. [DOI] [PubMed] [Google Scholar]
- SIMPSON D. A. The efferent fibres of the hippocampus in the monkey. J Neurol Neurosurg Psychiatry. 1952 May;15(2):79–92. doi: 10.1136/jnnp.15.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock G. K., Esiri M. M. Plaques, tangles and dementia. A quantitative study. J Neurol Sci. 1982 Nov;56(2-3):343–356. doi: 10.1016/0022-510x(82)90155-1. [DOI] [PubMed] [Google Scholar]


