Abstract
Plasmodium vivax is the most widespread and the second most prevalent malaria-causing species in the world. Current measures used to control the transmission of this disease would benefit from the development of an efficacious vaccine. In the case of the deadly parasite P. falciparum, the recombinant RTS,S vaccine containing the circumsporozoite antigen (CSP) consistently protects 30 to 50% of human volunteers against infection and is undergoing phase III clinical trials in Africa with similar efficacy. These findings encouraged us to develop a P. vivax vaccine containing the three circulating allelic forms of P. vivax CSP. Toward this goal, we generated three recombinant bacterial proteins representing the CSP alleles, as well as a hybrid polypeptide called PvCSP-All-CSP-epitopes. This hybrid contains the conserved N and C termini of P. vivax CSP and the three variant repeat domains in tandem. We also generated simian and human recombinant replication-defective adenovirus vectors expressing PvCSP-All-CSP-epitopes. Mice immunized with the mixture of recombinant proteins in a formulation containing the adjuvant poly(I·C) developed high and long-lasting serum IgG titers comparable to those elicited by proteins emulsified in complete Freund's adjuvant. Antibody titers were similar in mice immunized with homologous (protein-protein) and heterologous (adenovirus-protein) vaccine regimens. The antibodies recognized the three allelic forms of CSP, reacted to the repeated and nonrepeated regions of CSP, and recognized sporozoites expressing the alleles VK210 and VK247. The vaccine formulations described in this work should be useful for the further development of an anti-P. vivax vaccine.
INTRODUCTION
Human malaria infection starts when an Anopheles mosquito injects sporozoites of Plasmodium species into the skin of a person. The sporozoites traverse the skin, enter the blood circulation, and infect hepatocytes. While sporozoites are in the skin or migrating to the liver, their infectivity can be abolished by antibodies against the circumsporozoite antigen (CSP). The neutralizing antibodies are predominantly, but not exclusively, directed against the immunodominant B epitopes in the CSP repeat domain (reviewed in reference 1).
Multiple trials with experimental animals and more recently with humans provide a solid basis for the use of vaccines against CSP to prevent malaria. Thus far, the only vaccine against the deadly Plasmodium falciparum parasite tested in a phase III clinical trial is RTS,S, a fusion protein between portions of CSP and the hepatitis B surface antigen (S) that is administered in a powerful adjuvant system (AS), either an oil-in-water emulsion (AS02) or a liposomal suspension (AS01). These adjuvants contain monophosphoryl lipid A (a detoxified form of lipopolysaccharide [LPS]) and QS21 (purified saponin from Quillaja saponaria). Clinical phase IIa trials with naive, vaccinated volunteers who were challenged with bites from mosquitoes infected with P. falciparum reported protective efficacies of 32 to 50%. Protective immunity largely correlated with the serum levels of specific IgG antibodies against the repeats in the CSP antigen and, to a lesser extent, with the frequency of CD4+ T cells expressing two or more of the following cytokines: interleukin-2 (IL-2), tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ) (2).
One of these formulations (RTS,S/AS01E) is currently being tested in a large phase III clinical trial of African children living in areas where malaria is endemic. The results reported from this trial indicate 49.5 or 30.1% protective efficacy during a 14-month period postvaccination in 5- to 17-month-old or 6- to 12-week-old African children, respectively (3, 4). These trials and previous human trials have established that immunodominant CSP is a worthwhile candidate antigen to be included in future vaccine formulations to combat malaria infections, including infections with P. vivax.
P. vivax is the most widespread malaria-causing species in the world and is the second most prevalent. It is estimated that more than 2.8 billion people are at risk of contracting P. vivax infection (5). Nevertheless, only three clinical trials based on subunit P. vivax vaccines have been completed (http://www.clinicaltrials.gov/). One complication of P. vivax vaccine development is that, in contrast to P. falciparum, different allelic forms of P. vivax CSP have been described. The two most common CSP alleles are VK210 and VK247 (6, 7). A third allelic form exists at a low frequency (8–12). The main variation among these allelic forms is in the central repeat region of CSP, which is a possible target of neutralizing antibodies.
To generate a vaccine with universal coverage against P. vivax malaria strains, we tested a prime-boost regimen using recombinant proteins and adenovirus vectors expressing epitopes from the three CSP alleles as antigens. We used two approaches to generate these vaccines. The first consisted of mixing recombinant proteins expressing the three CSP alleles to generate a vaccine. Additionally, we generated a single recombinant fusion protein called PvCSP-All-CS-epitopes that contains epitopes from the three P. vivax CSP alleles. We primed animals with either a simian or a human recombinant replication-defective adenovirus vector expressing PvCSP-All-CS-epitopes in some experiments. In most of the experiments, recombinant antigens were administered in a formulation containing the adjuvant poly(I·C). We compared the immunogenicities of homologous (protein-protein) and heterologous (adenovirus-protein) immunization regimens. We primarily measured the magnitude and longevity of the serum IgG responses to all of CSP and the CSP domains. In selected experiments, we also evaluated the cell-mediated immune response to CSP.
MATERIALS AND METHODS
Ethics statement.
This study was carried out in strict accordance with the recommendations provided by Guide for the Care and Use of Laboratory Animals of the Brazilian National Council of Animal Experimentation (http://www.cobea.org.br/). The protocol was approved by the Committee on the Ethics of Animal Experiments of the Institutional Animal Care and Use Committee at the Federal University of Sao Paulo (ID number CEP 0307/09). Six- to 8-week-old isogenic female C57BL/6 (H-2b) mice were maintained under specific-pathogen-free conditions were purchased from the Center for Development of Experimental Models for Medicine and Biology (Federal University of São Paulo, São Paulo, Brazil). tlr4 knockout (tlr4−/−) C57BL/6 mice were kindly provided by Shizuo Akira, Osaka University, Osaka, Japan (13). Experiments were approved by the Ethics Committee in Research of the Federal University of São Paulo and São Paulo Hospital (CEP 0307/09).
Generation of recombinant bacterial proteins containing sequences of the CSP antigen of P. vivax.
For immunization, recombinant bacterial proteins representing the different allelic forms of P. vivax CSP were expressed and purified as described below. These recombinant proteins were named His6-PvCSP-VK210, His6-PvCSP-VK247, His6-PvCSP-Vivax-like, and His6-PvCSP-All-CSP-epitopes. The deduced amino acid sequences of the recombinant proteins are depicted in Fig. 1B. Codon-optimized synthetic genes were purchased from GenScript Inc. that contained the sequences of nucleotides PvCSP-vk210, PvCSP-vk247, PvCSP-Vivax-like, and PvCSP-All-CSP-epitopes. These genes were subcloned into the commercial pET28a expression vector (Novagen), expressed, and purified as follows. Recombinant Escherichia coli BL21(DE3) (Novagen) transformed with each recombinant plasmid was cultivated at 37°C in flasks containing Luria broth (LB) and kanamycin (30 μg/ml). Protein expression was induced at an optical density at 600 nm (OD600) of 0.6 with 0.1 mM isopropyl-β-d-thiogalactopyranoside (Invitrogen) for 4 h. After centrifugation, bacteria were lysed on ice with an ultrasonic processor (Sonics and Materials Inc. Vibra Cell VCX 750) in phosphate buffer (pH 8.0) with 1.0 mg/ml lysozyme (Sigma) and 1 mM phenylmethylsulfonyl fluoride (Sigma). The bacterial lysate was centrifuged, and the supernatant was resuspended in 8 M urea (Invitrogen). After being boiled at 100°C for 15 min, the proteins were applied to a column with Ni2+-nitrilotriacetic acid-agarose resin (Qiagen). After several washes, bound proteins were eluted with 0.5 M imidazole (Sigma). The eluted protein was dialyzed against 20 mM Tris-HCl (pH 8.0), and the recombinant proteins were purified by ion-exchange chromatography with a Resource Q column (GE Healthcare) coupled to a fast protein liquid chromatography system (GE Healthcare). Fractions containing the recombinant proteins with a high degree of purity were pooled and incubated with polymyxin B-agarose (Sigma) overnight. Unbound material was extensively dialyzed against phosphate-buffered saline (PBS; pH 7.2). Protein concentration was determined with the Bradford assay and by SDS-PAGE analyses. LPS determination was done with the E-TOXATE kit (Sigma). Tests were performed with different dilutions of each sample, and the results demonstrated that the LPS contamination varied between 300 and 3,000 endotoxin U/mg of purified protein.
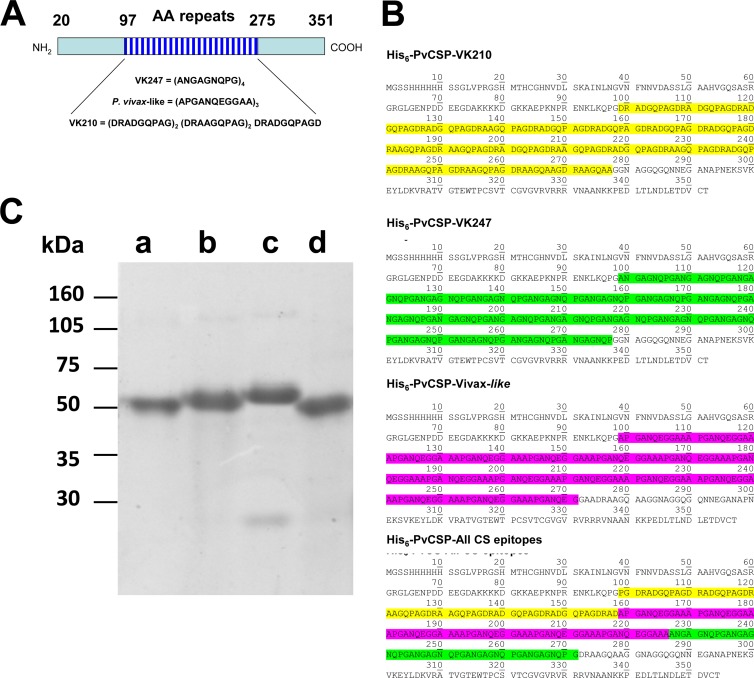
FIG 1.
Production and purification of P. vivax CSP recombinant proteins. (A) Schematic representation of the P. vivax CSP antigen and the sequences of the repeat regions present in each allelic form (PvCSP-VK210, PvCSP-VK247, or PvCSP-Vivax-like). (B) The predicted amino acid sequence of each recombinant protein. (C) Recombinant proteins were purified by affinity purification followed by anion-exchange chromatography and separated by SDS-PAGE under reducing conditions. Lanes: a, His6-PvCSP-VK210; b, His6-PvCSP-VK247; c, His6-PvCSP-Vivax-like; d, His6-PvCSP-All-CSP-epitopes. Each lane was loaded with 1 μg of protein.
In addition, for epitope mapping, we used His6-FliC, His6-FliC-PvCSP-VK210, His6-FliC-PvCSP-VK247, and His6-FliC-PvCSP-Vivax-like. These proteins were generated as described in detail in reference 14. Finally, a recombinant protein devoid of the repeat regions expressing the N- and C-terminal regions of CSP was generated and named His6-PvCSP-No-repeats. These recombinant proteins were expressed and purified as described previously (15).
Generation of recombinant replication-defective adenovirus vectors expressing sequences encoding the CSP antigens of P. vivax.
Recombinant replication-defective human adenovirus serotype 5 (AdHu5) and chimpanzee adenovirus serotype 68 (AdC68) vectors were generated, purified, quality controlled, and titrated as previously described (16), with the sequence ad-PvCSP. For the deduced amino acid sequence of the CS antigen, see Fig. 6B.
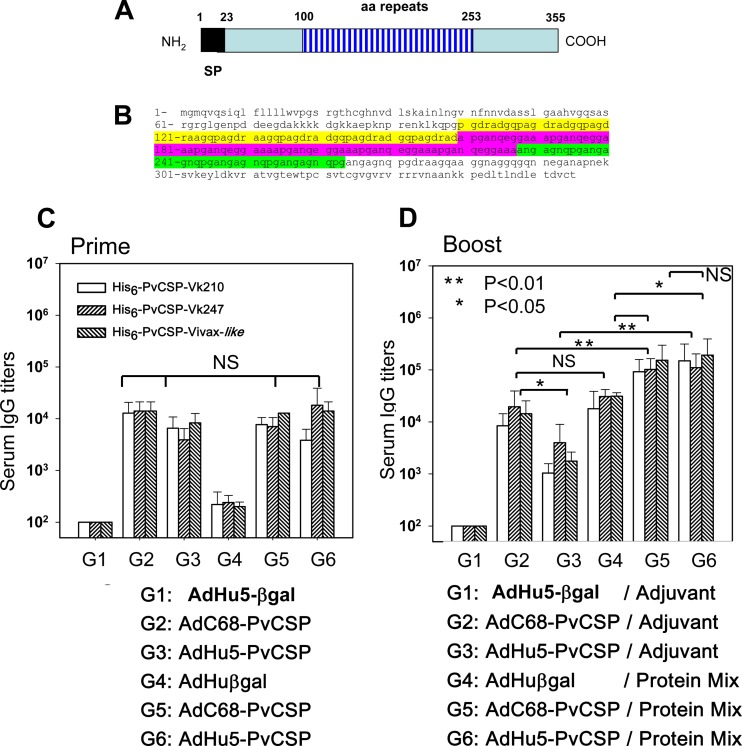
FIG 6.
Induction of specific antibody responses in mice immunized with recombinant adenoviruses expressing P. vivax CSP as part of a heterologous prime-boost regimen of vaccination. (A and B) Schematic representations and deduced amino acid (aa) sequences of the PvCSP-All-CSP-epitopes expressed by recombinant replication-defective simian or human adenovirus. (C) C57BL/6 mice were immunized i.m. at 2 × 107 PFU/mouse with AdHu5-βgal (control), AdC68-PvCSP, or AdHu5-PvCSP. Serum titers of IgG against recombinant proteins of each allelic form of PvCSP were determined 21 days later as indicated. Mice immunized with AdC68-PvCS and AdHu5-PvCS responded equally well according to statistical comparisons (one-way ANOVA, no statistically significant difference [NS]). The results are expressed as means ± SD (n = 5). (D) Mice were boosted s.c. 42 days after being primed with a formulation containing a protein mixture (His6-PvCSP-VK210, His6-PvCSP-VK247, and His6-PvCSP-Vivax-like, 3 μg/dose/mouse) in the presence of poly(I·C). The titers of specific antibodies to the different allelic forms of PvCSP were measured at day 63. According to statistical comparisons (one-way ANOVA, Tukey's HSD test), mice immunized with AdC68-PvCS and AdHu5-PvCS and boosted with a protein mixture (G5 and G6) had serum IgG antibody titers higher than those of the other groups, as shown.
Immunization regimens.
Mice were given three immunizations 3 weeks apart subcutaneously (s.c.) in the two hind footpads with a final volume of 50 μl for each footpad (first dose) and a final volume of 100 μl at the base of the tail (second and third doses). For each dose, the indicated amount of protein was used. Complete Freund's adjuvant (CFA) and incomplete Freund's adjuvant (IFA) (Sigma) were emulsified with the protein antigens (1:1, vol/vol) prior to injection. For the CFA-IFA immunization regimens, CFA was used for the first dose and IFA was used for subsequent doses. As additional adjuvants, 50 μg of high-molecular-weight poly(I·C) (InvivoGen) or Hiltonol (PolyICL, Oncovir Inc., kindly provided by A. M. Salazar) was mixed with the protein antigens in a final volume of 100 μl.
Recombinant adenovirus vectors were injected intramuscularly (i.m.) into the tibialis anterioris with a final volume of 50 μl for each leg.
Peptide synthesis.
Synthetic peptides were purchased from GenScript (Piscataway, NJ). Peptide purity was higher than 90%. Peptide identities were confirmed by a Q-TOF Micro equipped with an electrospray ionization source (Micromass UK Ltd., Manchester, United Kingdom).
Immunological assays.
Serum IgG anti-P. vivax CSP antibodies were detected by enzyme-linked immunosorbent assay (ELISA) as previously described (14, 15). The recombinant proteins (100 to 200 ng/well) were used as the solid-phase bound antigen. A peroxidase-conjugated goat anti-mouse IgG (Sigma) was applied at a final dilution of 1:1,000, while the sera from mice were tested at serial dilutions starting with 1:200. Specific antibody titers were determined as the highest dilution yielding an OD492 higher than 0.1. In parallel, we performed a standard curve of purified mouse IgG (Sigma) by using the anti-Fab (Sigma) capture antibody according to the protocol suggested in reference 17.
Detection of IgG subclass responses was performed as described above, except that the secondary antibody was specific for mouse IgG1, IgG2b, and IgG2c (Southern Technologies). The results are presented as means ± standard deviations (SD).
Monoclonal antibodies (MAbs) against P. vivax CS proteins representing the allelic forms VK210 (2F2) and VK247 (2E10.E9; Alan Cochrane, unpublished results) were generated after immunization with radiation-attenuated sporozoites (18). The hybridomas used were obtained from the Malaria Research and Reference Reagent Resource Center (MR4). Polyclonal antibodies against the P. vivax-like epitopes were obtained from C57BL/6 mice immunized with the His6-FliC-PvCSP-Vivax-like antigen as described previously (14). MAbs to the His tag were purchased from GE Healthcare Systems.
Cell-mediated immunity assays.
Splenocytes collected from immunized C57BL/6 mice were used for enzyme-linked immunospot (ELISPOT) assays to measure the secretion of IFN-γ and for in vivo cytotoxicity assays. These assays were performed as in our previous studies (19, 20).
For assays of the expression of the intracellular cytokines (ICs) IFN-γ and TNF-α, splenocytes collected from C57BL/6 mice were treated with ACK buffer (NH4Cl, 0.15 M; KHCO3, 10 mM; Na2-EDTA, 0.1 mM; pH 7.4) to lyse the erythrocytes. ICs were evaluated after in vitro culture of splenocytes in the presence or absence of the indicated antigenic stimulus. Cells were washed in cell culture medium consisting of RPMI 1640 medium, pH 7.4, supplemented with 10 mM HEPES, 0.2% sodium bicarbonate, 59 mg/liter of penicillin, 133 mg/liter of streptomycin, and 10% HyClone fetal bovine serum (HyClone, Logan, UT). The viability of the cells was evaluated with 0.2% trypan blue. The cell concentration was adjusted to 5 × 106/ml of cell culture medium containing anti-CD28 (2 μg/ml), brefeldin A (10 μg/ml), and monensin (5 μg/ml; BD Pharmingen). To half of the cultures, a final concentration of 10 μg/ml of the indicated peptides or recombinant proteins or 2 μg/ml of concanavalin A (ConA; Sigma) was added. The cells were cultivated in V-bottom 96-well plates (Corning) in a final volume of 200 μl in duplicate at 37°C in a humid environment containing 5% CO2. After a 12-h incubation, cells were stained for surface markers with peridinin chlorophyll protein-Cy5.5-labeled anti-CD4 (clone RM4-5) or PECy7-labeled CD8 (clone 53-6.7) on ice for 20 min. To detect IFN-γ and TNF-α by intracellular staining, cells were then washed twice in buffer containing PBS, 0.5% bovine serum albumin (BSA), and 2 mM EDTA, fixed in 4% PBS-paraformaldehyde solution for 10 min, and permeabilized for 15 min in a solution of PBS, 0.1% BSA, and 0.1% saponin. After being washed twice, cells were stained for intracellular markers with fluorescein isothiocyanate (FITC)-labeled anti-IL-2 antibody (JES6-5H4), allophycocyanin-labeled anti-IFN-γ antibody (clone XMG1.2), and phycoerythrin -labeled anti-TNF-α antibody (clone MP6-XT22) for 20 min on ice. Finally, cells were washed twice and fixed in 1% PBS-paraformaldehyde. At least 300,000 cells were acquired on a BD FacsCanto flow cytometer and then analyzed with FlowJo (Tree Star, Ashland, OR).
P. vivax slide preparation and indirect immunofluorescence assay (IIA).
Sera from animals immunized simultaneously with the three recombinant proteins (a mixture of the His6-PvCSP-VK210, His6-PvCSP-VK247, and His6-PvCSP-Vivax-like proteins) in a formulation containing the adjuvant poly(I·C) or from mice that received a heterologous prime-boost vaccination regimen (adenovirus-protein mixture) were selected for the immunofluorescence assay. P. vivax sporozoites expressing PvCSP VK210 or VK247 were obtained as previously described (21, 22). Slides containing sporozoites fixed in air were blocked with PBS–3% BSA for 30 min at room temperature. Slides were incubated in a humid chamber for 1 h at room temperature with 1 μg/ml of the MAb 2F2 (anti-PvCSP-VK210 MAb) as a positive control or with sera from mice were immunized with adjuvant only diluted 1:1,000 as a negative control. Additionally, we used sera from immunized animals that had been diluted 1:100, 1:1,000, 1:10,000, and 1:100,000. After immersion in PBS to wash the slides, the slides were incubated for 1 h at room temperature with PBS–3% BSA containing FITC-conjugated goat antibody reactive against murine IgG (Kirkegaard & Perry Laboratories, Inc.) at a concentration of 0.02 mg/ml. The slides were washed in PBS containing 50 mM glycine, slightly dried, and mounted with buffered glycerol. We analyzed the slides under an optical microscope with fluorescence at a magnification of ×100.
Statistical analyses.
One-way analysis of variance (ANOVA) and Tukey's honestly significant difference (HSD) test were used to compare the differences between the serum IgG titers of the different groups.
Nucleotide sequence accession numbers.
Sequences for PvCSP-vk210, PvCSP-vk247, PvCSP-Vivax-like, PvCSP-All-CSP-epitopes, and ad-PvCSP were deposited into GenBank under accession numbers KF971719 to KF971723, respectively.
RESULTS
Production and purification of P. vivax CSP recombinant proteins and recognition by MAbs.
Figure 1A shows a schematic representation of the P. vivax CSP antigen and the sequences of the repeated regions present in the allelic forms of CSP (VK210, VK247, and P. vivax-like). Codon-optimized genes were synthesized that contained the same coding regions for the N and C termini. The central domain encoded the different allelic forms of the P. vivax CSP protein. These genes were expressed as recombinant fusion proteins representing each individual allelic form of the P. vivax CSP antigen linked to a hexahistidine (His6) tag. These antigens were named His6-PvCSP-VK210, His6-PvCSP-VK247, and His6-PvCSP-Vivax-like. The predicted amino acid sequence of each recombinant fusion protein is shown in Fig. 1B. In addition, a fourth synthetic gene was constructed encoding the three distinct allelic forms expressed as a single fusion polypeptide called His6-PvCSP-All-CSP-epitopes (Fig. 1B).
Recombinant proteins were purified as described in Materials and Methods and separated by SDS-PAGE under reducing conditions. Although the predicted molecular masses of the recombinant proteins were 35,207.42, 33,633.48, 33,878.94, and 34,346.40 Da, the apparent molecular masses determined by SDS-PAGE varied from approximately 50,000 to 55,000 Da (Fig. 1C).
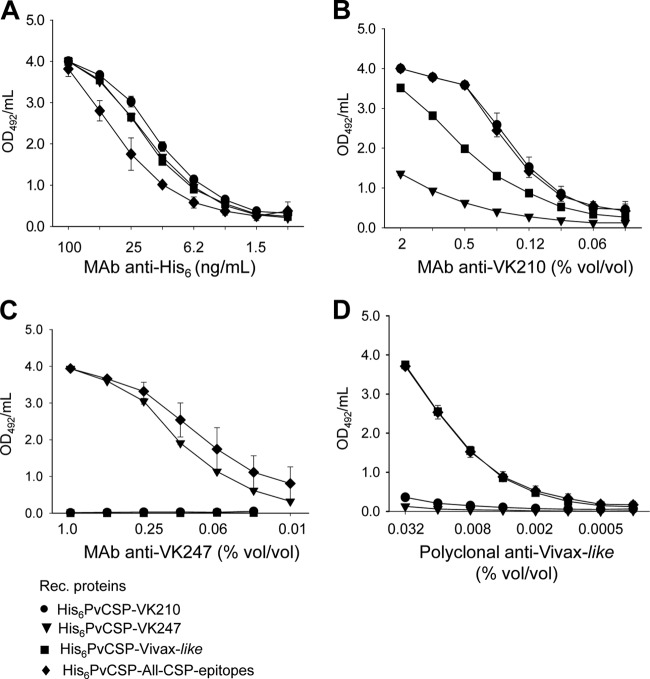
To determine whether the polypeptides retained the epitopes recognized by specific MAbs generated against radiation-attenuated P. vivax sporozoites, we performed an ELISA with each of the four recombinant proteins bound to the plates. As depicted in Fig. 2A, all four recombinant proteins were recognized well by a MAb against the His tag. The MAb against allelic variant VK210 (2F2) recognized recombinant proteins His6-PvCSP-VK210 and His6-PvCSP-All-CSP-epitopes equally well. This MAb extensively cross-reacted with the recombinant protein His6-PvCSP-Vivax-like and reacted to a minor extent with His6-PvCSP-VK247 (Fig. 2B).
FIG 2.
Antibody recognition of P. vivax CSP recombinant proteins. ELISAs were performed with each of the four PvCSP recombinant (Rec.) proteins bound to the plates to determine whether the polypeptides retained the epitopes recognized by specific MAbs generated against radiation-attenuated P. vivax sporozoites. (A) MAb against the His tag. (B) MAb 2F2 specific to the allelic form PvCSP-VK210. (C) MAb 2E10.E9 specific to the allelic form PvCSP-VK247. (D) Polyclonal antibodies to the PvCSP-Vivax-like repeat region.
The MAb against the VK247 allele (2E10.E9) reacted with the recombinant proteins His6-PvCSP-VK247 and His6-PvCSP-All-CSP-epitopes equally well. No cross-reactivity with the recombinant protein His6-PvCSP-VK210 or His6-PvCSP-Vivax-like was detected (Fig. 2C). Finally, polyclonal antibodies to the P. vivax-like repeat region reacted equally well with the recombinant proteins His6-PvCSP-Vivax-like and His6-PvCSP-All-CS-epitopes. No cross-reactivity was detected with the recombinant protein His6-PvCSP-VK210 or His6-PvCSP-VK247 (Fig. 2D). On the basis of these results, we concluded that the repeat epitopes present in the different allelic variants of P. vivax were preserved in the bacterial recombinant proteins. Most relevant, the recombinant His6-PvCSP-All-CSP-epitopes were equally recognized by all three different MAbs and polyclonal sera used, indicating the presence of the three distinct epitopes in this fusion polypeptide.
Induction of specific antibody responses in mice immunized with recombinant P. vivax CSP antigens.
The serum IgG responses to P. vivax CSP antigens were determined in C57BL/6 mice immunized s.c. with purified proteins in the presence of the adjuvant poly(I·C) or emulsified in complete/incomplete Freund's adjuvant (CFA-IFA). Mice were immunized with three doses 21 days apart, and the antibody titers were analyzed according to the timeline described in Fig. 3A. Twelve mouse groups were immunized with (i) poly(I·C), (ii) poly(I·C) plus His6-PvCSP-VK210 (10 μg/dose/mouse), (iii) poly(I·C) plus His6-PvCSP-VK247 (10 μg/dose/mouse), (iv) poly(I·C) plus His6-PvCSP-Vivax-like (10 μg/dose/mouse), (v) poly(I·C) plus a protein mixture (His6-PvCSP-VK210, His6-PvCSP-VK247, and His6-PvCSP-Vivax-like, 30 μg/dose/mouse), (vi) poly(I·C) plus His6-PvCSP-All-CSP-epitopes (30 μg/dose/mouse), (vii) CFA-IFA, (viii) CFA-IFA plus His6-PvCSP-VK210 (10 μg/dose/mouse), (ix) CFA-IFA plus His6-PvCSP-VK247 (10 μg/dose/mouse), (x) CFA-IFA plus His6-PvCSP-Vivax-like (10 μg/dose/mouse), (xi) CFA-IFA plus a protein mixture (His6-PvCSP-VK210, His6-PvCSP-VK247, and His6-PvCSP-Vivax-like, 30 μg/dose/mouse), and (xii) CFA-IFA plus His6-PvCSP-All-CSP-epitopes (30 μg/dose/mouse).
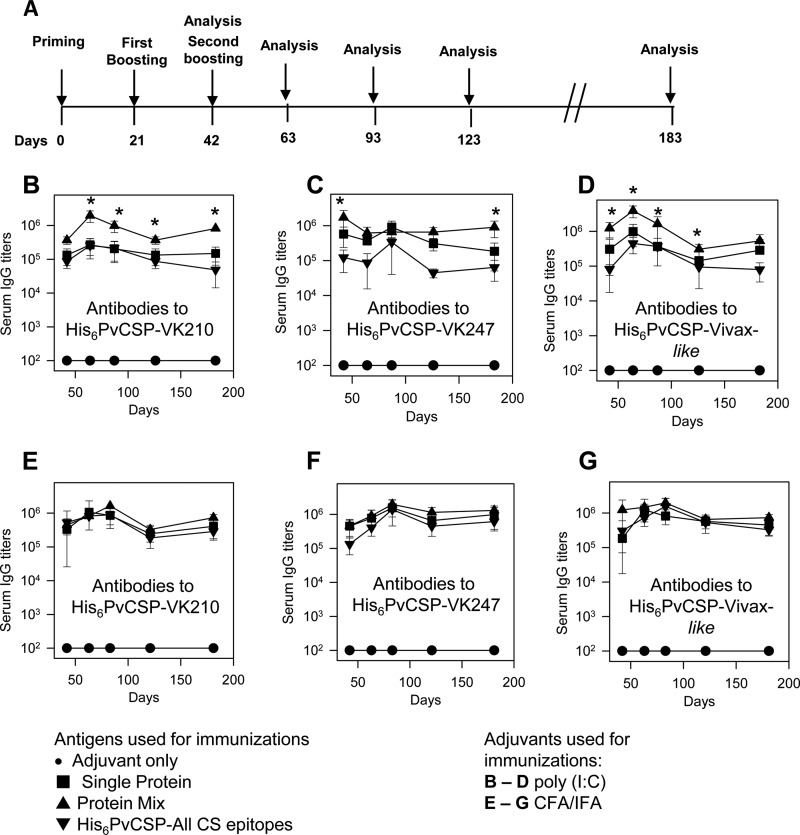
FIG 3.
Induction of specific antibody responses in mice immunized with recombinant P. vivax CSP antigens. (A) Mice were immunized with three doses 21 days apart, and the antibody titers were analyzed according to the timeline shown. The serum IgG responses to P. vivax CS antigens were determined in C57BL/6 mice immunized s.c. with the purified proteins in a formulation containing the adjuvant poly(I·C) (B to D) or emulsified in CFA or IFA (E to G). Mice were immunized with His6-PvCSP-VK210 (10 μg/dose/mouse), His6-PvCSP-VK247 (10 μg/dose/mouse), His6-PvCSP-Vivax-like (10 μg/dose/mouse), a protein mixture (His6-PvCSP-VK210, His6-PvCSP-VK247, and His6-PvCSP-Vivax-like, 30 μg/dose/mouse), or His6-PvCSP-All-CS-epitopes (30 μg/dose/mouse). Serum IgG titers of antibodies against recombinant proteins representing each allelic form of PvCSP were measured as indicated. The results are expressed as means ± SD (n = 6). Statistical comparisons by one-way ANOVA of the antibody titers from mice immunized with formulations containing the adjuvant poly(I·C) (B to D) show significantly higher antibody levels in animals immunized with the protein mixture (P < 0.01, asterisks). Comparisons of the antibody titers of mice immunized with formulations containing the adjuvant CFA-IFA and recombinant proteins did not show statistically significant differences.
At the indicated days, the titer of antibody to each individual allelic form of P. vivax CSP was determined by ELISA. In all of the groups of mice immunized with a formulation containing recombinant CSP antigen and adjuvant, the antibody titers were above 105 after the second dose. According to our standard curve of mouse IgG, this antibody titer represents approximately 50 μg/ml of specific IgG. After the third dose, in some cases, we observed an enhancement of these titers to ≥106. High antibody titers lasted for a period of 183 days after the first immunization. At that time, titers were still in the range of 105 to 106.
By comparing the antibody titers of groups of mice immunized with the recombinant antigens in the presence of the adjuvant poly(I·C) (Fig. 3B to D), we found that immunization with the protein mixture generated higher antibody titers than immunization with each individual protein or with the protein His6-PvCSP-All-CSP-epitopes (P < 0.01 in all cases for the period of 183 days). In contrast, all groups of mice immunized with the recombinant CSP antigens emulsified in CFA-IFA produced similar antibody titers (Fig. 3E to G; no statistically significant difference in all cases for the period of 183 days).
We then compared groups of mice injected with the protein mixture or His6-PvCSP-All-CSP-epitopes in formulations containing the adjuvants poly(I·C) or CFA-IFA. We found that immunization with the protein mixture generated similar antibody titers when emulsified in CFA-IFA or mixed with the adjuvant poly(I·C) (no statistically significant difference). In contrast, mice immunized with His6-PvCSP-All-CSP-epitopes emulsified in CFA-IFA displayed significantly higher antibody titers than animals injected with the antigen in the presence of poly(I·C). These experiments allowed us to conclude that immunization with the protein mixture was superior to immunization with His6-PvCSP-All-CSP-epitopes when poly(I·C) was used as the adjuvant. Additionally, we concluded that when using the protein mixture in the presence of poly(I·C), the antibody titers could be as high and long lasting as the titers generated by this same protein mixture emulsified in CFA-IFA.
Although the anti-PvCSP-specific IgG antibody titers after immunization with the protein mixture in formulations containing CFA-IFA or poly(I·C) were equally high, these immune responses differed significantly in terms of the subclasses of IgG that were elicited. Responses generated in the presence of CFA-IFA resulted in predominately IgG1 rather than IgG2c (ratio of 19.1), while responses generated in the presence of poly(I·C) resulted in an IgG1-to-IgG2c subclass ratio of 0.95.
To compare the potency of poly(I·C) with the commercially available Hiltonol [poly(ICLC)] that is being used in human trials (23), we performed side-by-side comparison experiments by immunizing mice with (i) poly(I·C), (ii) poly(I·C) plus a protein mixture (His6-PvCSP-VK210, His6-PvCSP-VK247, and His6-PvCSP-Vivax-like, 30 μg/dose/mouse), or (iii) Hiltonol plus the same protein mixture. We observed that the antibody titers of mice immunized with the protein mixture in the presence of either adjuvant elicited similarly high antibody titers that lasted until at least day 160 (no statistically significant difference; data not shown).
We then determined whether lower doses of the recombinant protein mixture could be as effective in eliciting high and sustained antibody immune responses. For this purpose, mice were immunized side by side with 10 or 1 μg of each of the three recombinant proteins in the presence of the adjuvant poly(I·C). Mice immunized with 30 or 3 μg of the total protein mixture responded equally well. Antibody titers above 105 lasted for more than 200 days (Fig. 4A to C; no statistically significant difference).
FIG 4.
Induction of specific antibody responses in mice immunized with recombinant P. vivax CSP antigens. C57BL/6 mice were immunized at 30 or 3 μg/dose/mouse with a formulation containing a protein mixture (His6-PvCSP-VK210, His6-PvCSP-VK247, and His6-PvCSP-Vivax-like) in the presence of the adjuvant poly(I·C). The immunizations were s.c. with three doses given 21 days apart according to the timeline shown in Fig. 3A. Serum IgG titers of antibodies against recombinant proteins representing each allelic form of PvCSP were measured as indicated. Mice immunized with 30 (inverted triangles) and 3 (squares) μg/dose/mouse of the protein mixture responded equally well according to statistical comparisons (one-way ANOVA, no statistically significant difference). The results as expressed as means ± SD (n = 6).
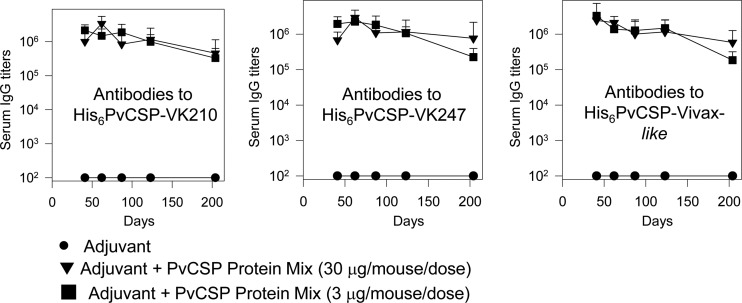
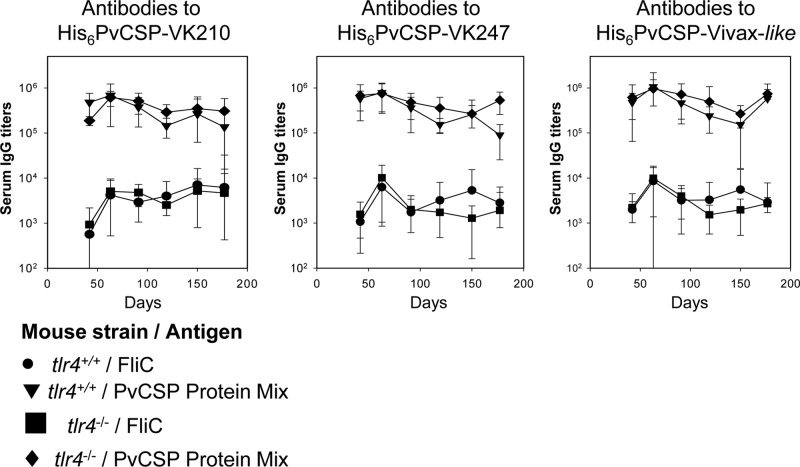
Purified bacterial recombinant proteins often contain traces of contaminating LPS that can interfere with immunogenicity. To show that contaminating LPS was not influencing the immunogenicity results, we performed a side-by-side comparison of the antibody immune responses to the protein mixture when administered to tlr4+/+ or tlr4−/− mice in the presence of poly(I·C). After immunization, both mouse strains responded equally well for the entire 177-day observation period (Fig. 5A to C; no statistically significant difference). These observations ruled out any possible interference from LPS contamination in our samples.
FIG 5.
Induction of specific antibody responses in tlr4−/− mice immunized with recombinant P. vivax CS antigens. Mice were immunized with three doses 21 days apart according to the protocol and timeline shown in Fig. 3A. The serum IgG responses against P. vivax CSP recombinant proteins were determined in tlr4+/+ and tlr4−/− mice immunized with formulations containing poly(I·C) and a PvCSP mixture (His6-PvCSP-VK210, His6-PvCSP-VK247, and His6-PvCSP-Vivax-like, 3 μg/dose/mouse) or control recombinant FliC (10 μg/dose/mouse). tlr4+/+ (inverted triangles) and tlr4−/− (diamonds) mice immunized with the PvCSP protein mixture responded well according to statistical comparisons (one-way ANOVA, no statistically significant difference). The results are expressed as means ± SD (n = 6).
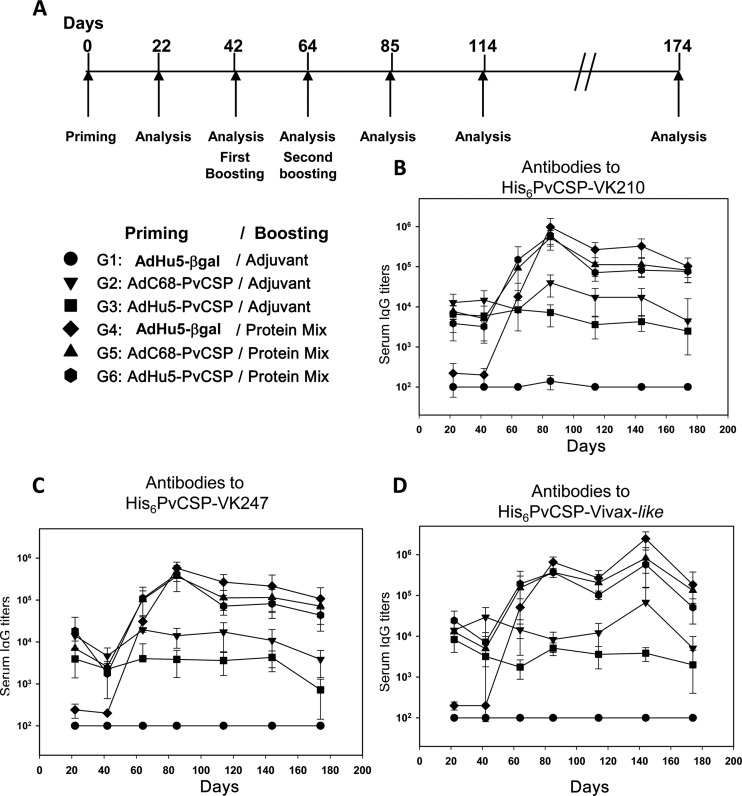
Induction of specific antibody responses in mice immunized with recombinant adenovirus vectors expressing P. vivax CSP as part of a heterologous prime-boost regimen for vaccination.
In addition to the recombinant proteins, we generated two recombinant replication-defective adenovirus vectors expressing the gene encoding the PvCSP-All-CSP-epitopes antigen. Schematic representations of the deduced amino acid sequences of the proteins expressed by these recombinant adenovirus vectors are depicted in Fig. 6A and B. Antigen expression was confirmed by infection of HEK-293 cells and immunofluorescence analyses (data not shown). The recombinant adenoviruses were designated AdC68-PvCSP and AdHu5-PvCSP. Immunization of C57BL/6 mice with 2 × 107 PFU of either of these recombinant adenovirus vectors elicited specific IgG antibodies against each individual allelic form of PvCSP. This dose was determined experimentally as the minimum dose to elicit antibody titers higher than 103. After 21 days, titers of antibodies to all three different alleles of recombinant PvCSP were in the range of 104. No statistically significant difference was observed in the serum IgG titers between the groups of mice vaccinated with AdC68-PvCSP or AdHu5-PvCSP (Fig. 6C). As expected, control mice injected with the same dose of AdHu5β-gal did not generate any P. vivax-specific antibodies. However, at a later time point, the antibody titers of mice immunized with AdC68-PvCSP were higher than those of animals immunized with AdHu5-PvCSP (P < 0.05; Fig. 6D).
To test whether recombinant adenovirus vectors could be used as priming immunogens in a heterologous prime-boost regimen, mice primed with the adenovirus vector were boosted s.c. 42 days after being primed with a protein mixture (His6-PvCSP-VK210, His6-PvCSP-VK247, and His6-PvCSP-Vivax-like 3 μg/dose/mouse) in a formulation containing poly(I·C). Serum titers of IgG antibodies against all three allelic forms of PvCSP at day 63 in mice primed with AdC68-PvCSP (G5) or AdHu5-PvCSP (G6) and boosted with the protein mixture were significantly higher than the titers in control groups (G1, G2, G3, and G4) (P < 0.05, Fig. 6D). No statistically significant differences were scored between mouse groups G5 and G6. These results provide evidence that priming with AdC68-PvCSP and AdHu5-PvCSP improves antibody responses upon subsequent immunization with the recombinant protein mixture.
These same mice described above received a second boost with a protein mixture in a formulation containing poly(I·C) or with only adjuvant (control), as depicted in Fig. 7A. We followed the titers of specific serum IgGs against the different allelic forms of PvCSP for 178 days (Fig. 7B to D). Antibody titers in the different mouse groups immunized (boosted) twice with the recombinant protein mixture were equally high, and we did not detect statistically significant differences among groups G4, G5, and G6 (no statistically significant difference in all cases). Not only were the titers of anti-PvCSP-specific IgG after immunization equally high, but these immune responses were equally sustained, with high antibody titers persisting until day 178. Additionally, these responses did not differ significantly in terms of the subclasses of IgG that were elicited. The IgG1/IgG2c ratios of groups G4 and G5 were 0.95 and 1.125, respectively.
FIG 7.
Induction of specific antibody responses in mice immunized with a heterologous (adenovirus-protein) or a homologous (protein-protein) vaccination regimen. (A) C57BL/6 mice were immunized with three doses of the indicated immunogens, and the antibody titers were analyzed according to the timeline shown. The first dose consisted of 2 × 107 PFU/mouse of AdHu5-βgal (control), AdC68-PvCSP, or AdHu5-PvCSP administered i.m. The first and second boosts consisted of a formulation containing a protein mixture (His6-PvCSP-VK210, His6-PvCSP-VK247, and His6-PvCSP-Vivax-like, 3 μg/dose/mouse) in the presence of poly(I·C). (B) Serum IgG titers against His6-PvCSP-VK210. (C) Serum IgG titers against His6-PvCSP-VK247. (D) Serum IgG titers against His6-PvCSP-Vivax-like. Mice immunized with AdHu5-βgal, AdC68-PvCS, and AdHu5-PvCS and boosted twice with a formulation containing a protein mixture in the presence of poly(I·C) (G4, G5, and G6) responded equally well according to statistical comparisons (one-way ANOVA, Tukey's HSD test, no statistically significant difference). The results are expressed as means ± SD (n = 5).
These experiments allow us to conclude that recombinant adenoviruses expressing the PvCSP protein can successfully be used in a heterologous prime-boost protocol for vaccination. However, no significant impact on the serum IgG antibody titers was observed when we used either the homologous (protein-protein) or the heterologous (adenovirus-protein) regimen.
Specificity of the antibodies elicited by the homologous (protein-protein) or heterologous (adenoviruses/protein) regimen.
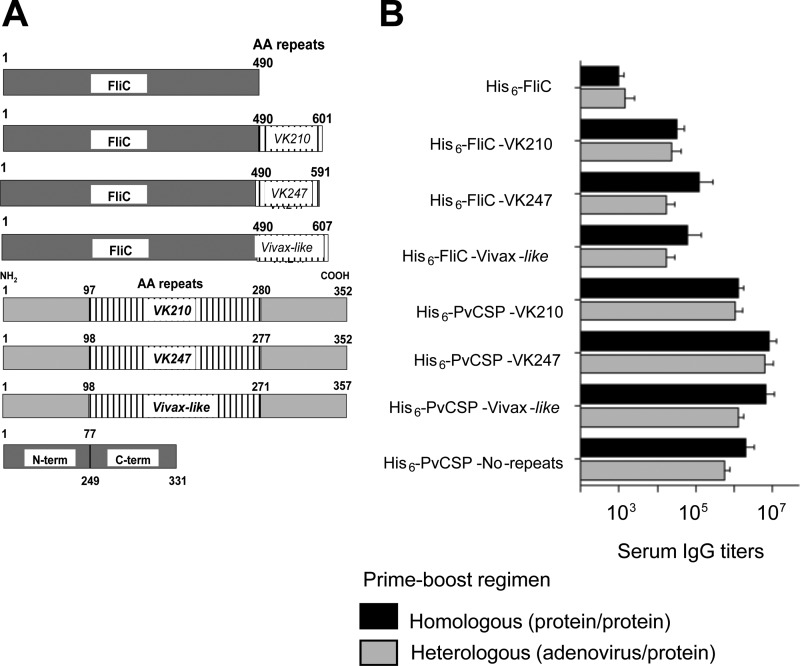
We then studied the specificity of the antibodies against PvCSP. We selected for the sera from mice that had been immunized with the homologous (protein-protein) or heterologous (adenovirus-protein) regimen. To aid in the determination of the specificity of anti-PvCSP serum IgG antibodies, we used a number of recombinant proteins containing different portions of the antigen. Schematic representations of the recombinant proteins used and the antibody titers are depicted in Fig. 8A.
FIG 8.
Specificity of the antibodies elicited by a homologous (protein-protein) or a heterologous (adenovirus-protein) immunization regimen. (A) Schematic representation of each recombinant protein used as a substrate bound to the plates in the ELISA. AA, amino acid; term, terminus. (B) Serum IgG antibodies from mice immunized with the homologous [protein-protein with poly(I·C)] or the heterologous (AdC68-PvCSP/protein mixture) regimen. The detailed immunization protocol is described in the legends to Fig. 3 (homologous) and 7 (heterologous). Sera were collected 2 weeks after the third dose was given. The results are expressed as means ± S.D (n = 5).
We found that antibodies from mice immunized with either the homologous or the heterologous regimen reacted with all of the recombinant proteins containing different portions of the three distinct allelic forms of the PvCSP antigen. These antibodies reacted with each repeat domain expressed as a part of a fusion protein in the C-terminal region of FliC. The mice also had significant titers of antibodies to a recombinant protein expressing the N- and C-terminal regions but not the repeat region of the PvCSP antigen (His6-PvCSP-No-repeats). As expected, however, the maximal reactivity was directed against the recombinant proteins containing all of PvCSP (Fig. 8B). We concluded that both the repeated and nonrepeated regions of PvCSP are targets for antibodies.
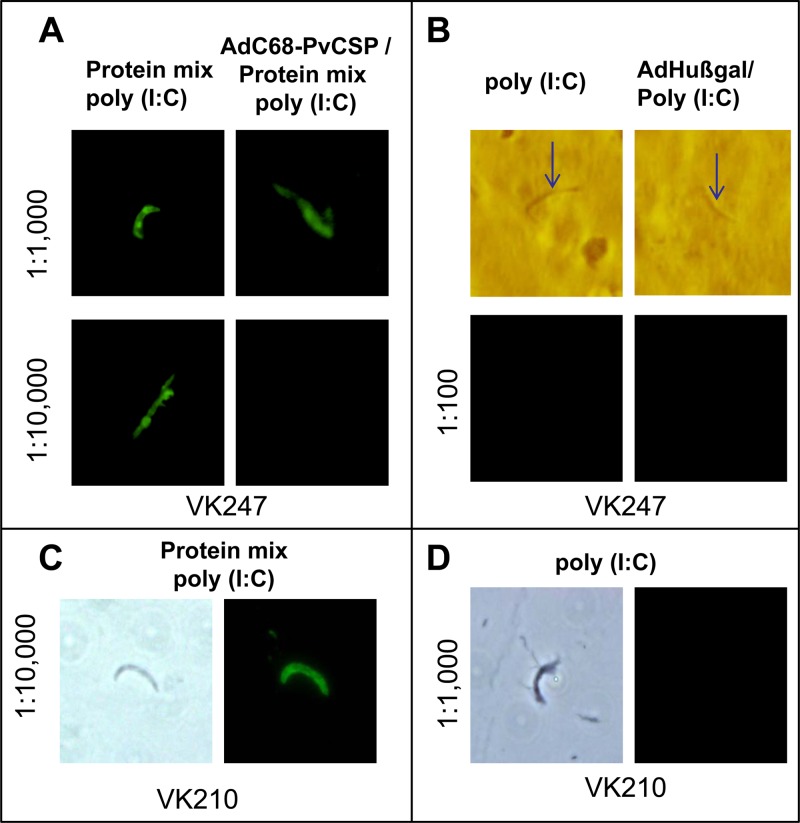
Finally, we determined whether sera from mice immunized with the homologous or heterologous immunization regimens reacted with P. vivax sporozoites in immunofluorescence assays. We observed that sera from both groups reacted to sporozoites of the P. vivax VK247 strain at a titer of 1:1,000 and in the case of the homologous immunization also at a titer of 1:10,000 (Fig. 9A). Antibody recognition was specific, as control sera from mice immunized with either the adjuvant poly(I·C) or AdHu5β-gal/poly(I·C) did not react, even at a 1:100 dilution (Fig. 9B). We also confirmed that sera from both groups of mice that were immunized with a formulation containing the protein mixture and poly(I·C) reacted to sporozoites of the VK210 strain at a dilution of 1:10,000 (Fig. 9C).
FIG 9.
IIA for the recognition of the native protein PvCSP. Pools of sera from mice immunized with the homologous [protein-protein with poly(I·C)] and heterologous (AdC68-PvCSP/protein mixture) regimens were used. The detailed immunization protocol is described in the legends to Fig. 3 (homologous) and 7 (heterologous).
Cell-mediated immune responses.
To determine whether the vaccination protocols elicited T cell-mediated immune responses, we employed several methods of analyses. Spleen cells from mice immunized with either the homologous or the heterologous regimen were stimulated in vitro with recombinant proteins or overlapping synthetic peptides (15-mers) covering all of PvCSP. We measured IFN-γ secretion by ELISPOT assay, by IC expression assays, and by detection of the cytokine in the supernatants. We also attempted to detect IL-2 and TNF by IC expression assays. Finally, we also used an in vivo cytotoxicity assay to estimate the presence of cytotoxic T lymphocytes in vaccinated mice (24). The results were negative in most cases independently of the timing of the analyses. Positive controls performed in parallel with experimental samples were consistently successful (data not shown).
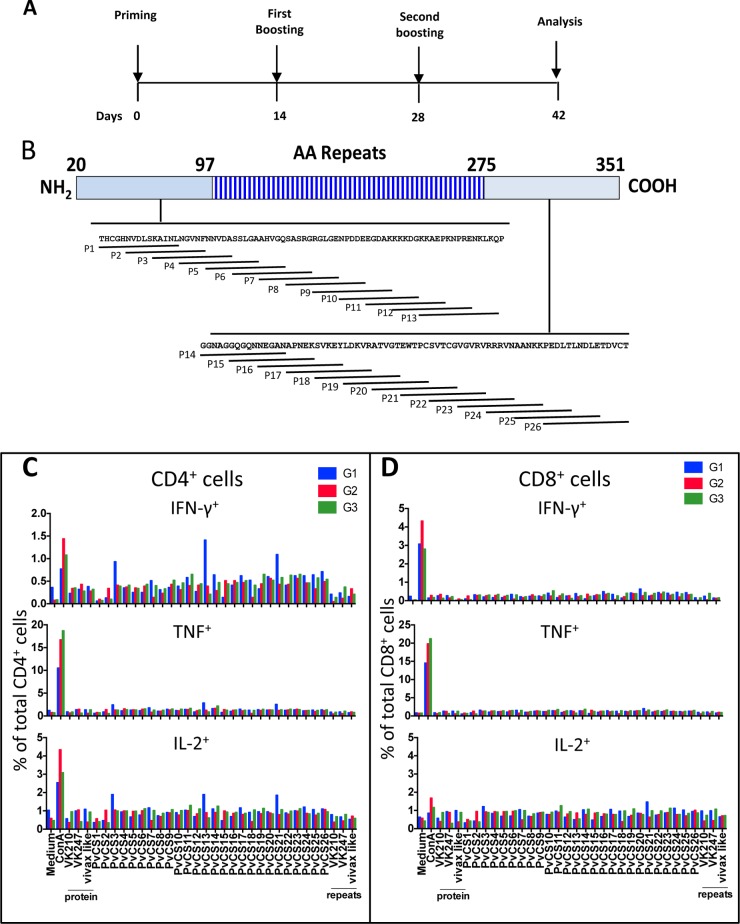
Examples of these studies are depicted in Fig. 10 and 11. Splenic cells of mice immunized with the heterologous or homologous prime-and-boost regimen (described in Fig. 10A) were restimulated in culture with the recombinant proteins or synthetic peptides spanning the entire N- or C-terminal region of P. vivax CSP (Fig. 10B). Significant differences in the frequencies of IFN-γ+, IL-2+, or TNF-a+ in CD4+ or CD8+ cells in the experimental and control groups were not detected (Fig. 10C and D).
FIG 10.
Cell-mediated immunity of mice immunized with recombinant P. vivax CSP antigens. (A) C57BL/6 mice were immunized with three doses given 14 days apart, and IC expression assays were performed according to the timeline shown. (B) Twenty-six overlapping peptides spanning the entire sequence of the N or C terminus of PvCSP were used to stimulate splenocytes. AA, amino acid. (C and D) G1 mice (control) were primed i.m. at 2 × 108 PFU/mouse with AdHu5-b-gal and boosted s.c. twice with poly(I·C). G2 mice were primed i.m. at 2 × 108 PFU/mouse with AdC68-PvCSP and boosted twice with a protein mixture (His6-PvCSP-VK210, His6-PvCSP-VK247, and His6-PvCSP-Vivax-like, 30 μg/dose/mouse) in the presence of poly(I·C). G3 mice were primed and boosted with a protein mixture (His6-PvCSP-VK210, His6-PvCSP-VK247, and His6-PvCSP-Vivax-like, 30 μg/dose/mouse). Fourteen days later, splenic cells of these mice were cultured in the presence of anti-CD28 antibody, monensin, and brefeldin A with or without the peptides or recombinant proteins or ConA as indicated. The PvCS-VK210, PvCS-VK247, and PvCS-Vivax-like peptides represent the repeat regions of the allelic variants. After 12 h, cells were stained with anti-CD4, anti-CD8, anti-IL-2, anti-IFN-γ, and anti-TNF-α antibodies. The results are expressed as the total frequency (%) of CD4+ or CD8+ cells stained for each molecule. Results were obtained from pooled cells from five mice.
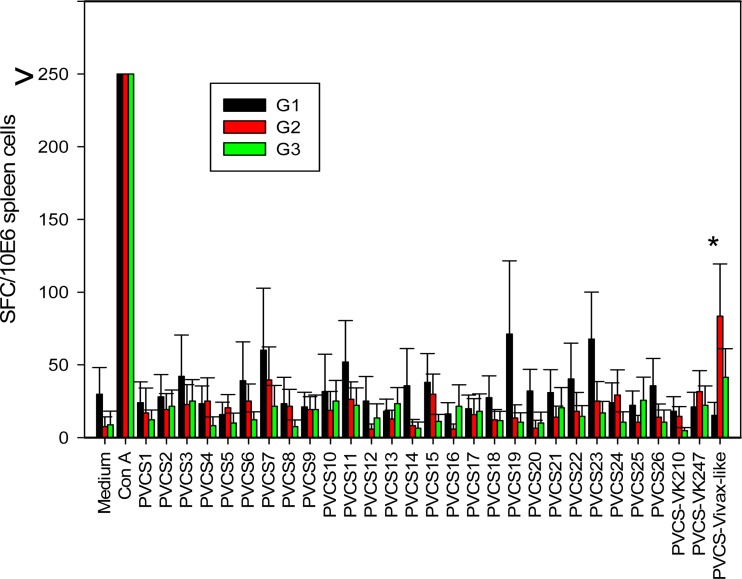
FIG 11.
Cell-mediated immunity of mice immunized with recombinant P. vivax CSP antigens. C57BL/6 mice were immunized as described in the legend to Fig. 10. Splenic cells of these mice were cultured in the presence or absence of the peptides or ConA, as indicated. The PvCS-VK210, PvCS-VK247, and PvCS-Vivax-like peptides represent the repeat regions of the allelic variants. After 48 h, IFN-γ-secreting cells (spot-forming cells [SFC]) were estimated by ELISPOT assay. The results are expressed as the frequency of SFC per 106 spleen cells obtained from pooled cells of five mice. The asterisk denotes a statistically significant difference (P < 0.01).
In parallel, these cells were subjected to ELISPOT assays. Likewise in the IC expression assay experiments, significant differences in the frequencies of IFN-γ-producing cells in the experimental and control groups were not detected (Fig. 11). One peptide only representing the repeat region of PvCSP of the P. vivax-like allele elicited a modest number of specific spot-forming cells. This peptide possibly represents a T cell epitope for C57BL/6 mice. We were able to obtain a stronger immune response by using IFA for immunization (data not shown). We concluded that although we were able to obtain high levels of antibody production following immunization, this response was not paralleled by high levels of T cell-mediated immunity.
DISCUSSION
In the present study, we tested whether recombinant proteins and adenovirus vectors could be used for the development a universal vaccine against P. vivax malaria. Using homologous or heterologous prime-boost immunization regimens, we were able to successfully elicit high titers of antibodies recognizing the three allelic forms of the PvCSP antigen. This malaria vaccine formulation could be used either as a protein mixture containing His6-PvCSP-VK210, His6-PvCSP-VK247, and His6-PvCSP-Vivax-like or a single recombinant protein containing epitopes of all three different allelic forms in a single fusion polypeptide (His6-PvCSP-All-CSP-epitopes). However, in the presence of poly(I·C), we found the protein mixture to be more immunogenic. This improved immunogenicity was not observed when the antigens were emulsified in CFA-IFA. The precise reasons for these differences are not clear. Nevertheless, these data highlight the importance of testing different formulations for vaccine selection.
Both homologous and heterologous vaccine protocols elicited specific antibody titers that were as high as those elicited following immunization with recombinant proteins emulsified in CFA-IFA. In addition, the antibody responses induced by both protocols were long lasting and significant titers were observed during the ∼180-day period of the study. We have focused our efforts on inducing persistently high titers of specific antibodies against the CSP antigen. Antibodies against CSP have been described previously as a critical mechanism of antisporozoite immunity in diverse malaria models (reviewed in reference 1). On the basis of phase II human trials performed with the RTS,S/AS candidate vaccine, antibodies continue to be the factor that strongly correlates with sterile immunity. In RTS,S/AS-vaccinated individuals who were experimentally challenged with P. falciparum sporozoites by mosquito bite, vaccine efficacy was correlated with higher titers of antibodies against CSP and sporozoites (2). Similarly, phase II vaccine trials performed with African children reported a nonlinear relationship between the titers of antibodies against CSP and a lower incidence of malaria (25).
Several antiparasitic mechanisms have been described that are mediated by antibodies against CSP. First, antibodies reduce the sporozoite inoculum and parasite viability during a mosquito bite. With an experimental rodent model, it has been shown that an immune complex formed during the mosquito bite reduces the number of sporozoites deposited during feeding (26). The parasites injected into immune mice were immobilized and not capable of invading dermal blood vessels (26). When the sporozoites are inoculated intravenously, thus bypassing the skin, anti-CSP antibodies efficiently block the infection of hepatocytes (26). The inhibition of hepatocyte infection may be due to a reduction of the sporozoites' ability to move or attach to hepatocytes (27–29).
The specificity of the inhibitory antibodies for the malaria CSP antigen was initially assigned to the central repeat domain (30). In the case of P. vivax, this postulate was recently confirmed by the use of a transgenic P. berghei sporozoite expressing the repeat domain from the VK210 strain. In vivo parasite infectivity in mice was drastically inhibited by the passive transfer of a MAb against the VK210 repeats (31). These observations further confirm similar data from other parasites, which showed that the repeat domain of CSP is a target of neutralizing antibodies.
In addition to the repeat domain, in the case of the P. vivax CSP protein, humans immunized with long synthetic peptides representing the N and C termini of CSP generated antibodies that can inhibit sporozoite invasion in vitro (32). These results indicate that vaccines that elicit high titers of antibodies against different regions of PvCSP should be pursued. In the vaccination protocols described here, we generated high titers of antibodies against all three allelic forms of the PvCSP protein that reacted with different domains of the protein.
IIA analyses with sporozoites of P. vivax strains VK210 and VK247 demonstrated that these vaccine-elicited antibodies can recognize the native CSP on sporozoites. However, the ability of these antibodies to neutralize sporozoites in vivo has yet to be evaluated with transgenic parasites (31).
In addition to antibodies, CD4+ and CD8+ T cells have been described as powerful mediators of preerythrocytic stage elimination in rodent experimental models (reviewed in references 32–38). In fact, a number of T cell epitopes have been described by us and others in CSP (reviewed in references 32–38). The results obtained with the mouse model have fueled a number of human trials with plasmid DNA, recombinant viruses, and, more recently, radiation-attenuated sporozoites as vaccine formulations. Unfortunately, in spite of the enthusiasm generated by the mouse model, T cell-based vaccines against preerythrocytic stages of malaria have provided sterile immunity against infection with P. falciparum to only a few vaccinated individuals to date (39–44). The precise reason for this failure is not clear. Perhaps the frequency of T cells generated by the different vaccine protocols was not sufficient, as these T cells have to patrol the entire human liver. In fact, since the first reports of adoptive T cell transfer more than 20 years ago, it has been known that large numbers of multifunctional effector CD8+ T cells must be transferred to a mouse to provide sterile immunity (45–47). More recently, elegant studies using the mouse model have confirmed that a large number of multifunctional effector CD8+ T cells must be generated to provide sterile protection (48).
The development of candidate vaccines against preerythrocytic stages of P. vivax has been performed mostly with preclinical immunization models (mice and nonhuman primates; 49–51). Only three phase I clinical trials of recombinant proteins or long synthetic peptides against this parasite have been reported (52–54). The reagents and protocols described in the present study, when used in homologous or heterologous regimens, can be immediately transferred to humans. Poly(ICLC) is currently being tested for human use (23). Similarly, recombinant human replication-defective adenoviruses of serotype 5 are being used in multiple human trials (55–58).
The fact that we did not detect significant differences in antibody titers or cell-mediated immunity when using either the homologous or the heterologous vaccination regimen should not be considered a failure of the second. Considering that our ultimate goal is human trials, the use of a recombinant adenovirus vector may still improve the immune response by the presence of human T-cell epitopes. On the basis of that, we believe that the formulations described here provide new P. vivax vaccine candidates with universal coverage that warrant further clinical testing.
ACKNOWLEDGMENTS
This work was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP Proc. 2009/15432-4 and Proc. 2012/13032-5), the National Institute for Vaccine Development and Technology (CNPq-INCTV; CNPq Universal Proc. 471087/2013-0), and PNPD and fellowships from CNPq (M.M.R., I.S.S., A.G.C., OBR), FAPESP (M.T.A.L., C.A.T., J.E.), and CAPES (L.H.T.).
We are in debt to Kim Lee Sim and Stephen L. Hoffman of Sanaria Inc. for providing P. vivax sporozoite slides. We thank Keyna M. Soares for helping during the experiment shown in Fig. 6.
L.H.T., C.A.T., M.O.L., O.B.R., I.S.S., R.S.N., H.E., V.N. and M.M.R. are named inventors on patent applications entitled “Plasmodium vivax vaccine compositions,” file reference no.:27522-0201WO1.
Footnotes
Published ahead of print 9 December 2013
REFERENCES
- 1.Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, Leach A. 2010. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum. Vaccin. 6:90–96. 10.4161/hv.6.1.9677 [DOI] [PubMed] [Google Scholar]
- 2.White MT, Bejon P, Olotu A, Griffin JT, Riley EM, Kester EK, Ockenhouse CF, Ghani AC. 2013. The relationship between RTS,S vaccine-induced antibodies, CD4+ T cell responses and protection against Plasmodium falciparum infection. PLoS One 8:e61395. 10.1371/journal.pone.0061395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BG, Doucka Y, Flamen A, Mordmüller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Nhamuave A, Quelhas D, Bassat Q, Mandjate S, Macete E, Alonso P, Abdulla S, Salim N, Juma O, Shomari M, Shubis K, Machera F, Hamad AS, Minja R, Mtoro A, Sykes A, Ahmed S, Urassa AM, Ali AM, Mwangoka G, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Tahita MC, Kaboré W, Ouédraogo S, Sandrine Y, Guiguemdé RT, Ouédraogo JB, Hamel MJ, Kariuki S, Odero C, Oneko M, Otieno K, Awino N, Omoto J, Williamson J, Muturi-Kioi V, Laserson KF, Slutsker L, Otieno W, Otieno L, Nekoye O, Gondi S, Otieno A, Ogutu B, Wasuna R, Owira V, Jones D, Onyango AA, Njuguna P, Chilengi R, Akoo P, Kerubo C, Gitaka J, Maingi C, Lang T, Olotu A, Tsofa B, Bejon P, Peshu N, Marsh K, Owusu-Agyei S, Asante KP, Osei-Kwakye K, Boahen O, Ayamba S, Kayan K, Owusu-Ofori R, Dosoo D, Asante I, Adjei G, Adjei G, Chandramohan D, Greenwood B, Lusingu J, Gesase S, Malabeja A, Abdul O, Kilavo H, Mahende C, Liheluka E, Lemnge M, Theander T, Drakeley C, Ansong D, Agbenyega T, Adjei S, Boateng HO, Rettig T, Bawa J, Sylverken J, Sambian D, Agyekum A, Owusu L, Martinson F, Hoffman I, Mvalo T, Kamthunzi P, Nkomo R, Msika A, Jumbe A, Chome N, Nyakuipa D, Chintedza J, Ballou WR, Bruls M, Cohen J, Guerra Y, Jongert E, Lapierre D, Leach A, Lievens M, Ofori-Anyinam O, Vekemans J, Carter T, Leboulleux D, Loucq C, Radford A, Savarese B, Schellenberg D, Sillman M, Vansadia P, RTS,S Clinical Trials Partnership 2011. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 365:1863–1875. 10.1056/NEJMoa1102287 [DOI] [PubMed] [Google Scholar]
- 4.Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, RTS,S Clinical Trials Partnership 2012. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 367:2284–2295. 10.1056/NEJMoa1208394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IR, Baird JK, Snow RW, Hay SI. 2010. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl. Trop. Dis. 4:e774. 10.1371/journal.pntd.0000774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnot DE, Barnwell JW, Tam JP, Nussenzweig V, Nussenzweig RS, Enea V. 1985. Circumsporozoite protein of Plasmodium vivax: gene cloning and characterization of the immunodominant epitope. Science 230:815–818. 10.1126/science.2414847 [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg R, Wirtz RA, Lanar DE, Sattabongkot J, Hall T, Waters AP, Prasittisuk C. 1989. Circumsporozoite protein heterogeneity in the human malaria parasite Plasmodium vivax. Science 245:973–976. 10.1126/science.2672336 [DOI] [PubMed] [Google Scholar]
- 8.Qari SH, Shi YP, Povoa MM, Alpers MP, Deloron P, Murphy GS, Harjosuwarno S, Lal AA. 1993. Global occurrence of Plasmodium vivax-like human malaria parasite. J. Infect. Dis. 168:1485–1489. 10.1093/infdis/168.6.1485 [DOI] [PubMed] [Google Scholar]
- 9.de Arruda M, Souza RC, Veiga ME, Ferreira AF, Zimmerman RH. 1998. Prevalence of Plasmodium vivax variants VK247 and P. vivax-like human malaria: a retrospective study in indigenous Indian populations of the Amazon region of Brazil. Trans. R. Soc. Trop. Med. Hyg. 92:628. 10.1016/S0035-9203(98)90788-X [DOI] [PubMed] [Google Scholar]
- 10.Marrelli MT, Branquinho MS, Hoffmann EH, Taipe-Lagos CB, Natal D, Kloetzel JK. 1998. Correlation between positive serology for Plasmodium vivax-like/Plasmodium simiovale malaria parasites in the human and anopheline populations in the State of Acre, Brazil. Trans. R. Soc. Trop. Med. Hyg. 92:149–151. 10.1016/S0035-9203(98)90723-4 [DOI] [PubMed] [Google Scholar]
- 11.Bonilla JA, Validum L, Cummings R, Palmer CJ. 2006. Genetic diversity of Plasmodium vivax Pvcsp and Pvmsp1 in Guyana, South America. Am. J. Trop. Med. Hyg. 75:830–835 http://www.ajtmh.org/content/75/5/830.full.pdf+html [PubMed] [Google Scholar]
- 12.Gopinath R, Wongsrichanalai C, Cordón-Rosales C, Mirabelli L, Kyle D, Kain KC. 1994. Failure to detect a Plasmodium vivax-like malaria parasite in globally collected blood samples. J. Infect. Dis. 170:1630–1633. 10.1093/infdis/170.6.1630 [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443–451. 10.1016/S1074-7613(00)80119-3 [DOI] [PubMed] [Google Scholar]
- 14.Leal MT, Ariza Camacho AG, Teixeira LH, Bargieri DY, Soares IS, Tararam CA, Rodrigues MM. 2013. Immunogenicity of recombinant proteins consisting of Plasmodium vivax circumsporozoite protein allelic variant-derived epitopes fused with Salmonella Typhimurium flagellin. Clin. Vaccine Immunol. 20:1418–1425. 10.1128/CVI.00312-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bargieri DY, Rosa DS, Braga CJ, Carvalho BO, Costa FT, Espíndola NM, Vaz AJ, Soares IS, Ferreira LC, Rodrigues MM. 2008. New malaria vaccine candidates based on the Plasmodium vivax merozoite surface protein-1 and the TLR-5 agonist Salmonella Typhimurium FliC flagellin. Vaccine 26:6132–6142. 10.1016/j.vaccine.2008.08.070 [DOI] [PubMed] [Google Scholar]
- 16.Zhou D, Zhou X, Bian A, Li H, Chen H, Small JC, Li Y, Giles-Davis W, Xiang Z, Ertl HC. 2010. An efficient method of directly cloning chimpanzee adenovirus as a vaccine vector. Nat. Protoc. 5:1775–1785. 10.1038/nprot.2010.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta RK, Siber GR. 1995. Method for quantitation of IgG subclass antibodies in mouse serum by enzyme-linked immunosorbent assay. J. Immunol. Methods 181:75–81. 10.1016/0022-1759(94)00331-P [DOI] [PubMed] [Google Scholar]
- 18.Nardin EH, Nussenzweig V, Nussenzweig RS, Collins WE, Harinasuta KT, Tapchaisri P, Chomcharn Y. 1982. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J. Exp. Med. 156:20–30. 10.1084/jem.156.1.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Alencar BC, Persechini PM, Haolla FA, de Oliveira G, Silvério JC, Lannes-Vieira J, Machado AV, Gazzinelli RT, Bruna-Romero O, Rodrigues MM. 2009. Perforin and gamma interferon expression are required for CD4+ and CD8+ T-cell-dependent protective immunity against a human parasite, Trypanosoma cruzi, elicited by heterologous plasmid DNA prime-recombinant adenovirus 5 boost vaccination. Infect. Immun. 77:4383–4395. 10.1128/IAI.01459-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasconcelos JR, Bruña-Romero O, Araújo AF, Dominguez MR, Ersching J, de Alencar BC, Machado AV, Gazzinelli RT, Bortoluci KR, Amarante-Mendes GP, Lopes MF, Rodrigues MM. 2012. Pathogen-induced proapoptotic phenotype and high CD95 (Fas) expression accompany a suboptimal CD8(+) T-cell response: reversal by adenoviral vaccine. PLoS Pathog. 8:e1002699. 10.1371/journal.ppat.1002699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chattopadhyay R, Velmurugan S, Chakiath C, Andrews Donkor L, Milhous W, Barnwell JW, Collins WE, Hoffman SL. 2010. Establishment of an in vitro assay for assessing the effects of drugs on the liver stages of Plasmodium vivax malaria. PLoS One 5:e14275. 10.1371/journal.pone.0014275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solarte Y, Manzano MR, Rocha L, Hurtado H, James MA, Arévalo-Herrera M, Herrera S. 2011. Plasmodium vivax sporozoite production in Anopheles albimanus mosquitoes for vaccine clinical trials. Am. J. Trop. Med. Hyg. 84(2 Suppl):28–34. 10.4269/ajtmh.2011.09-0499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caskey M, Lefebvre F, Filali-Mouhim A, Cameron MJ, Goulet JP, Haddad EK, Breton G, Trumpfheller C, Pollak S, Shimeliovich I, Duque-Alarcon A, Pan L, Nelkenbaum A, Salazar AM, Schlesinger SJ, Steinman RM, Sékaly RP. 2011. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J. Exp. Med. 208:2357–2366. 10.1084/jem.20111171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clemente T, Dominguez MR, Vieira NJ, Rodrigues MM, Amarante-Mendes GP. 2013. In vivo assessment of specific cytotoxic T lymphocyte killing. Methods 61:105–109. 10.1016/j.ymeth.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 25.Olotu A, Lusingu J, Leach A, Lievens M, Vekemans J, Msham S, Lang T, Gould J, Dubois MC, Jongert E, Vansadia P, Carter T, Njuguna P, Awuondo KO, Malabeja A, Abdul O, Gesase S, Mturi N, Drakeley CJ, Savarese B, Villafana T, Lapierre D, Ballou WR, Cohen J, Lemnge MM, Peshu N, Marsh K, Riley EM, von Seidlein L, Bejon P. 2011. Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5-17 months in Kenya and Tanzania: a randomised controlled trial. Lancet Infect. Dis. 11:102–109. 10.1016/S1473-3099(10)70262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kebaier C, Voza T, Vanderberg J. 2009. Kinetics of mosquito-injected Plasmodium sporozoites in mice: fewer sporozoites are injected into sporozoite-immunized mice. PLoS Pathog. 5:e1000399. 10.1371/journal.ppat.1000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacroix C, Ménard R. 2008. TRAP-like protein of Plasmodium sporozoites: linking gliding motility to host-cell traversal. Trends Parasitol. 24:431–434. 10.1016/j.pt.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 28.Coppi A, Natarajan R, Pradel G, Bennett BL, James ER, Roggero MA, Corradin G, Persson C, Tewari R, Sinnis P. 2011. The malaria circumsporozoite protein has two functional domains, each with distinct roles as sporozoites journey from mosquito to mammalian host. J. Exp. Med. 208:341–356. 10.1084/jem.20101488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montagna GN, Matuschewski K, Buscaglia CA. 2012. Plasmodium sporozoite motility: an update. Front. Biosci. 17:726–744. 10.2741/3954 [DOI] [PubMed] [Google Scholar]
- 30.Yoshida N, Nussenzweig RS, Potocnjak P, Nussenzweig V, Aikawa M. 1980. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science 207:71–73. 10.1126/science.6985745 [DOI] [PubMed] [Google Scholar]
- 31.Espinosa DA, Yadava A, Angov E, Maurizio PL, Ockenhouse CF, Zavala F. 2013. Development of a chimeric Plasmodium berghei strain expressing the repeat region of the P. vivax circumsporozoite protein for in vivo evaluation of vaccine efficacy. Infect. Immun. 81:2882–2887. 10.1128/IAI.00461-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arévalo-Herrera M, Soto L, Perlaza BL, Céspedes N, Vera O, Lenis AM, Bonelo A, Corradin G, Herrera S. 2011. Antibody-mediated and cellular immune responses induced in naive volunteers by vaccination with long synthetic peptides derived from the Plasmodium vivax circumsporozoite protein. Am. J. Trop. Med. Hyg. 84(2 Suppl):35–42. 10.4269/ajtmh.2011.09-0507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nardin EH, Nussenzweig RS. 1993. T cell responses to pre-erythrocytic stages of malaria: role in protection and vaccine development against pre-erythrocytic stages. Annu. Rev. Immunol. 11:687–727. 10.1146/annurev.iy.11.040193.003351 [DOI] [PubMed] [Google Scholar]
- 34.Schwenk RJ, Richie TL. 2011. Protective immunity to pre-erythrocytic stage malaria. Trends Parasitol. 27:306–314. 10.1016/j.pt.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 35.Douradinha B, Doolan DL. 2011. Harnessing immune responses against Plasmodium for rational vaccine design. Trends Parasitol. 27:274–283. 10.1016/j.pt.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 36.Sinnis P, Zavala F. 2012. The skin: where malaria infection and the host immune response begin. Semin. Immunopathol. 34:787–792. 10.1007/s00281-012-0345-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaughan AM, Kappe SH. 2012. Malaria vaccine development: persistent challenges. Curr. Opin. Immunol. 24:324–331. 10.1016/j.coi.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 38.Soares IS, Françoso KS, Jampaulo VO, Rodrigues MM. 2012. CD8(+) T-cell-mediated immunity against malaria: a novel heterologous prime-boost strategy. Expert Rev. Vaccines 11:1039–1041. 10.1586/erv.12.82 [DOI] [PubMed] [Google Scholar]
- 39.Dunachie SJ, Walther M, Epstein JE, Keating S, Berthoud T, Andrews L, Andersen RF, Bejon P, Goonetilleke N, Poulton I, Webster DP, Butcher G, Watkins K, Sinden RE, Levine GL, Richie TL, Schneider J, Kaslow D, Gilbert SC, Carucci DJ, Hill AV. 2006. A DNA prime-modified vaccinia virus Ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect. Immun. 74:5933–5942. 10.1128/IAI.00590-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, Gunasekera A, Chakravarty S, James ER, Sedegah M, Richman A, Velmurugan S, Reyes S, Li M, Tucker K, Ahumada A, Ruben AJ, Li T, Stafford R, Eappen AG, Tamminga C, Bennett JW, Ockenhouse CF, Murphy JR, Komisar J, Thomas N, Loyevsky M, Birkett A, Plowe CV, Loucq C, Edelman R, Richie TL, Seder RA, Hoffman SL. 2011. Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science 334:475–480. 10.1126/science.1211548 [DOI] [PubMed] [Google Scholar]
- 41.Sedegah M, Tamminga C, McGrath S, House B, Ganeshan H, Lejano J, Abot E, Banania GJ, Sayo R, Farooq F, Belmonte M, Manohar N, Richie NO, Wood C, Long CA, Regis D, Williams FT, Shi M, Chuang I, Spring M, Epstein JE, Mendoza-Silveiras J, Limbach K, Patterson NB, Bruder JT, Doolan DL, King CR, Soisson L, Diggs C, Carucci D, Dutta S, Hollingdale MR, Ockenhouse CF, Richie TL. 2011. Adenovirus 5-vectored P. falciparum vaccine expressing CSP and AMA1. Part A: safety and immunogenicity in seronegative adults. PLoS One 6:e24586. 10.1371/journal.pone.0024586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamminga C, Sedegah M, Regis D, Chuang I, Epstein JE, Spring M, Mendoza-Silveiras J, McGrath S, Maiolatesi S, Reyes S, Steinbeiss V, Fedders C, Smith K, House B, Ganeshan H, Lejano J, Abot E, Banania GJ, Sayo R, Farooq F, Belmonte M, Murphy J, Komisar J, Williams J, Shi M, Brambilla D, Manohar N, Richie NO, Wood C, Limbach K, Patterson NB, Bruder JT, Doolan DL, King CR, Diggs C, Soisson L, Carucci D, Levine G, Dutta S, Hollingdale MR, Ockenhouse CF, Richie TL. 2011. Adenovirus-5-vectored P. falciparum vaccine expressing CSP and AMA1. Part B: safety, immunogenicity and protective efficacy of the CSP component. PLoS One 6:e25868. 10.1371/journal.pone.0025868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richie TL, Charoenvit Y, Wang R, Epstein JE, Hedstrom RC, Kumar S, Luke TC, Freilich DA, Aguiar JC, Sacci JB, Jr, Sedegah M, Nosek RA, Jr, De La Vega P, Berzins MP, Majam VF, Abot EN, Ganeshan H, Richie NO, Banania JG, Baraceros MF, Geter TG, Mere R, Bebris L, Limbach K, Hickey BW, Lanar DE, Ng J, Shi M, Hobart PM, Norman JA, Soisson LA, Hollingdale MR, Rogers WO, Doolan DL, Hoffman SL. 2012. Clinical trial in healthy malaria-naïve adults to evaluate the safety, tolerability, immunogenicity and efficacy of MuStDO5, a five-gene, sporozoite/hepatic stage Plasmodium falciparum DNA vaccine combined with escalating dose human GM-CSF DNA. Hum. Vaccin. Immunother. 8:1564–1584. 10.4161/hv.22129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chuang I, Sedegah M, Cicatelli S, Spring M, Polhemus M, Tamminga C, Patterson N, Guerrero M, Bennett JW, McGrath S, Ganeshan H, Belmonte M, Farooq F, Abot E, Banania JG, Huang J, Newcomer R, Rein L, Litilit D, Richie NO, Wood C, Murphy J, Sauerwein R, Hermsen CC, McCoy AJ, Kamau E, Cummings J, Komisar J, Sutamihardja A, Shi M, Epstein JE, Maiolatesi S, Tosh D, Limbach K, Angov E, Bergmann-Leitner E, Bruder JT, Doolan DL, King CR, Carucci D, Dutta S, Soisson L, Diggs C, Hollingdale MR, Ockenhouse CF, Richie TL. 2013. DNA prime/adenovirus boost malaria vaccine encoding P. falciparum CSP and AMA1 induces sterile protection associated with cell-mediated immunity. PLoS One 8:e55571. 10.1371/journal.pone.0055571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero P, Maryanski JL, Corradin G, Nussenzweig RS, Nussenzweig V, Zavala F. 1989. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature 341:323–326. 10.1038/341323a0 [DOI] [PubMed] [Google Scholar]
- 46.Rodrigues MM, Cordey AS, Arreaza G, Corradin G, Romero P, Maryanski JL, Nussenzweig RS, Zavala F. 1991. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int. Immunol. 3:579–585. 10.1093/intimm/3.6.579 [DOI] [PubMed] [Google Scholar]
- 47.Rodrigues M, Nussenzweig RS, Romero P, Zavala F. 1992. The in vivo cytotoxic activity of CD8+ T cell clones correlates with their levels of expression of adhesion molecules. J. Exp. Med. 175:895–905. 10.1084/jem.175.4.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, Lauer P, Reyes-Sandoval A, Hutchings CL, Moore AC, Gilbert SC, Hill AV, Bartholomay LC, Harty JT. 2008. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc. Natl. Acad. Sci. U. S. A. 105:14017–14022. 10.1073/pnas.0805452105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yadava A, Sattabongkot J, Washington MA, Ware LA, Majam V, Zheng H, Kumar S, Ockenhouse CF. 2007. A novel chimeric Plasmodium vivax circumsporozoite protein induces biologically functional antibodies that recognize both VK210 and VK247 sporozoites. Infect. Immun. 75:1177–1185. 10.1128/IAI.01667-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lumsden JM, Pichyangkul S, Srichairatanakul U, Yongvanitchit K, Limsalakpetch A, Nurmukhambetova S, Klein J, Bertholet S, Vedvick TS, Reed SG, Sattabongkot J, Bennett JW, Polhemus ME, Ockenhouse CF, Howard RF, Yadava A. 2011. Evaluation of the safety and immunogenicity in rhesus monkeys of a recombinant malaria vaccine for Plasmodium vivax with a synthetic Toll-like receptor 4 agonist formulated in an emulsion. Infect. Immun. 79:3492–3500. 10.1128/IAI.05257-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. 2012. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc. Natl. Acad. Sci. U. S. A. 109:1080–1085. 10.1073/pnas.1112648109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corradin G, Céspedes N, Verdini A, Kajava AV, Arévalo-Herrera M, Herrera S. 2012. Malaria vaccine development using synthetic peptides as a technical platform. Adv. Immunol. 114:107–149. 10.1016/B978-0-12-396548-6.00005-6 [DOI] [PubMed] [Google Scholar]
- 53.Gordon DM, Cosgriff TM, Schneider I, Wasserman GF, Majarian WR, Hollingdale MR, Chulay JD. 1990. Safety and immunogenicity of a Plasmodium vivax sporozoite vaccine. Am. J. Trop. Med. Hyg. 42:527–531 [DOI] [PubMed] [Google Scholar]
- 54.Herrington DA, Nardin EH, Losonsky G, Bathurst IC, Barr PJ, Hollingdale MR, Edelman R, Levine MM. 1991. Safety and immunogenicity of a recombinant sporozoite malaria vaccine against Plasmodium vivax. Am. J. Trop. Med. Hyg. 45:695–701 [DOI] [PubMed] [Google Scholar]
- 55.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, Robertson MN, Mehrotra DV, Self SG, Corey L, Shiver JW, Casimiro DR, Step Study Protocol Team 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372:1894–1905. 10.1016/S0140-6736(08)61592-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Rosa SC, Thomas EP, Bui J, Huang Y, deCamp A, Morgan C, Kalams SA, Tomaras GD, Akondy R, Ahmed R, Lau CY, Graham BS, Nabel GJ, McElrath MJ, National Institute of Allergy and Infectious Diseases HIV Vaccine Trials Network 2011. HIV-DNA priming alters T cell responses to HIV-adenovirus vaccine even when responses to DNA are undetectable. J. Immunol. 187:3391–3401. 10.4049/jimmunol.1101421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frahm N, DeCamp AC, Friedrich DP, Carter DK, Defawe OD, Kublin JG, Casimiro DR, Duerr A, Robertson MN, Buchbinder SP, Huang Y, Spies GA, De Rosa SC, McElrath MJ. 2012. Human adenovirus-specific T cells modulate HIV-specific T cell responses to an Ad5-vectored HIV-1 vaccine. J. Clin. Invest. 122:359–367. 10.1172/JCI60202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casazza JP, Bowman KA, Adzaku S, Smith EC, Enama ME, Bailer RT, Price DA, Gostick E, Gordon IJ, Ambrozak DR, Nason MC, Roederer M, Andrews CA, Maldarelli FM, Wiegand A, Kearney MF, Persaud D, Ziemniak C, Gottardo R, Ledgerwood JE, Graham BS, Koup RA, VRC 101 Study Team 2013. Therapeutic vaccination expands and improves the function of the HIV-specific memory T-cell repertoire. J. Infect. Dis. 207:1829–1840. 10.1093/infdis/jit098 [DOI] [PMC free article] [PubMed] [Google Scholar]