Abstract
Streptococcus pneumoniae is the leading cause of respiratory infection worldwide. Although oral hygiene has been considered a risk factor for developing pneumonia, the relationship between oral bacteria and pneumococcal infection is unknown. In this study, we examined the synergic effects of Prevotella intermedia, a major periodontopathic bacterium, on pneumococcal pneumonia. The synergic effects of the supernatant of P. intermedia (PiSup) on pneumococcal pneumonia were investigated in mice, and the stimulation of pneumococcal adhesion to human alveolar (A549) cells by PiSup was assessed. The effects of PiSup on platelet-activating factor receptor (PAFR) transcript levels in vitro and in vivo were analyzed by quantitative real-time PCR, and the differences between the effects of pneumococcal infection induced by various periodontopathic bacterial species were verified in mice. Mice inoculated with S. pneumoniae plus PiSup exhibited a significantly lower survival rate, higher bacterial loads in the lungs, spleen, and blood, and higher inflammatory cytokine levels in the bronchoalveolar lavage fluid (macrophage inflammatory protein 2 and tumor necrosis factor alpha) than those infected without PiSup. In A549 cells, PiSup increased pneumococcal adhesion and PAFR transcript levels. PiSup also increased lung PAFR transcript levels in mice. Similar effects were not observed in the supernatants of Porphyromonas gingivalis or Fusobacterium nucleatum. Thus, P. intermedia has the potential to induce severe bacteremic pneumococcal pneumonia with enhanced pneumococcal adhesion to lower airway cells.
INTRODUCTION
Streptococcus pneumoniae is the leading cause of community-acquired respiratory infections worldwide (1). There are several known risk factors for pneumococcal disease but limited descriptive data concerning the relationship between oral hygiene and pneumococcal infection.
Poor oral hygiene has been suggested to be a risk factor for respiratory disease (2), and several studies indicate that oral care reduces the incidence and mortality of pneumonia in hospitals or nursing homes (3–5). Regarding the relationship between S. pneumoniae and oral hygiene, Okuda et al. reported that oral cleansing significantly reduced the detection rates of S. pneumoniae in patients who had undergone oral and maxillofacial surgeries (6).
Several oral anaerobes, mostly related to periodontitis, are known to interact in a synergistic or antagonistic manner (7, 8). To understand the interactions between microorganisms, the enhancement of reciprocal bacterial growth, adhesion/invasion into host cells, and effects on host immunity response have been examined (7–11). Regarding the synergic effects of anaerobes on pulmonary infection by Streptococcus species, Shinzato and Saito reported that Prevotella intermedia exhibits synergic effects on lower respiratory tract infections with Streptococcus constellatus in mice by enhancing reciprocal bacterial growth (9). However, whether oral bacteria exhibit synergic effects on pneumococcal infections remains unclear.
Here we hypothesized that an anaerobe that is ubiquitous in the oral cavity may have synergic effects on pneumococcal respiratory infection. To investigate our hypothesis, we focused on the anaerobe P. intermedia.
P. intermedia is a Gram-negative, black-pigmented obligate anaerobic rod which is often isolated from periodontal lesions associated with various forms of periodontal disease (12, 13). In addition, P. intermedia was recently detected in cystic fibrosis airway specimens (14–16). Ulrich et al. reported the pathogenic potential of P. intermedia in the respiratory tract and demonstrated that extracellular toxins of P. intermedia are cytotoxic for human alveolar type II cells and neutrophils (17).
In this study, we examined the effects of P. intermedia on pneumococcal pneumonia in a murine model. The aims of this study were to determine whether P. intermedia exhibits synergic effects on pneumococcal pneumonia and to examine its mechanism of interactions.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The Streptococcus pneumoniae strain NU83127 (MIC of penicillin G, 0.03 μg/ml; serotype 4), which was clinically isolated at Nagasaki University School of Medicine, was used in the present study. The obligate anaerobes examined are listed in Table 1. All obligate anaerobes were cultured on PV brucella HK agar (Kyokuto Pharmaceutical Industrial Co., Tokyo, Japan) for 48 to 96 h under anaerobic conditions and then scraped and suspended in modified GAM broth (Nissui Pharmaceutical Industrial Co., Tokyo, Japan). To prepare a bacterial suspension, P. intermedia was incubated with modified GAM broth in an anaerobic chamber until it reached its late logarithmic growth phase (24 h). Bacteria were then harvested by centrifugation (3,000 rpm, 10 min) and resuspended in normal saline.
TABLE 1.
Strains used in this study
| Microorganism | Strain | Sourcea |
|---|---|---|
| Prevotella intermedia | PINU499 | A |
| PINU046 | A | |
| ATCC 25611 | B | |
| Fusobacterium nucleatum | FNU191 | A |
| GAI 03017 | C | |
| ATCC 10953 | B | |
| Porphyromonas gingivalis | W83 | B |
| TBC60 | B | |
| ATCC 33277 | B |
A, Department of Laboratory Medicine, Nagasaki University Hospital, Nagasaki, Japan; B, Division of Microbiology and Oral Infection, Department of Molecular Microbiology and Immunology, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan; C, Division of Anaerobe Research, Life Science Research Center, Gifu University, Gifu City, Japan.
The supernatants of P. intermedia and the other anaerobes were obtained as previously reported (18, 19). Briefly, the anaerobes were incubated using modified GAM broth for 48 h in an anaerobic chamber. The supernatants were then collected by centrifugation at 10,000 rpm at 4°C for 50 min to remove the bacteria and were filter sterilized through a 0.22-μm-pore-size membrane filter (Millipore, Bedford, MA).
We conducted all experiments using the PINU499 strain, with the exception of the experiments performed to verify the differences between the effects of periodontopathic bacterial species and strains on pneumococcal infection. We also identified clinical strains at our institution by PCR amplification and 16S rRNA gene sequence analysis.
Mice.
Eight-week-old male BALB/c specific-pathogen-free mice were obtained from SLC Japan Inc., Shizuoka, Japan. All mouse experiments were performed according to the guidelines of the Laboratory Animal Center for Biomedical Research, Nagasaki University School of Medicine. The experimental protocol was approved by the Animal Care Ethics Review Committee at our institution.
Intratracheal infection procedure.
The S. pneumoniae strain was cultured on blood agar plates (Becton, Dickinson Co., Ltd., Japan) for 24 h at 37°C, scraped and suspended in brain heart infusion broth mixed with horse serum, and cultured with shaking at 37°C at 250 rpm for 4 h. Bacteria were then harvested by centrifugation (3,000 rpm, 10 min). The organism was resuspended in normal saline for a final concentration of approximately 108 CFU/ml, as determined by the optical density method. Mice were anesthetized with pentobarbital, and the trachea was inoculated with 0.05 ml of the bacterial suspension via insertion with a 24-gauge catheter. For mixed-infection experiments with S. pneumoniae and P. intermedia, the bacterial suspension of S. pneumoniae was mixed with the same amount of bacterial suspension of P. intermedia or modified GAM broth before inoculating mice. The final bacterial load of S. pneumoniae was approximately 2 × 106 to 2 × 107 CFU/ml (1 × 105 to 1 × 106 CFU/mouse), and the final bacterial load of P. intermedia was approximately 2 × 108 to 2 × 109 CFU/ml (1 × 107 to 1 × 108 CFU/mouse).
In experiments that examined the effects of culture supernatants of P. intermedia and the other periodontopathic bacteria on pneumococcal pneumonia, a bacterial suspension of S. pneumoniae was mixed with the same amount of culture supernatant of anaerobes or modified GAM broth before inoculating mice. The final bacterial load of S. pneumoniae was approximately 5 × 107 CFU/ml (2.5 × 106 CFU/mouse). The control group was inoculated with equal volumes of broth and normal saline. For the group inoculated with the supernatant of P. intermedia (PiSup) without S. pneumoniae, equal volumes of PiSup and normal saline were used. The pH of modified GAM broth was adjusted to that of the anaerobe's supernatant (pH 5.6 for PiSup and pH 6.8 for the supernatant of Fusobacterium nucleatum or Porphyromonas gingivalis).
Bacteriological and histopathological examinations.
Each group of animals was sacrificed at specific time intervals by cervical dislocation. After exsanguination, the lungs and spleen were dissected and removed under aseptic conditions. Blood was collected by right ventricular puncturing using heparin-coated syringes. For bacteriological analyses, the organs were suspended in normal saline (1 ml) and homogenized with a Polytron homogenizer (AS One Co., Osaka, Japan). Each specimen (blood, lung, and spleen) was quantitatively inoculated onto blood agar plates by serial dilution, followed by incubation at 37°C for 24 h. The lowest level of detectable CFU/ml was 50 CFU/ml (1.7 log CFU/ml). The lung tissue used for histological examination was fixed in 10% buffered formalin and stained with hematoxylin-eosin.
BAL and cytokine ELISA.
Bronchoalveolar lavage (BAL) was performed as previously described (20). The recovered fluid fractions were pooled for each animal, and the total cell counts were calculated using Turk staining. For differential cell counts, cells were centrifuged at 850 rpm for 2 min onto slides that were then stained with Diff-Quick stain. Differential cell counts were performed by counting 100 cells. Various concentrations of macrophage inflammatory protein 2 (MIP-2) and tumor necrosis factor alpha (TNF-α) in BAL fluid (BALF) were assayed using mouse cytokine enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Cell culture.
The NCI-A549 cell line (human type II pneumocyte cell line) was cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. The cells were grown at 37°C with 5% CO2 in fully humidified air. Cells were exposed to PiSup for pneumococcal adhesion studies. For controls, cells were incubated with modified GAM broth, and the pH was adjusted to that of PiSup.
Pneumococcal adhesion to airway cells exposed to PiSup in vitro.
The adhesion of pneumococci to airway cells in vitro was performed as previously described (21). Briefly, A549 cells were seeded in 24-well plates. PiSup was added to cell monolayers, incubated at 37°C for 4 h, and subsequently removed by washing twice with RPMI medium. Pneumococci were then added and incubated for 2 h. Cell monolayers were washed five times, and cells were removed from the tissue culture plate with trypsin-EDTA and lysed with ice-cold sterile distilled water for 10 min. The lysates were then plated to determine the CFU/ml.
The functional relevance of platelet-activating factor receptor (PAFR) was also assessed by coincubating cells with the competitive PAFR antagonist CV-3988 (Sigma-Aldrich). A stock solution of CV-3988 was prepared in ethanol and then diluted in medium to a final concentration of 10 μM. The adhesion data are representative of at least three separate experiments performed on different days.
PAFR transcript levels in airway cells exposed to PiSup in vitro.
Transcript levels of PAFR were assessed in A549 cells by using quantitative real-time PCR. The total RNA was extracted from A549 cells cultured in 6-well plates by use of QuickGene-Mini80 and QuickGene RNA cultured cell kits (Fujifilm Co., Tokyo, Japan) according to the manufacturer's instructions. The total RNA (1 μg) was reverse transcribed into cDNA by using oligo(dT) primers and SuperScript III reverse transcriptase (Invitrogen) and then treated with RNase H. To quantify the expression of the PAFR gene, PCR primers and TaqMan probes were used as previously reported (Hs00265399_S1) (21). To normalize PAFR expression, the housekeeping gene hypoxanthine phosphoribosyltransferase 1 (HPRT1) was also measured using the primer set Hs01003267_m1 according to the manufacturer's instructions (Life Technologies). The data are presented as ratios relative to HPRT1.
Lung PAFR transcript levels in mice exposed to PiSup in vivo.
Lung PAFR transcript levels were examined in PiSup-inoculated mice and S. pneumoniae-infected mice with and without PiSup. Each group of animals was sacrificed at specific time intervals, and a partial lung was preserved in RNA Later (Life Technologies). The tissue samples were homogenized, and RNA was extracted using an RNeasy minikit (Qiagen) according to the manufacturer's instructions. First-strand cDNA synthesis was performed as described above. mRNA transcript levels of PAFR and the housekeeping gene HPRT1 were determined by quantitative real-time PCR using the TaqMan primer and probe sets Mm02621061_m1 and Mm00446968_m1, respectively. Mouse PAFR mRNA transcript levels were normalized to the housekeeping gene HPRT1 (22).
Statistical analysis.
All data were expressed as means and standard errors of the means (SEM). Differences between groups were evaluated using the Mann-Whitney U test. Survival analysis was performed using the log rank test, and the survival rates were calculated by the Kaplan-Meier method. P values of <0.05 were considered to be statistically significant.
RESULTS
Mixed infection of S. pneumoniae and P. intermedia.
There were no significant differences observed between the survival rates of mixed-infection experiments of S. pneumoniae with and without the bacterial suspension of P. intermedia (data not shown). In preliminary experiments in which BALB/c mice were inoculated with only P. intermedia via the trachea, changes in inflammation and the proliferation of P. intermedia in the lungs were not observed. Based on these results, the synergic effects of P. intermedia on pneumococcal pneumonia were difficult to assess in the mixed-infection experiments, because the virulence of only P. intermedia was less significant. Therefore, we did not conduct additional experiments using bacterial suspensions of P. intermedia.
Pneumococcal infection with P. intermedia supernatant caused severe bacteremic pneumonia.
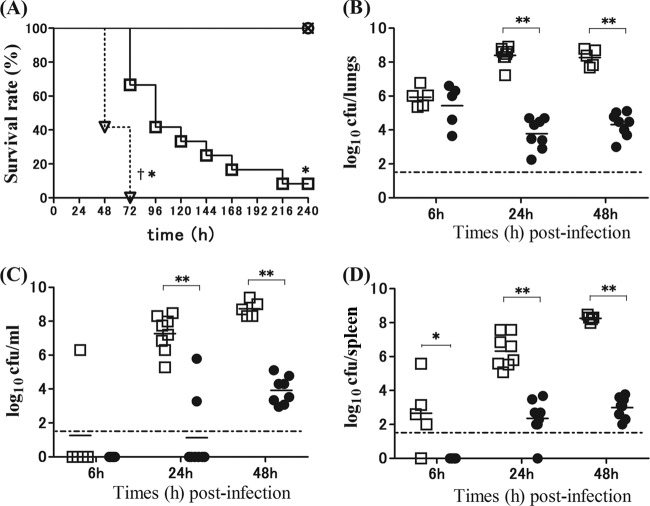
Figure 1A illustrates the survival rates of S. pneumoniae-infected mice with and without PiSup. In the controls (broth- or PiSup-inoculated mice), no deaths were observed during the 10-day observation period. In contrast, 90% of S. pneumoniae-infected mice without PiSup died 3 days after inoculation, and all S. pneumoniae-infected mice with PiSup died within 3 days. The survival rates of S. pneumoniae-infected mice with PiSup were significantly lower than those of S. pneumoniae-infected mice without PiSup (P < 0.01). The changes in the numbers of viable S. pneumoniae cells in the lungs, blood, and spleen over time following infection are shown in Fig. 1B to D. The mean bacterial count in each organ/blood of S. pneumoniae-infected mice with PiSup began to increase 24 h after inoculation (P < 0.005 for S. pneumoniae with PiSup versus S. pneumoniae without PiSup), with the exception of the spleen, in which the increase was observed starting as early as 6 h after inoculation (P < 0.05). Because these results indicate that PiSup induces early exacerbation of S. pneumoniae infection in mice within 6 to 48 h, we examined the pathological changes in the lungs 24 h after inoculation (Fig. 2). Pathological examination of the lungs of S. pneumoniae-infected mice with PiSup showed severe bronchopneumonia with massive hemorrhaging (Fig. 2d). PiSup-inoculated mice also exhibited mild hemorrhaging (Fig. 2b), whereas the lungs of S. pneumoniae-infected mice without PiSup exhibited only mild pneumonia 24 h after inoculation (Fig. 2c). Broth-inoculated (control) mice did not exhibit any inflammatory changes in the lungs.
FIG 1.
(A) Survival rates of mice infected by Streptococcus pneumoniae with or without supernatant of Prevotella intermedia (PiSup). Inocula for all groups contained equal amounts of modified GAM broth and normal saline. Each group was composed of 6 to 12 mice. ○, broth-inoculated mice; ×, PiSup-inoculated mice; □, S. pneumoniae-infected mice without PiSup; ▽, S. pneumoniae-infected mice with PiSup. The survival rates of both S. pneumoniae-infected groups were significantly lower than those of broth- and PiSup-inoculated groups (*, P < 0.05). The survival rates of S. pneumoniae-infected mice with PiSup were also significantly lower than those of S. pneumoniae-infected mice without PiSup (†, P < 0.01). Similar results were obtained in two independent experiments. (B to D) Bacterial load in the lungs (B), blood (C), and spleen (D) of S. pneumoniae-infected mice with and without PiSup were compared at different times (6 h, 24 h, and 48 h) after inoculation. Each point represents the value for a mouse (●, S. pneumoniae-infected mice without PiSup; □, S. pneumoniae-infected mice with PiSup). The mean bacterial count in each organ/blood of S. pneumoniae-infected mice with PiSup increased 24 h after inoculation (**, P < 0.005 [S. pneumoniae with PiSup versus S. pneumoniae without PiSup]), with the exception of the spleen, which showed an increase as early as 6 h after inoculation (*, P < 0.05 [S. pneumoniae with PiSup versus S. pneumoniae without PiSup]). The bars represent mean bacterial counts. The broken horizontal line represents the detection limit (1.7 log CFU/ml or organ). The data represent two independent experiments.
FIG 2.
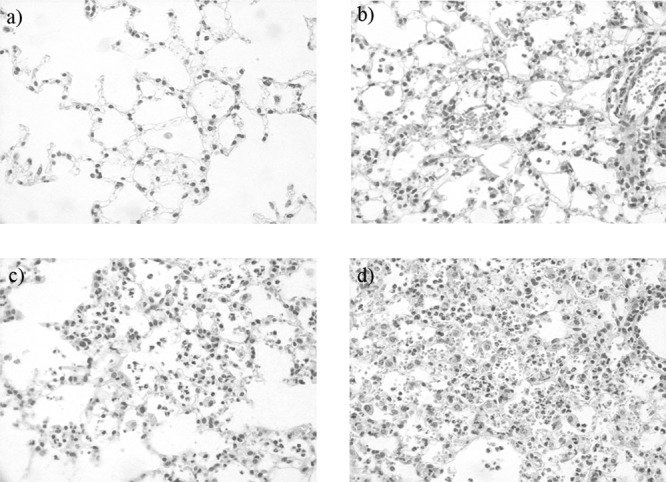
Pathological analysis of the lungs of Streptococcus pneumoniae-infected mice with or without supernatant of Prevotella intermedia (PiSup). Lungs were collected 24 h after inoculation. (a to d) Hematoxylin-eosin-stained tissue sections. Magnification, ×400. (a) Broth-inoculated (control) mice; (b) PiSup-inoculated mice; (c) S. pneumoniae-infected mice with broth; (d) S. pneumoniae-infected mice with PiSup.
In order to examine peak inflammatory changes in the lungs of S. pneumoniae-infected mice with PiSup, we performed BAL 36 h after inoculation. The total cell and neutrophil counts (Table 2) were significantly higher in S. pneumoniae-infected mice with PiSup and in PiSup-inoculated mice than in S. pneumoniae-infected mice without PiSup. To further examine the differences, inflammatory cytokine levels in BALF were analyzed. TNF-α and MIP-2 concentrations were significantly higher in S. pneumoniae-infected mice with PiSup than in any other group (Fig. 3). TNF-α levels also increased slightly in PiSup-inoculated groups and were still significantly higher than those of S. pneumoniae-infected mice without PiSup. To confirm the inflammatory effects of PiSup, we also performed BAL 12 h and 24 h after PiSup inoculation. BALF of PiSup-inoculated mice demonstrated that the total cell and neutrophil counts increased 12 h after inoculation, and the concentrations of MIP-2 and TNF-α also increased after inoculation. However, the peak concentrations of TNF-α and MIP-2 in PiSup-inoculated mice were 183.0 ± 30.3 ng/ml (12 h) and 58.4 ± 39.4 ng/ml (24 h), respectively (data not shown), which were lower than those of S. pneumoniae-infected mice with PiSup.
TABLE 2.
Inflammatory cells in BALF from mice infected with Streptococcus pneumoniae with or without supernatant of Prevotella intermedia 36 h after inoculation
| Cell type | Cell density (104 cells ml−1)a |
|||
|---|---|---|---|---|
| Control | S. pneumoniae | PiSup | S. pneumoniae plus PiSup | |
| Total cells | 7.2 ± 3.0 | 15.1 ± 4.2*† | 45.1 ± 2.0*# | 63.3 ± 16.9*# |
| Neutrophils | 0.82 ± 0.86 | 6.8 ± 3.0*† | 38.2 ± 19.8*# | 58.6 ± 16.0*# |
| Macrophages | 6.0 ± 3.0 | 8.0 ± 4.5 | 6.3 ± 3.0 | 4.2 ± 3.9 |
| Lymphocytes | 0.33 ± 0.29 | 0.35 ± 0.29 | 0.49 ± 0.56 | 0.45 ± 0.55 |
Data are presented as means ± SEM (n = 6 to 9). *, P < 0.05 versus control group mice; #, P < 0.05 versus S. pneumoniae-infected mice; †, P < 0.05 versus PiSup-inoculated mice and S. pneumoniae-plus-PiSup-inoculated mice.
FIG 3.
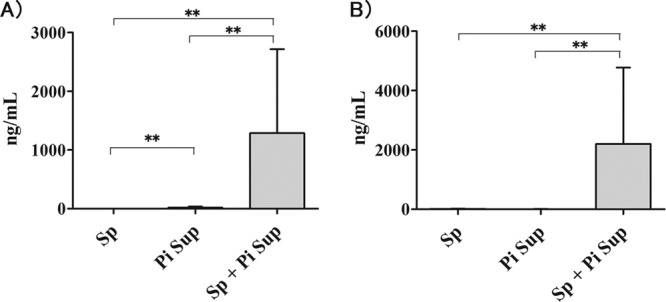
Changes in levels of the inflammatory cytokines TNF-α (A) and MIP-2 (B) (36 h after inoculation) in bronchoalveolar lavage fluid from Streptococcus pneumoniae (Sp)-infected mice with or without supernatant of Prevotella intermedia (PiSup) (n = 8 for each group) and from PiSup-inoculated mice (n = 7). All groups contained equal amounts of modified GAM broth and normal saline. TNF-α and MIP-2 levels were significantly higher in S. pneumoniae-infected mice with PiSup than in other groups. TNF-α levels also increased slightly in the PiSup-inoculated group. The data are expressed as means with SEM. Statistically significant differences are indicated as follows: **, P < 0.001.
Culture supernatant of P. intermedia stimulated PAFR in vitro and in vivo.
To further understand the effects of PiSup on pneumococcal pneumonia, we hypothesized that PiSup possesses a stimulatory effect on pneumococcal adhesion to lower airway cells, contributing to rapid bacterial proliferation and invasion. Regarding pneumococcal adhesion, there is increasing evidence that PAFR is a major epithelial receptor used by S. pneumoniae to invade airway epithelium cells (23). Upregulation of PAFR transcripts in vivo, as a result of interleukin 1 stimulation (24), influenza virus infection (25), and exposure to cigarette smoke (21), has been described for several animal models. However, the relationship between periodontopathic bacteria and PAFR transcript levels has not been described previously. Thus, we sought to examine the effects of PiSup on pneumococcal adhesion and PAFR expression.
PiSup increased pneumococcal adhesion to A549 cells (P < 0.05 versus control) (Fig. 4A). CV-3988 decreased pneumococcal adhesion stimulated by PiSup (P < 0.05 for PiSup plus antagonist versus PiSup plus ethanol) (Fig. 4B), and PAFR mRNA levels increased in PiSup-stimulated cells (P < 0.005 versus control) (Fig. 4C).
FIG 4.
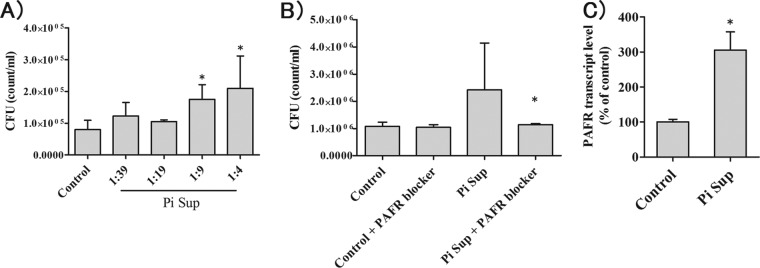
Pneumococcal adhesion to airway cells (A549 cells) exposed to the supernatant of Prevotella intermedia (PiSup) in vitro. (A) Incubation with 5- to 10-fold-diluted PiSup increased Streptococcus pneumoniae CFU, indicating increased adhesion (*, P < 0.05 versus modified GAM broth control). The data are representative of three separate experiments. (B) Coinfection with a PAFR blocker (10 μM CV-3988) reduced PiSup-stimulated adhesion (*, P < 0.05 versus infection without PAFR blocker). The data are representative of three separate experiments. (C) PiSup increased PAFR transcript levels (*, P < 0.01 versus the broth control). The data are representative of two experiments with six replicates. All data represent means and SEM.
In mice, PiSup increased lung PAFR transcript levels 6 to 24 h after inoculation (Fig. 5A). To examine the differences between the PAFR transcript levels of S. pneumoniae-infected mice with and without PiSup, we collected the lungs of mice 24 h after inoculation. The highest increase in PAFR transcript levels was observed in the lungs of S. pneumoniae-infected mice with PiSup (P < 0.005 versus S. pneumoniae without PiSup; P < 0.05 versus PiSup group). The PiSup-inoculated group exhibited higher PAFR transcript levels than S. pneumoniae-infected mice without PiSup (P < 0.005).
FIG 5.
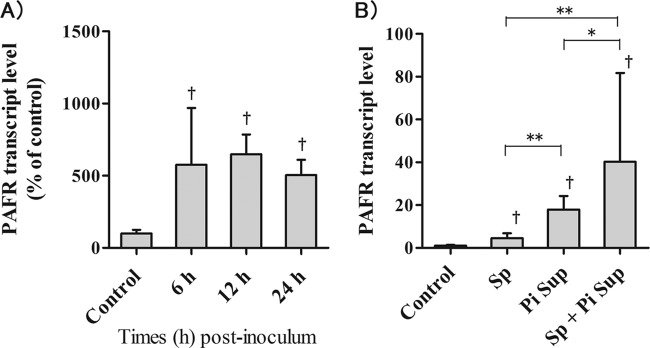
(A) Pulmonary PAFR transcript levels in mice inoculated with the supernatant of Prevotella intermedia (PiSup) were examined over time. PAFR expression significantly increased 6 h after inoculation with PiSup for up to 24 h compared to that of control mice (†, P < 0.05 versus control). (B) PAFR transcript levels in the lungs of Streptococcus pneumoniae (Sp)-infected mice with or without PiSup. Statistically significant differences are indicated as follows: *, P < 0.05; **, P < 0.001. All groups were inoculated with equal amounts of modified GAM broth and normal saline. Each group was composed of 6 mice. The data represent means and SEM.
In vivo effects of culture supernatant of periodontal bacteria on pneumococcal pneumonia.
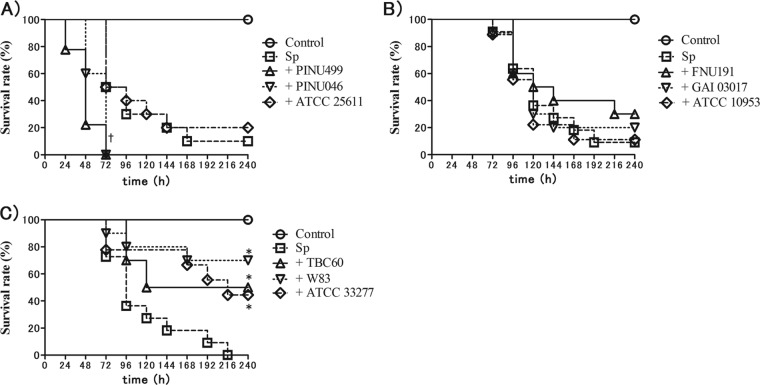
To estimate the effects of periodontopathic bacteria on pneumococcal infection, we examined the survival rates of S. pneumoniae-infected mice inoculated with the supernatants of Prevotella intermedia (Fig. 6A), Fusobacterium nucleatum (Fig. 6B), and Porphyromonas gingivalis (Fig. 6C). Each group was composed of three different strains, including a reference strain. The survival rates of S. pneumoniae-infected mice with the supernatant of PINU499 were significantly lower than that of S. pneumoniae-infected mice without PiSup (P < 0.01). The survival rates of S. pneumoniae-infected mice with P. gingivalis supernatant (PgSup) were significantly higher than those of S. pneumoniae-infected mice without PgSup (P < 0.05), whereas there was no significant difference between the survival rates of S. pneumoniae-infected mice with and without F. nucleatum supernatant (FnSup).
FIG 6.
Survival rates of mice infected by Streptococcus pneumoniae (Sp) with supernatant (Sup) of Prevotella intermedia (A), Fusobacterium nucleatum (B), and Porphyromonas gingivalis (C). All groups contained equal amounts of modified GAM broth and normal saline. The survival rates of S. pneumoniae-infected mice with PINU499 Sup were significantly lower than those of S. pneumoniae-infected mice without PiSup (†, P < 0.01). The survival rates of S. pneumoniae-infected mice with PgSup were significantly higher than those of S. pneumoniae-infected mice without PgSup (*, P < 0.05), whereas there was no significant difference between S. pneumoniae-infected mice with and without FnSup.
DISCUSSION
The present study is the first to demonstrate that the products of P. intermedia induce severe bacteremic pneumococcal pneumonia as well as the enhancement of pneumococcal adhesion to lower airway cells. Several lines of evidence support this notion.
First, S. pneumoniae-infected mice with PiSup exhibited significantly lower survival rates, with earlier increases in S. pneumoniae bacterial loads in the lungs, spleen, and blood, than those of S. pneumoniae-infected mice without PiSup. Significant increases in inflammatory cytokines were observed in the early phases of S. pneumoniae-infected mice with PiSup, indicating the severity of bacteremia compared to that of S. pneumoniae-infected mice without PiSup. Although belated bacteremia was observed in S. pneumoniae-infected mice without PiSup, a high bacterial load in the lungs was observed only in S. pneumoniae-infected mice with PiSup. These data suggest that PiSup enhances S. pneumoniae invasion into blood circulation, as well as S. pneumoniae adhesion and proliferation in the lungs.
Second, PiSup enhanced pneumococcal adhesion to lower airway cells in vitro. We also observed the upregulation of PAFR expression in airway cells upon PiSup stimulation and attenuation of pneumococcal adhesion by CV-3988, suggesting that PiSup enhances pneumococcal adhesion via PAFR upregulation.
Third, we also observed PAFR upregulation by PiSup in vivo. Higher levels of PAFR upregulation were observed in S. pneumoniae-infected mice with PiSup than in PiSup-inoculated mice, suggesting that PiSup may possess synergic effects on PAFR upregulation with pneumococcal infection. PAFR is a major epithelial receptor that binds to phosphorylcholine in the bacterial cell wall. Thus, the effects of PiSup on PAFR expression could be synergic not only for S. pneumoniae infection but also for other bacteria containing phosphorylcholine, including Pseudomonas aeruginosa (26) and Acinetobacter baumannii (27).
Because P. intermedia itself does not exhibit significant inflammatory or synergic effects on pneumococcal pneumonia in mice, we consider the instability of P. intermedia in lungs. Because of the aerobic environment in the lungs, P. intermedia may not be stable in the lungs, preventing proliferation and the secretion of virulent products.
The main goal of our study was to determine the extent by which PAFR expression affects the susceptibility of S. pneumoniae in mice administered PiSup, and the data obtained were inconclusive. We treated S. pneumoniae-infected mice with PiSup with CV-3988 (a PAFR antagonist) but could not determine any significant improvement in survival or attenuation of pneumococcal bacterial load in the lungs or blood (data not shown). However, PiSup-induced PAFR upregulation in our murine model was consistent for at least 24 h after inoculation. As were able to administer CV-3988 only once, at the initiation of inoculation, we could not thoroughly determine that treatment failure by CV-3988 was due to insufficient drug administration. To investigate the role of PAFR expression induced by PiSup in S. pneumoniae-infected mice, additional experiments that focus on specific P. intermedia products and use PAFR knockout mice will be necessary.
In this study, we also examined the effects of other periodontopathic bacteria on our murine model. P. gingivalis is a major pathogen of chronic periodontitis (28), and F. nucleatum is a pathogen frequently detected in the lesions of gingivitis, chronic periodontitis, and lower respiratory tract specimens (29, 30).
One possible mechanism that could increase the presence of periodontopathic bacteria in the pathogenesis of respiratory infection is saliva aspiration, as saliva contains periodontal disease-associated enzymes, cytokines, or other biologically active molecules (31, 32). Marik and Kaplan reported that approximately half of all healthy adults aspirate small amounts of oropharyngeal secretions while sleeping (33). On the basis of these reports, periodontopathic bacteria may have pathogenic effects on the respiratory tract via saliva aspiration. The results of our study indicate that the presence of P. intermedia in the oral cavity or lower respiratory tract may be a risk factor for severe pneumococcal pneumonia. In addition, our study suggests that differences in the pathogenicity of pneumococcal pneumonia may exist among periodontopathic bacterial species. Based on our data, there is a possibility that the constituents of periodontopathic species could play an important role in how periodontitis affects pneumococcal pneumonia.
Our results provide novel evidence that P. intermedia may contribute to the pathophysiology of pneumococcal pneumonia. Additional studies are required to elucidate a more detailed mechanism of interactions between P. intermedia and S. pneumoniae.
ACKNOWLEDGMENTS
We thank Mariko Naito and Koji Nakayama in the Division of Microbiology and Oral Infection, Department of Molecular Microbiology and Immunology, Nagasaki University Graduate School of Biomedical Sciences, Japan. We also thank Kaori Tanaka and Kunitomo Watanabe in the Division of Anaerobe Research, Life Science Research Center, Gifu University, Japan, for providing bacterial strains. We are grateful to Takeshi Yamanaka and Kazuyoshi Yamane from the Department of Bacteriology, Osaka Dental University, Japan, for their technical advice regarding Prevotella intermedia, and we also thank Atsushi Yokoyama for providing technical support.
This research was funded by the Department of Laboratory Medicine and Second Department of Internal Medicine, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan. This study was not sponsored by any grants, gifts, or fellowships.
Footnotes
Published ahead of print 18 November 2013
REFERENCES
- 1.van der Poll T, Opal SM. 2009. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 374:1543–1556. 10.1016/S0140-6736(09)61114-4 [DOI] [PubMed] [Google Scholar]
- 2.Sjögren P, Nilsson E, Forsell M, Johansson O, Hoogstraate J. 2008. A systematic review of the preventive effect of oral hygiene on pneumonia and respiratory tract infection in elderly people in hospitals and nursing homes: effect estimates and methodological quality of randomized controlled trials. J. Am. Geriatr. Soc. 56:2124–2130. 10.1111/j.1532-5415.2008.01926.x [DOI] [PubMed] [Google Scholar]
- 3.Yoneyama T, Yoshida M, Matsui T, Sasaki H. 1999. Oral care and pneumonia. Lancet 354:515. [DOI] [PubMed] [Google Scholar]
- 4.DeRiso AJ, 2nd, Ladowski JS, Dillon TA, Justice JW, Peterson AC. 1996. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest 109:1556–1561. 10.1378/chest.109.6.1556 [DOI] [PubMed] [Google Scholar]
- 5.Yoneyama T, Yoshida M, Ohrui T, Mukaiyama H, Okamoto H, Hoshiba K, Ihara S, Yanagisawa S, Ariumi S, Morita T, Mizuno Y, Ohsawa T, Akagawa Y, Hashimoto K, Sasaki H, Oral Care Working Group 2002. Oral care reduces pneumonia in older patients in nursing homes. J. Am. Geriatr. Soc. 50:430–433. 10.1046/j.1532-5415.2002.50106.x [DOI] [PubMed] [Google Scholar]
- 6.Okuda M, Kaneko Y, Ichinohe T, Ishihara K, Okuda K. 2003. Reduction of potential respiratory pathogens by oral hygienic treatment in patients undergoing endotracheal anesthesia. J. Anesth. 17:84–91. 10.1007/s005400300022 [DOI] [PubMed] [Google Scholar]
- 7.Feuille F, Ebersole JL, Kesavalu L, Stepfen MJ, Holt SC. 1996. Mixed infection with Porphyromonas gingivalis and Fusobacterium nucleatum in a murine lesion model: potential synergistic effects on virulence. Infect. Immun. 64:2094–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kesavalu L, Sathishkumar S, Bakthavatchalu V, Matthews C, Dawson D, Steffen M, Ebersole JL. 2007. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect. Immun. 75:1704–1712. 10.1128/IAI.00733-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinzato T, Saito A. 1994. A mechanism of pathogenicity of “Streptococcus milleri group” in pulmonary infection: synergy with an anaerobe. J. Med. Microbiol. 40:118–123. 10.1099/00222615-40-2-118 [DOI] [PubMed] [Google Scholar]
- 10.Saito A, Kokubu E, Inagaki S, Imamura K, Kita D, Lamont RJ, Ishihara K. 2012. Porphyromonas gingivalis entry into gingival epithelial cells modulated by Fusobacterium nucleatum is dependent on lipid rafts. Microb. Pathog. 53:234–242. 10.1016/j.micpath.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimizuka R, Kato T, Ishihara K, Okuda K. 2003. Mixed infections with Porphyromonas gingivalis and Treponema denticola cause excessive inflammatory responses in a mouse pneumonia model compared with monoinfections. Microbes Infect. 5:1357–1362. 10.1016/j.micinf.2003.09.015 [DOI] [PubMed] [Google Scholar]
- 12.Ximénez-Fyvie LA, Haffajee AD, Socransky SS. 2000. Microbial composition of supra- and subgingival plaque in subjects with adult periodontitis. J. Clin. Periodontol. 27:722–732. 10.1034/j.1600-051x.2000.027010722.x [DOI] [PubMed] [Google Scholar]
- 13.Gharbia SE, Haapasalo M, Shah HN, Kotiranta A, Lounatmaa K, Pearce MA, Devine DA. 1994. Characterization of Prevotella intermedia and Prevotella nigrescens isolates from periodontic and endodontic infections. J. Periodontol. 65:56–61. 10.1902/jop.1994.65.1.56 [DOI] [PubMed] [Google Scholar]
- 14.Tunney MM, Field TR, Moriarty TF, Patrick S, Doering G, Muhlebach MS, Wolfgang MC, Boucher R, Gilpin DF, McDowell A, Elborn JS. 2008. Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 177:995–1001. 10.1164/rccm.200708-1151OC [DOI] [PubMed] [Google Scholar]
- 15.Worlitzsch D, Rintelen C, Böhm K, Wollschläger B, Merkel N, Borneff-Lipp M, Döring G. 2009. Antibiotic-resistant obligate anaerobes during exacerbations of cystic fibrosis patients. Clin. Microbiol. Infect. 15:454–460. 10.1111/j.1469-0691.2008.02659.x [DOI] [PubMed] [Google Scholar]
- 16.Bittar F, Richet H, Dubus JC, Reynaud-Gaubert M, Stremler N, Sarles J, Raoult D, Rolain JM. 2008. Molecular detection of multiple emerging pathogens in sputa from cystic fibrosis patients. PLoS One 3:e2908. 10.1371/journal.pone.0002908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulrich M, Beer I, Braitmaier P, Dierkes M, Kummer F, Krismer B, Schumacher U, Gräpler-Mainka U, Riethmüller J, Jensen PØ, Bjarnsholt T, Høiby N, Bellon G, Döring G. 2010. Relative contribution of Prevotella intermedia and Pseudomonas aeruginosa to lung pathology in airways of patients with cystic fibrosis. Thorax 65:978–984. 10.1136/thx.2010.137745 [DOI] [PubMed] [Google Scholar]
- 18.Imai K, Yamada K, Tamura M, Ochiai K, Okamoto T. 2012. Reactivation of latent HIV-1 by a wide variety of butyric acid-producing bacteria. Cell. Mol. Life Sci. 69:2583–2592. 10.1007/s00018-012-0936-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagaoka K, Yanagihara K, Harada Y, Yamada K, Migiyama Y, Morinaga Y, Hasegawa H, Izumikawa K, Kakeya H, Nishimura M, Kohno S. 2013. Macrolides inhibit Fusobacterium nucleatum-induced MUC5AC production in human airway epithelial cells. Antimicrob. Agents Chemother. 57:1844–1849. 10.1128/AAC.02466-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura S, Yanagihara K, Araki N, Morinaga Y, Izumikawa K, Seki M, Kakeya H, Yamamoto Y, Kamihira S, Kohno S. 2009. In vivo efficacy of sitafloxacin in a new murine model of non-typeable Haemophilus influenzae pneumonia by sterile intratracheal tube. Int. J. Antimicrob. Agents 34:210–214. 10.1016/j.ijantimicag.2009.03.011 [DOI] [PubMed] [Google Scholar]
- 21.Grigg J, Walters H, Sohal SS, Wood-Baker R, Reid DW, Xu CB, Edvinsson L, Morissette MC, Stämpfli MR, Kirwan M, Koh L, Suri R, Mushtaq N. 2012. Cigarette smoke and platelet-activating factor receptor dependent adhesion of Streptococcus pneumoniae to lower airway cells. Thorax 67:908–913. 10.1136/thoraxjnl-2011-200835 [DOI] [PubMed] [Google Scholar]
- 22.Seki M, Kosai K, Hara A, Imamura Y, Nakamura S, Kurihara S, Izumikawa K, Kakeya H, Yamamoto Y, Yanagihara K, Miyazaki Y, Mukae H, Tashiro T, Kohno S. 2009. Expression and DNA microarray analysis of a platelet activating factor-related molecule in severe pneumonia in mice due to influenza virus and bacterial co-infection. Jpn. J. Infect. Dis. 62:6–10 [PubMed] [Google Scholar]
- 23.Grigg J. 2012. The platelet activating factor receptor: a new anti-infective target in respiratory disease? Thorax 67:840–841. 10.1136/thoraxjnl-2012-202206 [DOI] [PubMed] [Google Scholar]
- 24.Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. 1995. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377:435–438. 10.1038/377435a0 [DOI] [PubMed] [Google Scholar]
- 25.van der Sluijs KF, van Elden LJ, Nijhuis M, Schuurman R, Florquin S, Shimizu T, Ishii S, Jansen HM, Lutter R, van der Poll T. 2006. Involvement of the platelet-activating factor receptor in host defense against Streptococcus pneumoniae during postinfluenza pneumonia. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:194–199. 10.1152/ajplung.00050.2005 [DOI] [PubMed] [Google Scholar]
- 26.Barbier M, Oliver A, Rao J, Hanna SL, Goldberg JB, Albertí S. 2008. Novel phosphorylcholine-containing protein of Pseudomonas aeruginosa chronic infection isolates interacts with airway epithelial cells. J. Infect. Dis. 197:465–473. 10.1086/525048 [DOI] [PubMed] [Google Scholar]
- 27.Smani Y, Docobo-Pérez F, López-Rojas R, Domínguez-Herrera J, Ibáñez-Martínez J, Pachón J. 2012. Platelet-activating factor receptor initiates contact of Acinetobacter baumannii expressing phosphorylcholine with host cells. J. Biol. Chem. 287:26901–26910. 10.1074/jbc.M112.344556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grenier D, La VD. 2011. Proteases of Porphyromonas gingivalis as important virulence factors in periodontal disease and potential targets for plant-derived compounds: a review article. Curr. Drug Targets 12:322–331. 10.2174/138945011794815310 [DOI] [PubMed] [Google Scholar]
- 29.Teles RP, Haffajee AD, Socransky SS. 2006. Microbiological goals of periodontal therapy. Periodontol. 2000 42:180–218. 10.1111/j.1600-0757.2006.00192.x [DOI] [PubMed] [Google Scholar]
- 30.Kawanami T, Fukuda K, Yatera K, Kido M, Mukae H, Taniguchi H. 2011. A higher significance of anaerobes: the clone library analysis of bacterial pleurisy. Chest 139:600–608. 10.1378/chest.10-0460 [DOI] [PubMed] [Google Scholar]
- 31.Azarpazhooh A, Leake JL. 2006. Systematic review of the association between respiratory diseases and oral health. J. Periodontol. 77:1465–1482. 10.1902/jop.2006.060010 [DOI] [PubMed] [Google Scholar]
- 32.Scannapieco FA. 1999. Role of oral bacteria in respiratory infection. J. Periodontol. 70:793–802. 10.1902/jop.1999.70.7.793 [DOI] [PubMed] [Google Scholar]
- 33.Marik PE, Kaplan D. 2003. Aspiration pneumonia and dysphagia in the elderly. Chest 124:328–336. 10.1378/chest.124.1.328 [DOI] [PubMed] [Google Scholar]