Abstract
Alveolar macrophages (AM) seem to constitute the main cellular target of inhaled brucellae. Here, we show that Brucella abortus invades and replicates in murine AM without inducing cytotoxicity. B. abortus infection induced a statistically significant increase of tumor necrosis factor alpha (TNF-α), CXCL1 or keratinocyte chemoattractant (KC), interleukin-1β (IL-1β), IL-6, and IL-12 in AM from C57BL/6 mice and BALB/c mice, but these responses were generally weaker and/or delayed compared to those elicited in peritoneal macrophages. Studies using knockout mice for TLR2, TLR4, and TLR9 revealed that TNF-α and KC responses were mediated by TLR2 recognition. Brucella infection reduced in a multiplicity of infection-dependent manner the expression of major histocompatibility complex class II (MHC-II) molecules induced by gamma interferon (IFN-γ) in AM. The same phenomenon was induced by incubation with heat-killed B. abortus (HKBA) or the lipidated form of the 19-kDa outer membrane protein of Brucella (L-Omp19), and it was shown to be mediated by TLR2 recognition. In contrast, no significant downregulation of MHC-II was induced by either unlipidated Omp19 or Brucella LPS. In a functional assay, treatment of AM with either L-Omp19 or HKBA reduced the MHC-II-restricted presentation of OVA peptides to specific T cells. One week after intratracheal infection, viable B. abortus was detected in AM from both wild-type and TLR2 KO mice, but CFU counts were higher in the latter. These results suggest that B. abortus survives in AM after inhalatory infection in spite of a certain degree of immune control exerted by the TLR2-mediated inflammatory response. Both the modest nature of the latter and the modulation of MHC-II expression by the bacterium may contribute to such survival.
INTRODUCTION
Brucellosis is zoonotic disease caused by Brucella species, which is distributed worldwide and affects over 500,000 people annually (1) and for which there is no approved efficacious human vaccine available. Brucella melitensis, B. suis, and B. abortus are the most pathogenic species for humans and are responsible for the vast majority of human cases (2). The infection can be transmitted to humans by several ways, among which inhalation of infected aerosols is one of the most frequent. The easy aerosolization and airborne transmission of Brucella species has contributed to their consideration as potential biological weapons (1) and their classification by the CDC and NIAID as category B bioterrorism agents. Outbreaks of human brucellosis due to airborne transmission have been reported in different settings, including abattoirs, vaccine production laboratories, and rural areas (3–5). Notably, aerosols have been implicated in most cases of laboratory-acquired brucellosis, which is considered the commonest laboratory-acquired infection (6).
Lung epithelial cells and alveolar macrophages (AM) are the first cells to be contacted by inhaled microorganisms. AM constitute the first line of pulmonary defense and are capable of initiating a local immune response to pathogens, maintaining the functional integrity of pulmonary epithelium. Such immune response relies to a great extent on the recognition of foreign antigens by toll-like receptors (TLRs), which detect different pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), peptidoglycan, lipoproteins, flagellin, CpG DNA motifs, double-stranded RNA, etc., and trigger the activation of MyD88- and TRIF-dependent signaling pathways that lead to a wide range of cellular responses, including the secretion of proinflammatory cytokines and chemokines and type I interferons, which contribute to the protective response to microbial pathogens (7). Previous studies have shown that TLR2, TLR4, and TLR9 are involved in the recognition of Brucella by macrophages and dendritic cells (8), but the importance of TLRs in the recognition of Brucella by AM has not been characterized.
The interaction of Brucella with the pulmonary cells has scarcely been studied. We have previously shown that Brucella species can infect and replicate within human lung epithelial cells and can induce these cells to produce the monocyte chemoattractant MCP-1 (9, 10). More recently, a study performed in mice after intranasal inoculation of B. abortus revealed that AM constitute the main cellular target of these inhaled bacteria. Moreover, confocal microscopy studies indicated that the number of bacteria per AM increased between days 2 and 5 postinfection (p.i.) (11). Notably, in another study of mice infected with aerosolized B. abortus, lung CFU counts increased steadily until week 4 p.i. and remained high at least until week 8 p.i. (12), suggesting that B. abortus can replicate and persist within the murine lung. It can be speculated that this long-term survival of Brucella in the lung takes place, at least in part, in AM.
The above speculation is also supported by the fact that several studies have shown that Brucella can survive and replicate inside human and murine macrophagic cells (13, 14). Key determinants of such survival are the ability of the bacterium to avoid the fusion of the Brucella-containing vacuole with lysosomes (15) and several physiologic adaptations of the brucellae to their intracellular niche (16). In addition, to avoid the T cell receptor (TCR)-mediated recognition of infected monocytes/macrophages and an eventual T cell-mediated activation of such cells, the bacterium downmodulates the expression of MHC-II molecules on the cellular surface of these phagocytes (17).
To the best of our knowledge, all of the studies regarding Brucella invasion, replication, and immune evasion in monocytic/macrophagic cells have been performed using cell lines, peripheral blood monocytes, or murine peritoneal or bone marrow-derived macrophages. No studies have been performed using isolated AM. This lack of knowledge is not trivial, since several studies have shown that AM differ from other monocytic/macrophagic populations in several ways, including the cytokine response to infection or to stimulation with microbial antigens (18–23), the susceptibility to infection or the intracellular replication kinetics for certain pathogens (22, 23), the signaling pathways or innate immunity receptors involved in responses to antigenic stimuli (24, 25), and several other aspects (26). Notably, a recent study showed that AM can constitutively carry pathogens from the lung to the draining lymph nodes even before dendritic cells (27). Therefore, the main goals of the present study were to evaluate the time course of Brucella survival and replication in murine AM, to assess the cytokine response of these cells to infection, and to determine whether Brucella can also downregulate MHC-II expression in AM as a strategy to avoid or delay specific adaptive immune responses. The role of TLRs in the Brucella-mediated effects under study was also evaluated.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The smooth strain Brucella abortus 2308 was grown in tryptic soy broth at 37°C with agitation. Bacteria were washed twice with sterile phosphate-buffered saline (PBS), and inocula were prepared in sterile PBS on the basis of the optical density (OD) readings, but the actual concentration was later checked by plating on agar. All live Brucella manipulations were performed in biosafety level 3 facilities. When indicated, Brucella organisms were washed five times for 10 min each in sterile PBS, heat killed at 70°C for 20 min, aliquoted, and stored at −70°C until they were used. The absence of B. abortus viability after heat killing was verified by the absence of bacterial growth on tryptose soy agar (TSA).
Reagents.
B. abortus lipidated outer membrane protein 19 (L-Omp19) and unlipidated Omp19 (U-Omp19) were obtained as described previously (28). Both recombinant proteins contained less than 0.25 endotoxin U/μg of protein as assessed by Limulus amebocyte lysates (Associates of Cape Cod). Protein concentration was determined by the bicinchoninic acid method (Pierce, Rockford, IL) using bovine serum albumin (BSA) as the standard. B. abortus 2308 LPS and Escherichia coli O111 strain K58H2 LPS were provided by I. Moriyón (University of Navarra, Pamplona, Spain). The purity and characteristics of these preparations have been described elsewhere (29). The synthetic bacterial lipohexapeptide Pam3CysSerLys4 (Pam3Cys) was obtained from InvivoGen (San Diego, CA).
Mice.
Specific-pathogen-free, female BALB/c mice were purchased from University of La Plata, La Plata, Argentina, and used at 8 weeks of age. C57BL/6 mice and TLR2, TLR4, TLR9, and MAL/TIRAP gene knockout (KO) mice bred on a C57BL/6 background were provided by Federal University of Minas Gerais (UFMG), Brazil. Animals were housed in groups of 5 animals under controlled temperature (22°C ± 2°C) and artificial light under a 12-h cycle period. KO mice were kept under specific-pathogen-free conditions in positive-pressure cabinets and provided with sterile food and water ad libitum. All animal procedures were performed according to the rules and standards for the use of laboratory animals of the National Institutes of Health. Animal experiments were approved by the Ethical Committee of the IDEHU Institute and the Institutional Animal Care and Use Committee of the UFMG.
Murine alveolar macrophages.
Murine alveolar macrophages were isolated as previously described, with some modifications (30). Briefly, mice were euthanized by an intraperitoneal injection of a lethal dose of ketamine and xylazine, their tracheas were cannulated, and the airways were perfused several times with 0.7 ml of sterile cold PBS containing 1 mM EDTA to provide 4 ml of bronchoalveolar lavage (BAL) fluid. BAL fluid samples were centrifuged at 400 × g for 10 min at 4°C, and the cells present in the pellet were resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco-BRL Life Technologies, Grand Island, NY), 100 U of penicillin per ml, and 50 μg of streptomycin per ml. Cell viability was routinely greater than 95%, as determined by trypan blue exclusion. Cells were dispensed in culture plates of 48 wells at 2 × 105 or 5 × 105 cells/well and incubated in a 5% CO2 humidified atmosphere for 2 h at 37°C for adhesion before stimulation. After incubation, culture supernatants were discarded and cells were washed several times with culture medium to remove nonadherent cells. The identity of adherent cells as alveolar macrophages was confirmed by flow cytometry using anti-CD11c and anti-F4/80 antibodies as described below.
Murine peritoneal macrophages.
Peritoneal macrophages were extracted from BALB/c and C57BL/6 mice by peritoneal washing with ice-cold PBS-EDTA 1 mM and were enriched by plastic adherence in 48-well plates incubated for 1 h at 37°C in a 5% CO2 atmosphere. Cells (5 × 105 cells/well) were washed with fresh RPMI and then cultured overnight in RPMI, 2 mM glutamine, 100 U/ml of penicillin, and 50 μg/ml streptomycin supplemented with 10% FBS before infection.
Cellular infections.
Infections were performed at multiplicities of infection (MOI) of 100 bacteria/cell in culture medium containing no antibiotics. After dispensing the bacterial suspension, the culture plates were centrifuged (10 min at 300 × g at room temperature) and then incubated for 2 h at 37°C under 5% CO2 atmosphere. At the end of incubation (time zero p.i.), each well was washed three times with sterile PBS. For quantification of intracellular bacteria, the infected monolayers were incubated in the presence of 100 μg/ml of gentamicin (Sigma, USA) and 50 μg/ml of streptomycin (Sigma, USA) to kill extracellular bacteria. At different times after antibiotic addition (2, 24, or 48 h), culture supernatants were harvested for cytokine determinations, whereas cells were washed with sterile PBS and lysed with 0.2% Triton X-100. Serial dilutions of the lysates were plated on TSA to enumerate CFU.
Evaluation of cytotoxicity.
The reduction of the barrier function of the cell membrane is a marker of cytotoxicity. To analyze the effect of infection on cell membrane permeability, the release of lactate dehydrogenase (LDH) from infected macrophages was determined. Cells were infected with B. abortus 2308 at an MOI of 100 as described above. Culture supernatants were harvested at 24 and 48 h p.i., and LDH levels were measured using the CytoTox 96 nonradioactive cytotoxicity assay (Promega, USA) according to the instructions of the manufacturer. Other AM cultured in parallel were left uninfected to determine basal LDH release. In order to determine the LDH value corresponding to the lysis of all the cells (maximum lysis), other wells containing AM were treated for 60 min at 37°C with the lysis buffer provided in the kit (freeze-thaw lysis was employed in other experiments with similar results). Fifty microliters of each culture medium (infected or uninfected AM and maximum lysis control) were collected and transferred to a 96-well plate. After mixing each sample with 50 μl of substrate mix, the plate was covered and incubated for 30 min at room temperature protected from light. Finally, 50 μl of stop solution was added and the OD at 490 nm of each well was measured in a microplate reader. To account for the endogenous LDH activity of the fetal bovine serum, 50 μl of fresh culture medium was processed in the same way in parallel, and this background value was subtracted from the readings of all other samples. The relative LDH release (indicator of the percentage of cytotoxicity) was calculated as the ratio between the OD value measured for infected or uninfected cells and that corresponding to maximum lysis.
Measurement of cytokine concentrations.
Murine CXCL1 or keratinocyte chemoattractant (KC), interleukin-6 (IL-6), IL-1β, monocyte chemoattractant protein 1 (MCP-1), IL-12 p40/p70, and TNF-α were measured in culture supernatants of Brucella-infected AM by sandwich enzyme-linked immunosorbent assay (ELISA) (all from BD Biosciences, San Diego, CA, except for KC, which was from R&D, Minneapolis, MN) using paired cytokine-specific monoclonal antibody according to the manufacturer's instructions.
Effect of infection or Brucella antigens on MHC-II expression.
Alveolar macrophages from wild-type (wt) and TLR KO mice (0.5 × 106 cells/well) were incubated with 150 U/ml of recombinant murine IFN-γ (Thermo Fisher Scientific, USA) in the absence or presence of heat-killed B. abortus (HKBA; 107 to 109 CFU/ml), L-Omp19, U-Omp19, Pam3Cys, CpG, E. coli LPS, or Mycobacterium tuberculosis lysate for 48 h at the indicated concentrations. At the end of culture, cells were washed three times with sterile PBS and resuspended in Fc block (at a concentration of 2 μg/ml in 0.1% BSA in PBS) for 15 min on ice. The cells then were incubated for 30 min on ice with a phycoerythrin-labeled antibody against murine MHC-II (including I-Ab and I-Ad molecules; clone M5/114.15.2; eBioscience), a fluorescein isothiocyanate-labeled anti-mouse CD11c antibody (clone HL3; BD Bioscience), an allophycocyanin-labeled anti-mouse F4/80 antibody (clone CI:A3-1; Serotec), or an isotype-matched control antibody (BD Bioscience). The cells were then washed in BSA in 0.1% PBS and fixed with 1% paraformaldehyde in PBS (pH 7.4). Samples were stored in the dark at 4°C for subsequent analysis with a FACScan flow cytometer (Becton, Dickinson, Franklin Lakes, NJ) using CellQuest software (Becton, Dickinson). A total of 10,000 to 20,000 ungated events were collected. The results were expressed as mean fluorescence intensities (MFI; arithmetic means ± standard errors of the means [SEM]).
In infection experiments, 0.5 × 106 cells/ml were infected with B. abortus at MOIs of 10, 50, or 100 in the presence of IFN-γ for 2 h in standard medium containing no antibiotics. The cells then were extensively washed to remove noninternalized bacteria, and the infected cells were maintained in the presence of IFN-γ for an additional 48 h. After this, flow cytometry was conducted as indicated above.
Antigen presentation assay.
Alveolar macrophages were harvested as described above, plated onto 96-well flat-bottom plates (1.5 × 105 cells/well) in complete medium, and allowed to adhere overnight at 37°C. Cells were washed five times and treated with complete medium with or without L-Omp19 or HKBA in the presence of IFN-γ. After 48 h of incubation, alveolar macrophages were washed extensively with warm culture medium to remove residual IFN-γ and antigens. Cells then were incubated with 50 to 5,000 μg/ml of ovalbumin (OVA; Sigma) for 6 h, followed by incubation with BO97.10 T hybridoma cells (105 cells/well) specific for the OVA323-339 peptide presented on I-Ab (31). Culture supernatants were removed after 24 h and stored at −70°C until they were assayed for IL-2 levels by commercial ELISA (BD Biosciences).
Intratracheal infection and lung processing.
Mice were anesthetized by intraperitoneal injection of ketamine and xylazine and were inoculated intratracheally with 100 μl of B. abortus suspension (1 × 106 CFU/ml) by following the procedure described by Revelli et al. (32), with minor modifications. At 7 days postinfection, mice were euthanized by intraperitoneal overdose of ketamine/xylazine and BAL samples were obtained as described above. Cells from BAL fluid were resuspended in RPMI-10% FBS without antibiotics, and aliquots were taken for cell counting. Cell suspensions were adjusted to 5 × 105 cells/ml, and 1 ml per well was dispensed in 24-wells plates. Cells were incubated for 2 h at 37°C in a 5% CO2 atmosphere and were washed 3 times with sterile PBS to remove nonadherent cells. Adherent cells were lysed and plated for CFU determinations as described for in vitro infections. After the BAL procedure, lungs were homogenized in sterile PBS using a tissue grinder, and different dilutions of the resulting cell suspensions were plated on agar for CFU counting.
Statistical analysis.
Data were analyzed using analysis of variance (ANOVA). Comparisons between groups of data were performed with the Tukey's posttest, and those against a control group were performed with Dunnett's test. All of the statistical analyses were performed using GraphPad software (San Diego, USA).
RESULTS
Brucella abortus invades and replicates within murine alveolar macrophages.
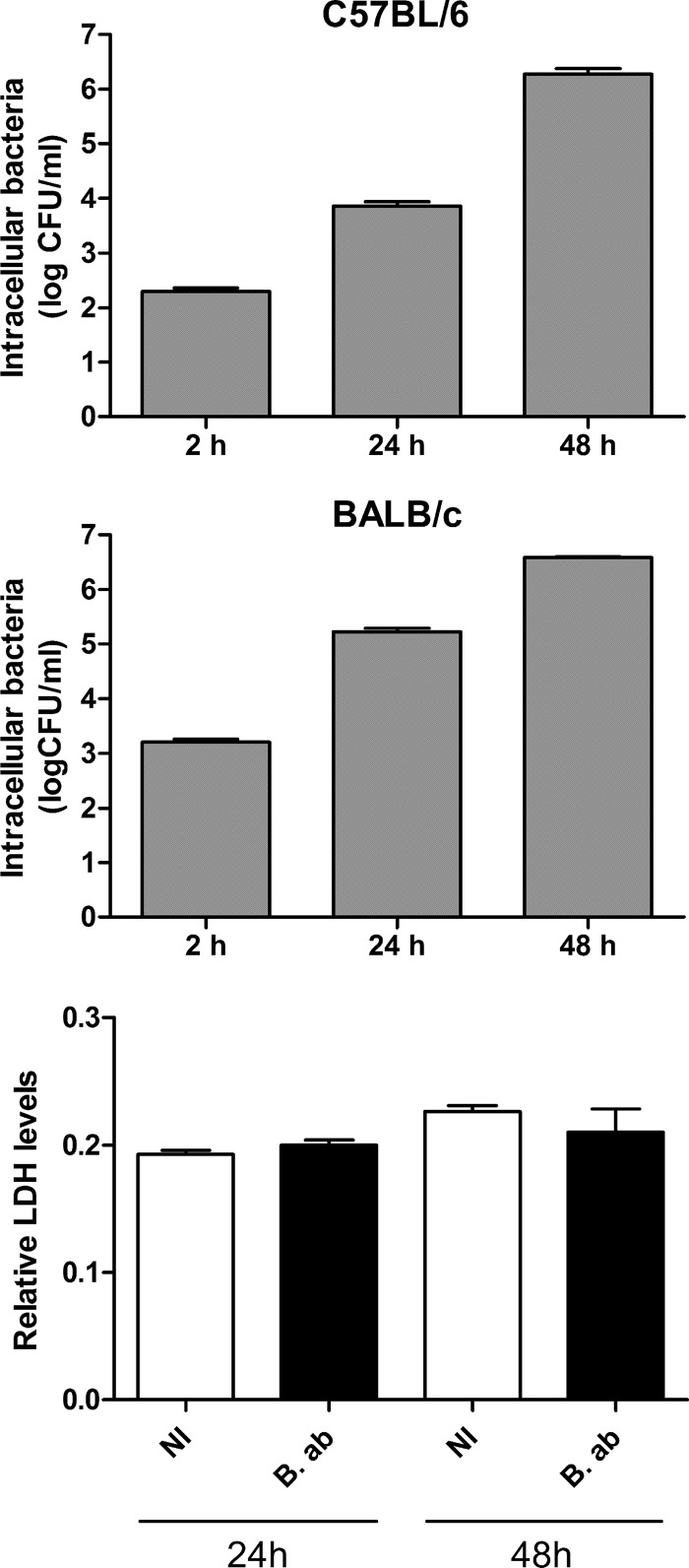
Experiments were carried out to determine whether B. abortus invades alveolar macrophages (AM) and establishes a replication niche within these cells. Murine AM were infected for 2 h at an MOI of 100 bacteria/cell, extracellular bacteria were killed by treatment with antibiotics, and intracellular CFU were determined at different times postinfection (p.i.) by plating cellular lysates in agar. As shown in Fig. 1 (upper and middle), B. abortus was able to invade and replicate within AM from both C57BL/6 and BALB/c mice, as CFU counts of intracellular bacteria increased steadily during the whole follow-up period. The detection of released lactate dehydrogenase (LDH) is widely used as a measure of cell damage or death for most cell types, including AM (33). To evaluate a potential cytotoxic effect of Brucella infection on AM, levels of LDH activity were measured in culture supernatants from B. abortus-infected AM at 24 and 48 h p.i. At both time points no significant difference in LDH activity was detected between supernatants from Brucella-infected cells and those from uninfected AM from both BALB/c mice (Fig. 1, lower) and C57BL/6 mice (not shown). Taken together, these results indicate that virulent B. abortus can survive and replicate inside alveolar macrophages, and that the increasing load of intracellular bacteria does not result in cytotoxic effects on these cells.
FIG 1.
Invasion and intracellular replication of B. abortus in murine alveolar macrophages (AM). AM (2 × 105 cells/well) from C57BL/6 mice (upper panel) or BALB/c mice (middle panel) were infected at an MOI of 100 for 2 h, and intracellular CFU were measured at different times p.i. Results are expressed as means ± SEM of values measured in triplicate in each experiment. Data are representative of an experiment from three performed with similar results. (Lower) Effect of Brucella infection on the cell membrane permeability of AM from BALB/c mice. Cells were infected as described above, and culture supernatants were collected at 24 and 48 h p.i. to measure LDH levels. Results are expressed as the ratio between LDH levels found in infected or noninfected (NI) cells and those found in a whole cellular lysate (100% lysis). B. ab., B. abortus.
Proinflammatory response of AM to B. abortus infection.
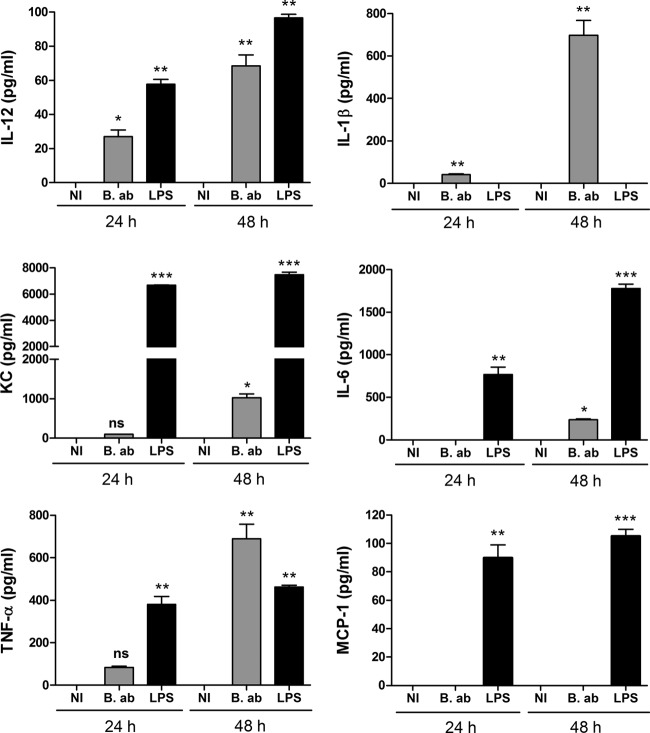
The levels of several proinflammatory cytokines, including TNF-α, IL-1-β, KC, IL-6, MCP-1, and IL-12, were measured at different p.i. times in culture supernatants from B. abortus-infected AM. As shown in Fig. 2, at 24 h p.i. cytokines were either not detected or were present at very low levels in supernatants from infected AM from C57BL/6 mice. Except for MCP-1, which was not detected at any p.i. time, cytokine levels increased at 48 h p.i. compared to those at 24 h p.i. Nevertheless, levels were rather low for IL-6 and IL-12, whereas the highest levels corresponded to TNF-α, IL-1β, and KC.
FIG 2.
Secretion of proinflammatory cytokines by AM from C57BL/6 mice in response to B. abortus infection. Alveolar macrophages were infected as described for Fig. 1, and culture supernatants were harvested at 24 and 48 h to measure cytokines by sandwich ELISA. NI, noninfected cells. B. ab., B. abortus-infected cells. LPS, cells stimulated with lipopolysaccharide from E. coli (100 ng/ml). Data represent means ± standard deviations (SD) from values measured in triplicate in each experiment. The figure shows a representative experiment from two performed with similar results. Asterisks indicate significant differences between infected and uninfected cells at each time point (*, P < 0.05; **, P < 0.01; ***, P < 0.001) (ANOVA followed by Dunnett's posttest).
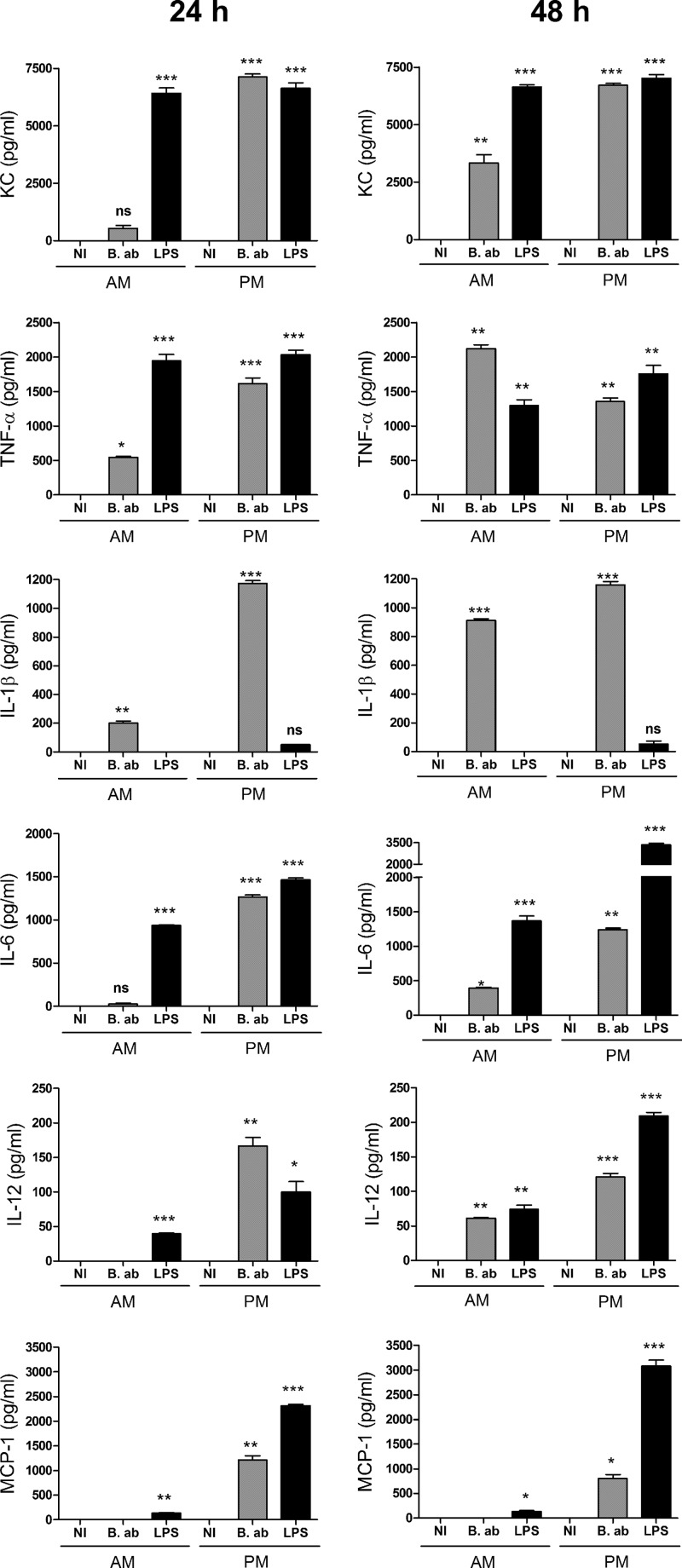
A similar profile of cytokine response was observed for AM from BALB/c mice (Fig. 3). Again, all of the cytokines were at low levels or were absent at 24 h p.i., and all of them (except MCP-1, which was never detected) increased at 48 h p.i., with the highest increases for TNF-α and KC and, to a lesser extent, IL-1β. As the cytokine response of AM to bacterial antigens usually differs from that of macrophages from other localizations, generally resulting in a milder proinflammatory profile (22, 23), cytokine levels were also measured in culture supernatants from B. abortus-infected peritoneal macrophages (PM) from BALB/c mice and compared to those obtained for AM from the same animals. As shown in Fig. 3, cytokine responses were generally weaker and/or delayed compared to those elicited in PM. While, as mentioned, cytokines were either absent or low at 24 h p.i. in AM, all were already produced by infected PM at this time point. For all of the cytokines, levels detected at 24 h p.i. were significantly higher for PM than for AM (P < 0.001 in all cases). MCP-1 was not detected in culture supernatants from B. abortus-infected AM at any time point but was detected in Brucella-infected PM at both p.i. times.
FIG 3.
Secretion of proinflammatory cytokines by AM and peritoneal macrophages (PM) from BALB/c mice in response to B. abortus infection. Alveolar macrophages were infected as described for Fig. 1, and culture supernatants were harvested at 24 and 48 h to measure cytokines by sandwich ELISA. NI, noninfected cells. B. ab., B. abortus-infected cells. LPS, cells stimulated with lipopolysaccharide from E. coli (100 ng/ml). Data represent means ± SD from values measured in triplicate in each experiment. The figure shows a representative experiment from two performed with similar results. Asterisks indicate significant differences between infected and uninfected cells at each time point (*, P < 0.05; **, P < 0.01; ***, P < 0.001) (ANOVA followed by Dunnett's posttest).
Overall, these results indicate that murine AM respond to B. abortus infection with a delayed and restricted panel of proinflammatory mediators (mainly represented by TNF-α and KC) compared to murine PM.
The proinflammatory response of AM to Brucella is mainly TLR2 mediated.
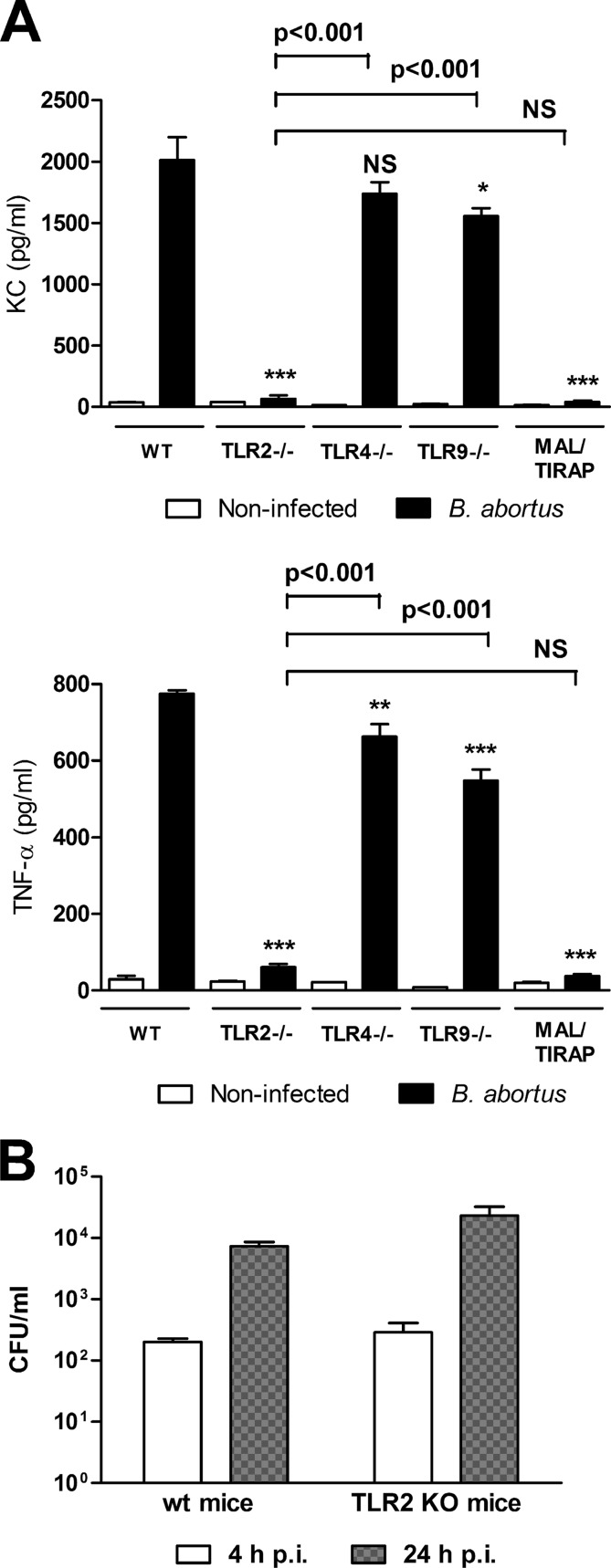
To determine whether Toll-like receptors (TLR) are involved in the induction of cytokine responses, infections were performed in parallel in AM from C57BL/6 mice (wt) and from mice of the same background genetically deficient for TLR2, TLR4, or TLR9. We chose to evaluate these receptors since they were demonstrated to be important for cytokine production in response to Brucella in PM and dendritic cells (8). Since KC and TNF-α were markedly induced by infection in AM from wt mice, these cytokines were measured in experiments with AM from TLR KO mice. As shown in Fig. 4A, TNF-α and KC were greatly reduced in supernatants of AM from TLR2 KO mice compared to those from wt mice (92% and 97% reduction, respectively). In contrast, the production of both cytokines was much less reduced in cells from TLR4 KO mice (significant reduction only for TNF-α) and TLR9 KO mice (29% and 23% reduction, both statistically significant). TNF-α production in response to infection was significantly lower in AM from TLR2 KO mice than in those from wt, TLR4 KO, or TLR9 KO mice (P < 0.001 in all cases), and the same was true for KC production (P < 0.001 in all cases). These results suggest that TNF-α and KC production by AM in response to B. abortus infection depends mainly on TLR2 recognition, with significantly smaller contributions of TLR4 and TLR9. In agreement with the results obtained with TLR2 and TLR4 KO mice, the TNF-α and KC responses of AM from mice deficient in the adaptor protein MAL/TIRAP were also greatly reduced (95% and 98% reduction, respectively) compared to wt mice.
FIG 4.
(A) Cytokine secretion by AM from C57BL/6 mice in response to B. abortus infection depends mainly on TLR2 recognition. Alveolar macrophages from wild-type (WT) and TLR or MAL/TIRAP mutant mice were infected as described for Fig. 1, and culture supernatants were harvested at 48 h to measure TNF-α and KC by sandwich ELISA. NI, noninfected. Data represent means ± SD from values measured in triplicate in each experiment. The figure shows a representative experiment from two performed with similar results. Asterisks indicate significant differences between mutant mice and WT mice (*, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, nonsignificant) (ANOVA followed by Tukey's posttest). Bru, B. abortus. (B) TLR2 is not involved in Brucella invasion to AM. AM from WT and TLR2 KO mice were infected as described above, and cell lysates were obtained at 4 and 24 h p.i. to determine intracellular CFU counts. Data represent means ± SD from values measured in duplicate in each experiment. The figure shows a representative experiment from two performed with similar results.
To rule out that differences in TNF-α and KC production between AM from wt and TLR2 KO mice were due to differences between these cells in the rate of Brucella infection or replication, CFU counts in AM lysates were determined at 2 and 24 h p.i. As shown in Fig. 4B, no significant differences in B. abortus CFU counts were found between AM from wt and TLR2 KO mice at both p.i. times.
Both live and heat-killed Brucella organisms downregulate MHC-II expression in AM.
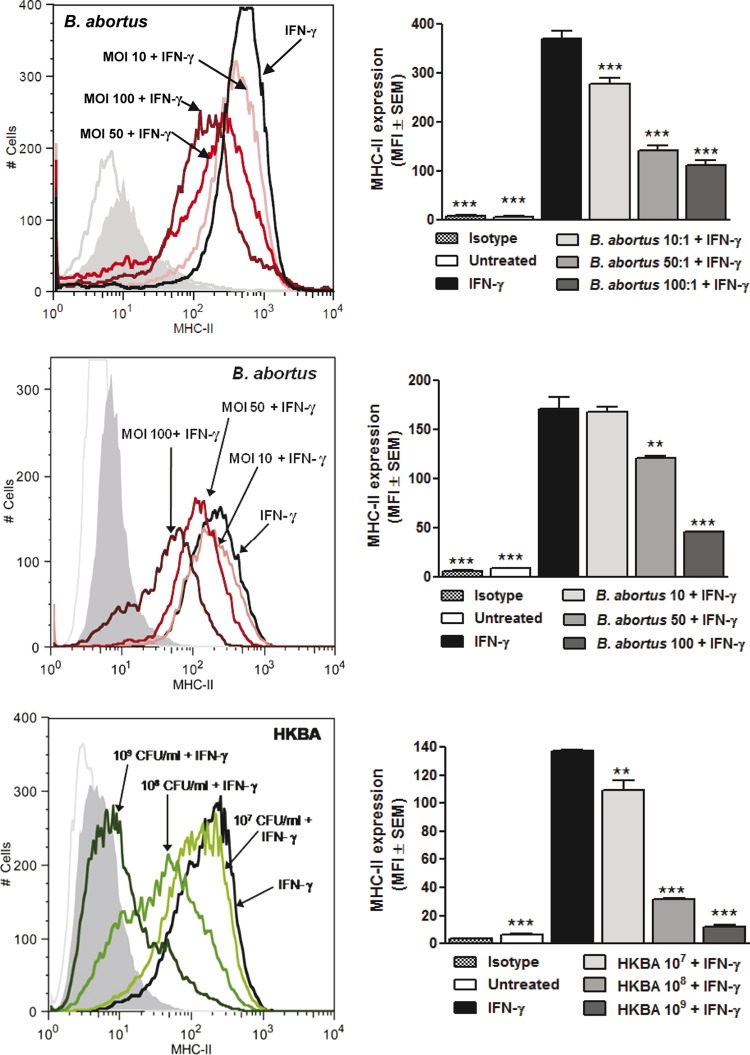
As mentioned, previous studies have shown that B. abortus can replicate and survive for several weeks in lungs of mice infected through the nasal route (12), suggesting that the bacterium displays mechanisms of immune evasion at the pulmonary level. Since it has been shown that mycobacteria can downregulate the expression of MHC-II molecules in macrophages (30, 34), we decided to test whether this evasion mechanism also operates in Brucella infections. AM from C57BL/6 and BALB/c mice were left uninfected or were infected with B. abortus at different multiplicities of infection for 2 h in the presence of IFN-γ (as an inducer of MHC-II expression), washed extensively, and maintained in the presence of IFN-γ for an additional 48 h. After this, the expression of MHC-II (I-Ad) was evaluated by flow cytometry. As shown in Fig. 5, the expression of MHC-II was significantly reduced in cells that had been incubated with live B. abortus during the first 2 h of IFN-γ stimulation compared to cells incubated with IFN-γ alone. For AM from both mouse strains, the downregulating effect of B. abortus infection was MOI dependent, with mean fluorescence intensity (MFI) reductions of 25%, 62%, and 70% for MOIs of 10, 50, and 100, respectively, in AM from C57BL/6 mice and 25%, 35%, and 44% in AM from BALB/c mice. To determine whether this downregulation is mediated by structural components of the bacterium, AM from BALB/c mice were stimulated with IFN-γ in the absence or presence of heat-killed B. abortus (HKBA) for 48 h, and the expression of MHC-II molecules was evaluated as described above. As shown in Fig. 5 (lower), the heat-killed bacterium also induced a significant MHC-II downregulation, suggesting that this effect was due to a structural component of Brucella. The downregulating effect of HKBA was dose dependent, with MFI reductions of 20%, 77%, and 91% for HKBA doses of 107, 108, and 109 CFU/ml, respectively.
FIG 5.
Brucella infection or stimulation with heat-killed bacteria (HKBA) downregulates IFN-γ-induced MHC-II expression in murine AM. Alveolar macrophages from C57BL/6 (upper panels) or BALB/c mice (middle panels) were incubated for 2 h with IFN-γ in the presence or absence of different MOIs of live B. abortus and then were maintained in the presence of IFN-γ for an additional 48 h. The expression of MHC-II molecules (I-Ad) was measured by flow cytometry using a specific antibody. Similar experiments were performed with AM from BALB/c mice but replacing the infection with stimulation with HKBA (lower panels). Panels on the right show mean fluorescence intensity (MFI) from values measured in duplicate in each experiment. The figure shows a representative experiment from three performed with similar results. Asterisks indicate significant differences between untreated cells or cells stimulated with IFN-γ plus live bacteria or HKBA compared to those stimulated with IFN-γ alone (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ANOVA followed by Dunnett's posttest).
These results indicate that infection with B. abortus induces a downregulation of MHC-II expression in AM, and that this phenomenon is mediated by a structural component of the bacterium.
Lipoproteins mediate the downregulation of MHC-II by Brucella.
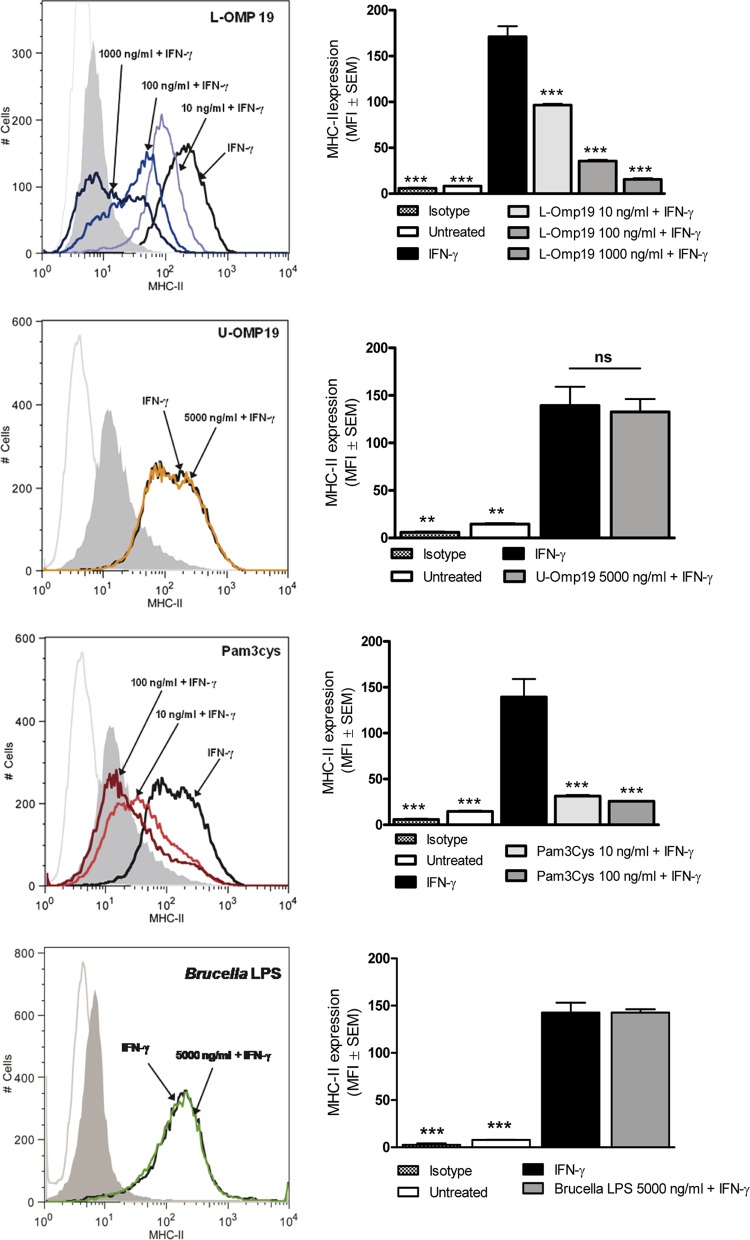
Previous studies on MHC-II downregulation by M. bovis have shown that such an effect may be mediated by a 19-kDa lipoprotein of this bacterium (30). Interestingly, previous studies from our group have shown that Brucella lipoproteins, and in particular the lipidated form of a 19-kDa lipoprotein (L-Omp19), may mediate important biological activities in human peripheral monocytes, such as the induction of proinflammatory responses and the downregulation of FcγRI (CD64) and MHC-II expression (17, 28, 35). Therefore, we decided to test whether L-Omp19 also is involved in the downregulation of MHC-II molecules induced by Brucella in AM. To do this, AM were incubated with murine IFN-γ in the absence or presence of this lipoprotein. As shown in Fig. 6, incubation with L-Omp19 induced a downregulation of MHC-II compared to cells treated only with IFN-γ. The downregulating effect of L-Omp19 was dose dependent, with MFI reductions of 44%, 79%, and 92% for L-Omp19 doses of 10, 100, and 1000 ng/ml, respectively. The downregulating effect of L-Omp19 on MHC-II expression depended on the lipid moiety of the lipoprotein, since its unlipidated version (U-Omp19) did not decrease MHC-II expression even when used at high concentrations (5,000 ng/ml). The requirement for lipidation was further supported by the fact that Pam3Cys, a synthetic lipohexapeptide that mimics the lipid moiety of lipoproteins, also downregulated in a dose-dependent manner the expression of MHC-II induced by IFN-γ (Fig. 6). These results suggest that L-Omp19 (and probably other Omps) is involved in the downregulating effect of B. abortus on the IFN-γ-induced MHC-II expression in murine AM.
FIG 6.
Downregulation of IFN-γ-induced MHC-II expression in murine AM is mediated by Brucella lipoproteins. Cells were incubated for 2 h with IFN-γ in the presence or absence of different doses of L-Omp19, U-Omp19, Pam3Cys, or Brucella LPS and were then maintained in the presence of IFN-γ for an additional 48 h. The expression of MHC-II molecules (I-Ad) was measured by flow cytometry using a specific antibody. Panels on the right show mean fluorescence intensity (MFI) from values measured in duplicate in each experiment. The figure shows a representative experiment from three performed with similar results. Asterisks indicate significant differences between untreated cells or cells stimulated with IFN-γ plus Brucella antigens or Pam3Cys compared to those stimulated with IFN-γ alone (**, P < 0.01; ***, P < 0.001; ns, nonsignificant; ANOVA followed by Dunnett's posttest).
Since LPS from some bacteria have been shown to diminish MHC-II expression in peritoneal macrophages (36), the LPS from B. abortus was considered another candidate molecule potentially involved in the inhibition of MHC-II expression in AM. Thus, experiments were conducted to evaluate the contribution of B. abortus LPS to such an effect. As shown in Fig. 6, B. abortus LPS was unable to inhibit the expression of MHC-II molecules even when used at high concentrations (5,000 ng/ml). In contrast, LPS from E. coli inhibited in a dose-dependent manner the IFN-γ-induced MHC-II expression, attaining MFI reductions of 95.5% and 88.2% at doses 10 and 50 times lower than those used for Brucella LPS (1,000 and 100 ng/ml, respectively) (data not shown).
Taken together, these results indicate that B. abortus lipoproteins, but not its LPS, contribute to the downmodulation of MHC-II in murine AM induced by HKBA stimulation or B. abortus infection.
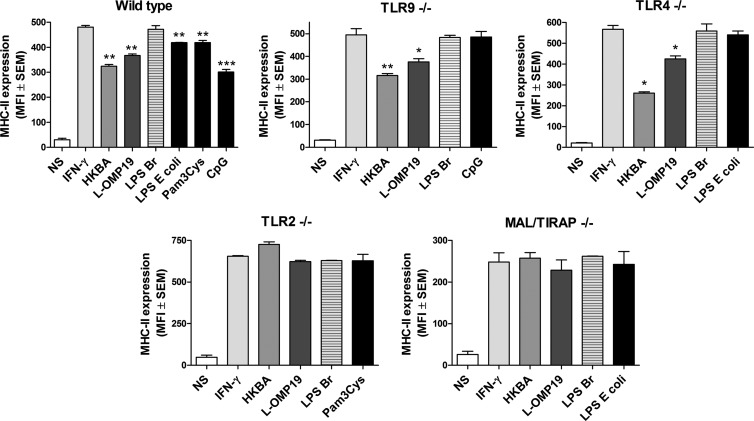
MHC-II downregulation by Brucella is TLR2 mediated.
Since TLR2, TLR4, and TLR9 have been shown to be involved in immune responses to Brucella infection and to Brucella antigens (8), experiments were carried out to determine if one or more of these receptors was involved in the downregulation of MHC-II expression induced by B. abortus antigens in murine AM. To do this, AM from wt C57BL/6 mice or from TLR2, TLR4, and TLR9 KO mice of the C57BL/6 background were incubated with murine IFN-γ in the absence or presence of HKBA, L-Omp19, or LPS from B. abortus. Pam3Cys, E. coli LPS, and CpG were included in parallel experiments, since TLR2, TLR4, and TLR9 agonists have been shown to downregulate MHC-II expression in murine peritoneal macrophages (36, 37).
As shown in Fig. 7, the IFN-γ-induced MHC-II expression on AM from wt C57BL/6 mice was downregulated by coincubation with HKBA and L-Omp19 but not by coincubation with LPS from B. abortus, in agreement with results obtained with AM from BALB/c mice (Fig. 6). Results obtained with AM from TLR4 and TLR9 KO mice were similar to those yielded by AM from wt mice, indicating that these receptors are not involved in the downregulation of MHC-II expression by Brucella antigens. In contrast, the downregulating effect of HKBA and L-Omp19 on MHC-II expression was abolished in AM obtained from TLR2 KO mice, indicating that this receptor is involved in such an effect. The downregulating effect was also abolished in AM from MAL/TIRAP KO mice, in agreement with the involvement of TLR2 in MHC-II downregulation by Brucella antigens.
FIG 7.
Brucella-induced downregulation of MHC-II depends on TLR2 and MAL/TIRAP signaling. AM from wt C57BL/6 mice or from TLR2, TLR4, TLR9, and MAL/TIRAP KO mice from a C57BL/6 background were incubated with murine IFN-γ in the absence or presence of HKBA, L-Omp19, or LPS from B. abortus (LPS Br) for 48 h. LPS from E. coli, Pam3Cys, and CpG were included as control agonists for TLR4, TLR2, and TLR9, respectively. The expression of MHC-II was assessed by flow cytometry. Results are expressed as mean fluorescence intensity (MFI) with their corresponding SEM values. Data are representative of an experiment from three performed with similar results. *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus IFN-γ alone (ANOVA followed by Tukey's posttest).
These results indicate that TLR2 recognition mediates the downregulating effect of Brucella antigens on MHC-II expression in murine AM.
Functional evidence of MHC-II downregulation.
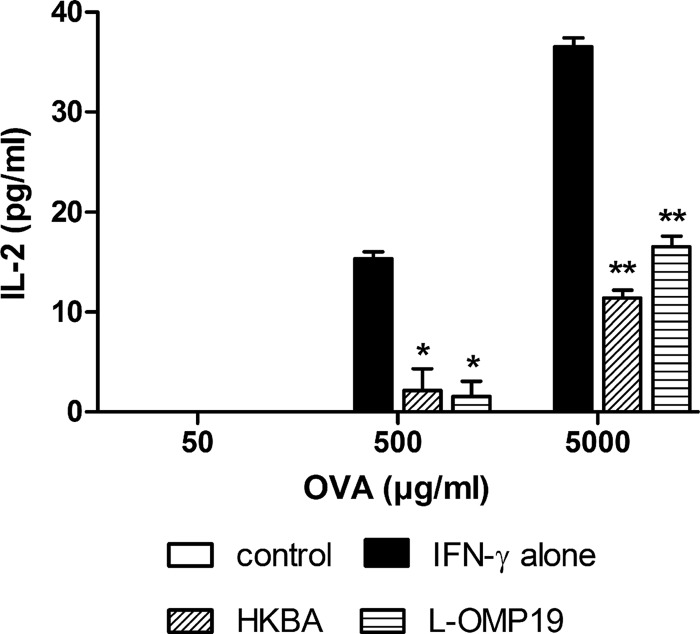
To examine whether MHC-II downregulation by Brucella has a functional consequence on the ability of AM to present antigenic peptides to specific T cells, AM from C57BL/6 mice were treated with IFN-γ alone or with the addition of L-Omp19 or HKBA. After 48 h, these cells were incubated with different amounts of ovalbumin and with a T cell hybridoma specific for an ovalbumin peptide presented in the context of I-Ab. As a measure of T cell activation, the levels of IL-2 were determined in culture supernatants at 24 h of coculture. As shown in Fig. 8, IL-2 levels were significantly lower in wells in which AM had been pretreated with L-Omp19 or HKBA than in nonpretreated cells. These results suggest that the reduction of MHC-II expression on AM induced by Brucella affects the ability of these cells to present MHC-II-restricted antigens to specific T cells, reducing the activation of the latter.
FIG 8.
MHC-II downregulation by Brucella antigens impairs the ability of AM to stimulate OVA-specific T cells. AM from C57BL/6 mice were treated with IFN-γ alone or with the addition of L-Omp19 or HKBA. After 48 h, these cells were incubated with different amounts of OVA and with a T cell hybridoma specific for an OVA peptide presented in the context of I-Ab (MHC-II). IL-2 levels were measured in the culture supernatants 24 h later. Data are presented as means ± SD of IL-2 concentrations assayed in triplicate in each experiment. The figure shows a representative experiment from three performed with similar results. P < 0.05 (*) and P < 0.01 (**) versus IFN-γ (ANOVA followed by Dunnett's posttest).
Impact of TLR2 absence on Brucella survival in AM.
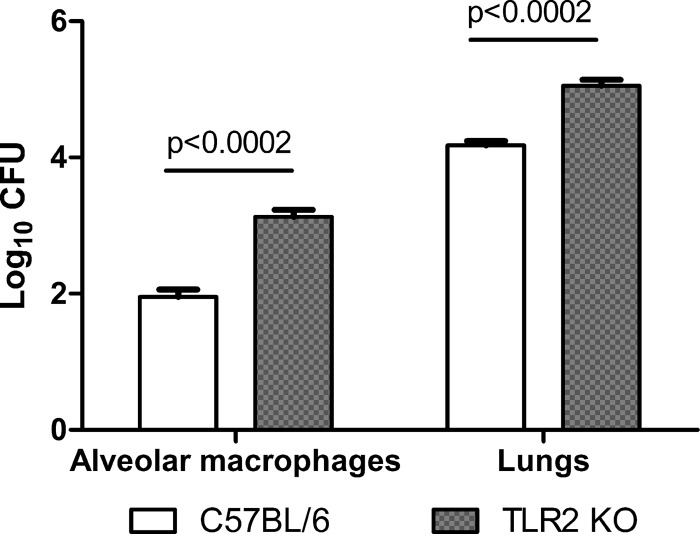
Our in vitro experiments revealed two TLR2-mediated phenomena that may have opposite effects on bacterial clearance: the production of proinflammatory cytokines (which may favor clearance) and the downregulation of MHC-II (which may favor bacterial persistence). The net effect of TLR2 recognition on Brucella survival in AM probably results from a balance between these two TLR2-mediated phenomena. To test the in vivo effect of TLR2 absence on Brucella survival within AM, wt and TLR2 KO C57BL/6 mice were infected with B. abortus through the intratracheal route. AM were obtained at 1 week p.i., lysed, and plated on agar to determine intracellular CFU counts. After bronchoalveolar lavage to obtain AM, lungs were processed and homogenates were also plated on agar. As shown in Fig. 9, viable bacteria were recovered from AM and lung homogenates from both mouse strains. However, bacterial counts were significantly higher in AM and lung homogenates from TLR2 KO mice than in those from wt mice. These results suggest that although the TLR2-mediated inflammatory response is important for controlling B. abortus infection in AM, the bacterium may still survive in these cells for at least 1 week after inhalatory infection.
FIG 9.
TLR2 absence impacts the ability of AM to control B. abortus infection. Wild-type C57BL/6 mice and TLR2 KO mice were infected intratracheally with B. abortus and were euthanized 1 week later. Cells obtained by bronchoalveolar lavage were adjusted to 5 × 105/ml and were dispensed in wells of a 24-well plate. Adherent cells were lysed, and lysates were plated for CFU determination. After bronchoalveolar lavage was performed, lungs were homogenized in sterile PBS and different dilutions of the resulting cell suspensions were plated on agar for CFU counting. Data represent means ± SD of CFU/well (AM) or CFU/lungs determined in duplicate in one experiment from two performed with similar results. Data for each type of sample were analyzed using an unpaired t test.
DISCUSSION
Airborne transmission is an important route for the acquisition of Brucella species infections by humans (4, 5) and may be also involved in animal cases. In spite of this, the interaction of brucellae with cells of the respiratory system has been scarcely documented, and in particular, the immunological aspects of such interaction have only recently begun to be studied (9, 11).
Alveolar macrophages (AM) and alveolar epithelial cells are the first cells contacted by inhaled bacteria immediately after reaching the lungs through infected aerosols. In the case of B. abortus, a recent study in mice has shown by confocal microscopy that AM constitute the main cellular target of these bacteria after intranasal infection (11). While an extensive body of literature has examined many aspects of the interaction between brucellae and different types of human or animal monocytic/macrophagic cells, there are no reports on the interaction between these bacteria and AM. Taking into account that AM differ from other monocytic/macrophagic populations in several ways, including cytokine response to infection or innate immunity receptors involved (19–21, 24, 25), we decided to characterize these aspects in the case of the interaction between brucellae and murine AM. In addition, since Mycobacterium tuberculosis has been shown to downregulate MHC-II expression in AM (37), we wondered whether a similar phenomenon occurs in Brucella-infected AM.
Our results show that B. abortus invades and replicates in murine AM. The ability of brucellae to replicate inside AM is in line with the findings of Archambaud et al. (11), who observed the same phenomenon by confocal microscopy, and is also in line with similar findings in several types of monocytic/macrophagic cells (13, 14). Notably, the infection did not induce cytotoxicity as measured by LDH release, suggesting that the viability of AM is not affected by B. abortus in spite of the marked increase in intracellular bacterial load. These results suggest that AM constitute a durable replicative niche for brucellae in the lung.
Previous studies have shown that mouse peritoneal macrophages and macrophagic cell lines respond to Brucella infection or stimulation with the production of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 and the Th1-inducing cytokine IL-12 (38–43). In contrast, the cytokine response of AM to Brucella has not been reported. In the present study, we found that AM from both BALB/c and C57BL/6 mice respond to Brucella infection with a marked increase in the secretion of TNF-α, KC (neutrophil chemoattractant), and IL-1β at 48 h p.i. but only low levels of IL-12 and IL-6. In addition, these cells do not produce MCP-1 in response to Brucella infection. Therefore, upon Brucella recognition AM seem to elicit a relatively attenuated inflammatory response with preserved KC and TNF-α secretion. The significant production of TNF-α and KC by Brucella-infected AM suggests that these cells contribute to the recruitment of neutrophils to the infection focus. A moderate amount of neutrophilic inflammation has been reported in the few histological studies performed in animal models of acute inhalational brucellosis (44, 45).
The profile of cytokine response of murine AM to Brucella infection differed from that found in PM. While infection elicited a marked increase of TNF-α in both AM and PM, the AM response was delayed compared to that of PM. The significant increase of TNF-α levels in response to Brucella agrees with data previously reported for murine PM and J774.A1 cells (39, 43). Differences in the kinetics of AM and PM responses were even more marked for KC, which was produced at very high levels at 24 h p.i. by Brucella-infected PM but did not exhibit a significant increase in AM at that time. Whereas IL-6 has been shown to be induced by Brucella infection in PM and J774.A1 cells (41, 43) and was also significantly induced in PM at 24 and 48 h p.i. in the present study, this cytokine was not increased in Brucella-infected AM at 24 h p.i. and exhibited only a small increase at 48 h p.i. IL-12 was secreted by B. abortus-infected PM, in agreement with previous studies (40), but its production by infected AM was comparatively low and delayed. The monocyte chemoattractant MCP-1 has been shown to be induced by Brucella infection in the murine macrophagic cell line RAW 264.7 and microglia cells (46, 47). In the present study, MCP-1 was produced early (24 h p.i.) by PM in response to B. abortus infection but was not produced by infected AM at any time point. Taken together, these results suggest that AM produce a weaker and/or delayed cytokine response to B. abortus compared to PM. This restricted response may be beneficial for Brucella infection, since some of the affected cytokines are known to be involved in the control of such infection. It is known that TNF-α is important for the influx of phagocytes to the site of infection and for macrophage activation and is critically involved in immune responses to intracellular pathogens. Previous studies have shown that the spleen load of Brucella is increased in mice treated with neutralizing antibodies against TNF-α (48). Although studies on localized effects of TNF-α deficiency at mucosal surfaces are lacking, it can be speculated that the comparatively delayed TNF-α production by AM in response to B. abortus favors the bacterium. It is known that IL-12 promotes efficient immune responses against intracellular pathogens not only by inducing a Th1 profile but also by promoting the migration of dendritic cells to draining lymph nodes (49). A central role for IL-12 in the control of B. abortus infection also has been demonstrated (42). Thus, a reduced local production of IL-12 may contribute to the long-term persistence of Brucella in the lung.
In the present study, IL-1β was among the cytokines induced by Brucella infection in AM, although its production by these cells was delayed compared to PM responses. While studies have shown that the genetic deficiency of IL-1β does not alter the splenic load of B. abortus in mice infected through the intraperitoneal route (50), the importance of this cytokine for the response to inhaled Brucella has not been explored. AM seemed to be poor producers of IL-1β upon LPS stimulation, since this was the only cytokine not elicited by the dose of E. coli LPS used as a control in cytokine assays (0.1 μg/ml). In line with these findings, murine AM have been reported to respond to a 10-fold higher dose of LPS than the one used here (1 μg/ml), with low levels of IL-1β (around 35 pg/ml) but high levels of KC (around 10 ng/ml) (51).
TLRs are involved in the inflammatory response of macrophages and other cell types to infection by different pathogens or to stimulation with their antigens. In the case of Brucella, previous studies have shown that TLR2 recognition is centrally involved in the inflammatory response of human or murine macrophages to these bacteria (28, 39). In agreement with those previous reports, our studies using KO mice for TLR2, TLR4, TLR9, and MAL/TIRAP revealed that TNF-α and KC responses of AM to Brucella infection were mainly mediated by TLR2 recognition; both responses were almost abolished in AM from TLR2 KO and MAL/TIRAP KO mice. In addition, a slight but significant reduction of TNF-α and KC secretion was observed in AM from TLR9 KO mice, and a slight diminution of TNF-α was also observed in AM from TLR4 KO mice. The participation of multiple TLRs in the inflammatory response to Brucella infection or antigens agrees with the published literature (8), although this study shows that TLR2 recognition has a preponderant role in the case of AM. Although not formally shown in the present study, it can be inferred from the existing literature that the Brucella antigens involved in the TLR2-mediated cytokine response of AM are outer membrane lipoproteins (28).
Alveolar macrophages perform several protective functions in the lung, including the phagocytosis and killing of invading pathogens and the secretion of cytokines and chemokines that contribute to the innate and adaptive immune response. In addition, like macrophages located in other tissues, AM process microbial antigens and display antigenic peptides in the context of MHC molecules for their recognition by specific T cells. Gamma interferon (IFN-γ) activates macrophages and induces their expression of MHC-II molecules, resulting in an enhanced antigen presentation to specific CD4+ T cells and the consequent activation of the latter. This partially explains the central role of IFN-γ in the protection against infections by intracellular pathogens. Therefore, pathogens that can induce a downregulation of the MHC-II expression induced by IFN-γ on macrophages may hinder the recognition of infected cells by specific T lymphocytes, thus evading some adaptive immune responses. Interestingly, Brucella has been reported to persist for a long time in the lungs of infected mice (12), a phenomenon that can be speculated to be due, at least in part, to the ability of the bacterium to evade the immune recognition of infected AM. Of note, it has been previously shown that B. abortus infection can downregulate the IFN-γ-induced expression of MHC-II molecules in human monocytes, leading to a reduced antigen-specific T cell proliferation (17). Against this background, we decided to evaluate whether Brucella infection can also modulate MHC-II expression and antigen presentation in AM. We found that infection of AM with B. abortus reduced in an MOI-dependent manner the expression of MHC-II molecules induced by IFN-γ. These findings agree not only with our previous studies in human monocytes but also with results from other groups showing that M. tuberculosis-infected AM have decreased MHC class II molecule expression and decreased antigen presentation to specific CD4+ T cells (30).
Of note, it has been shown that the effects of Brucella on MHC-II expression and antigen presentation mentioned above do not require viable bacteria and can be similarly induced by HKBA and also by L-Omp19, an outer membrane lipoprotein, in both cases through TLR2-mediated recognition (17). Similarly, it has been reported that viable M. tuberculosis is not required for inhibition of macrophage MHC class II expression, which can be achieved by exposure of macrophages to M. tuberculosis lysate and also to M. tuberculosis lipoproteins, which are recognized by TLR2 (52). In line with those previous reports, we found that MHC-II downregulation was induced in AM by incubation with HKBA or L-Omp19, and that for both stimulants the effect was mediated by TLR2 recognition. Moreover, we found that the presence of either L-Omp19 or HKBA during IFN-γ stimulation reduced significantly the ability of AM to activate an MHC-II-restricted OVA-specific T cell hybridoma. These results strongly suggest that AM infected with Brucella remain undetected by specific T cells, ensuring the long-term survival of such cells and, consequently, the long-term persistence of the bacterium in the lung. The absence of a significant cytotoxic effect of Brucella on AM may also contribute to the maintenance of these cells as a long-term reservoir of bacteria.
The results obtained in the present study indicate that TLR2 recognition is involved both in the cytokine/chemokine response of AM to Brucella infection and also in the downregulation of MHC-II expression and antigen presentation induced by Brucella and its antigens in AM. This dual effect of TLR2 recognition also has been reported for the interaction of M. tuberculosis (or its antigens) with macrophages. While activation of TLR2 by the 19-kDa lipoprotein of M. tuberculosis induces the killing of intracellular bacteria in both NO-dependent and -independent pathways in murine macrophages and in human monocytes and alveolar macrophages (53), prolonged TLR signaling by M. tuberculosis or its 19-kDa lipoprotein has been shown to inhibit several IFN-γ-induced genes (54). It has been speculated that the latter mechanism provides homeostatic feedback regulation to limit the extent of the induced responses (37). In particular, it has been postulated that the downregulation of antigen presentation is especially pronounced during persistent infection with intravacuolar pathogens that survive microbicidal mechanisms and that can persistently colocalize with TLRs in phagosomes for prolonged TLR signaling (37). Like M. tuberculosis, Brucella can establish a prolonged residence in the phagosomes of tissue macrophages and can display TLR2 agonists (like L-Omp19 and other lipoproteins), probably allowing a chronic stimulation of TLR2 that may lead to the inhibition of MHC-II expression and antigen presentation.
As mentioned, the TLR2-mediated recognition of Brucella antigens induces the production of proinflammatory cytokines, which may favor bacterial clearance, but also the downregulation of MHC-II, which may favor bacterial persistence. The net effect of TLR2 recognition on Brucella survival in AM probably results from a balance between these two TLR2-mediated phenomena. Notably, we detected viable B. abortus in AM of mice that had been infected 1 week earlier through the intratracheal route, indicating the ability of B. abortus to survive in vivo for a prolonged postinfection time within these cells. However, bacterial counts were significantly higher in AM from TLR2 KO mice, indicating the involvement of TLR2-mediated inflammation in the control of lung infection by B. abortus. A previous study in intranasally infected mice has shown that Brucella-infected AM migrate to the draining lymph node, where they are detected as early as 1.5 days postinfection (11). The fact that we detected Brucella-infected AM in the lungs at 1 week p.i. suggests that, upon infection, some infected AM migrate early to lymph nodes, whereas others remain in the lung.
Collectively, these results suggest that B. abortus survives in AM after inhalatory infection in spite of a certain degree of immune control exerted by the TLR2-mediated inflammatory response. Both the modest nature of the latter and the modulation of MHC-II expression by the bacterium may contribute to such survival. Further studies will be needed to determine whether AM contribute to the reported persistence of brucellae in the lungs or to a late dissemination of the bacterium from the lungs to distal tissues.
ACKNOWLEDGMENTS
We are grateful to Ignacio Moriyón (University of Navarra, Spain) for providing LPS from B. abortus and E. coli. We thank the staff of the Centro Nacional de Referencia del Sida, University of Buenos Aires, for their assistance with biosafety level 3 laboratory use.
This work was supported by grants 2006-1335 and 2008-0764 from Agencia Nacional de Promoción Científica (ANPCYT) and grant UBACYT B015 and 20020090100083 from Universidad de Buenos Aires. The work was also supported by grants from CNPq, FAPEMIG, FAPEMIG (PRONEX), CAPES (PNPD), INCT-Vacinas, CNPq/FAPEMIG (REPENSA), CNPq/ANPCyt (490528/2008-2), and CNPq/CONICET (490398/2010-3) to S.C.O. M.C.F. is the recipient of a fellowship from Consejo Nacional de Investigaciones Científicas (CONICET). P.B., G.H.G., and P.C.B. are members of the Research Career of CONICET.
Footnotes
Published ahead of print 25 November 2013
REFERENCES
- 1.Pappas G, Panagopoulou P, Christou L, Akritidis N. 2006. Brucella as a biological weapon. Cell. Mol. Life Sci. 63:2229–2236. 10.1007/s00018-006-6311-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. 2006. The new global map of human brucellosis. Lancet Infect. Dis. 6:91–99. 10.1016/S1473-3099(06)70382-6 [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann AF, Fox MD, Boyce JM, Anderson DC, Potter ME, Martone WJ, Patton CM. 1980. Airborne spread of brucellosis. Ann. N. Y. Acad. Sci. 353:105–114. 10.1111/j.1749-6632.1980.tb18912.x [DOI] [PubMed] [Google Scholar]
- 4.Olle-Goig JE, Canela-Soler J. 1987. An outbreak of Brucella melitensis infection by airborne transmission among laboratory workers. Am. J. Public Health 77:335–338. 10.2105/AJPH.77.3.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallach JC, Samartino LE, Efron A, Baldi PC. 1997. Human infection by Brucella melitensis: an outbreak attributed to contact with infected goats. FEMS Immunol. Med. Microbiol. 19:315–321. 10.1016/S0928-8244(97)00098-9 [DOI] [PubMed] [Google Scholar]
- 6.Yagupsky P, Baron EJ. 2005. Laboratory exposures to brucellae and implications for bioterrorism. Emerg. Infect. Dis. 11:1180–1185. 10.3201/eid1108.041197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawai T, Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11:373–384. 10.1038/ni.1863 [DOI] [PubMed] [Google Scholar]
- 8.Oliveira SC, de Oliveira FS, Macedo GC, de Almeida LA, Carvalho NB. 2008. The role of innate immune receptors in the control of Brucella abortus infection: toll-like receptors and beyond. Microbes Infect. 10:1005–1009. 10.1016/j.micinf.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 9.Ferrero MC, Fossati CA, Baldi PC. 2010. Direct and monocyte-induced innate immune response of human lung epithelial cells to Brucella abortus infection. Microbes Infect. 12:736–747. 10.1016/j.micinf.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 10.Ferrero MC, Fossati CA, Baldi PC. 2009. Smooth Brucella strains invade and replicate in human lung epithelial cells without inducing cell death. Microbes Infect. 11:476–483. 10.1016/j.micinf.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 11.Archambaud C, Salcedo SP, Lelouard H, Devilard E, de Bovis B, Van Rooijen N, Gorvel JP, Malissen B. 2010. Contrasting roles of macrophages and dendritic cells in controlling initial pulmonary Brucella infection. Eur. J. Immunol. 40:3458–3471. 10.1002/eji.201040497 [DOI] [PubMed] [Google Scholar]
- 12.Kahl-McDonagh MM, Arenas-Gamboa AM, Ficht TA. 2007. Aerosol infection of BALB/c mice with Brucella melitensis and Brucella abortus and protective efficacy against aerosol challenge. Infect. Immun. 75:4923–4932. 10.1128/IAI.00451-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elzer PH, Phillips RW, Robertson GT, Roop RM., Jr 1996. The HtrA stress response protease contributes to resistance of Brucella abortus to killing by murine phagocytes. Infect. Immun. 64:4838–4841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jimenez de Bagues MP, Dudal S, Dornand J, Gross A. 2005. Cellular bioterrorism: how Brucella corrupts macrophage physiology to promote invasion and proliferation. Clin. Immunol. 114:227–238. 10.1016/j.clim.2004.07.010 [DOI] [PubMed] [Google Scholar]
- 15.Celli J. 2006. Surviving inside a macrophage: the many ways of Brucella. Res. Microbiol. 157:93–98. 10.1016/j.resmic.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 16.Roop RM, Jr, Gaines JM, Anderson ES, Caswell CC, Martin DW. 2009. Survival of the fittest: how Brucella strains adapt to their intracellular niche in the host. Med. Microbiol. Immunol. 198:221–238. 10.1007/s00430-009-0123-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrionuevo P, Cassataro J, Delpino MV, Zwerdling A, Pasquevich KA, Garcia Samartino C, Wallach JC, Fossati CA, Giambartolomei GH. 2008. Brucella abortus inhibits major histocompatibility complex class II expression and antigen processing through interleukin-6 secretion via Toll-like receptor 2. Infect. Immun. 76:250–262. 10.1128/IAI.00949-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ettensohn DB, Roberts NJ., Jr 1984. Influenza virus infection of human alveolar and blood-derived macrophages: differences in accessory cell function and interferon production. J. Infect. Dis. 149:942–949. 10.1093/infdis/149.6.942 [DOI] [PubMed] [Google Scholar]
- 19.Hawn TR, Berrington WR, Smith IA, Uematsu S, Akira S, Aderem A, Smith KD, Skerrett SJ. 2007. Altered inflammatory responses in TLR5-deficient mice infected with Legionella pneumophila. J. Immunol. 179:6981–6987 [DOI] [PubMed] [Google Scholar]
- 20.Loving CL, Brockmeier SL, Ma W, Richt JA, Sacco RE. 2006. Innate cytokine responses in porcine macrophage populations: evidence for differential recognition of double-stranded RNA. J. Immunol. 177:8432–8439 [DOI] [PubMed] [Google Scholar]
- 21.Salez L, Singer M, Balloy V, Creminon C, Chignard M. 2000. Lack of IL-10 synthesis by murine alveolar macrophages upon lipopolysaccharide exposure. Comparison with peritoneal macrophages. J. Leukoc. Biol. 67:545–552 [DOI] [PubMed] [Google Scholar]
- 22.van Riel D, Leijten LM, van der Eerden M, Hoogsteden HC, Boven LA, Lambrecht BN, Osterhaus AD, Kuiken T. 2011. Highly pathogenic avian influenza virus H5N1 infects alveolar macrophages without virus production or excessive TNF-alpha induction. PLoS Pathog. 7:e1002099. 10.1371/journal.ppat.1002099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu WC, Chan RW, Wang J, Travanty EA, Nicholls JM, Peiris JS, Mason RJ, Chan MC. 2011. Viral replication and innate host responses in primary human alveolar epithelial cells and alveolar macrophages infected with influenza H5N1 and H1N1 viruses. J. Virol. 85:6844–6855. 10.1128/JVI.02200-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapetanovic R, Parlato M, Fitting C, Quesniaux V, Cavaillon JM, Adib-Conquy M. 2011. Mechanisms of TNF induction by heat-killed Staphylococcus aureus differ upon the origin of mononuclear phagocytes. Am. J. Physiol. Cell Physiol. 300:C850–859. 10.1152/ajpcell.00187.2010 [DOI] [PubMed] [Google Scholar]
- 25.Surewicz K, Aung H, Kanost RA, Jones L, Hejal R, Toossi Z. 2004. The differential interaction of p38 MAP kinase and tumor necrosis factor-alpha in human alveolar macrophages and monocytes induced by Mycobacterium tuberculosis. Cell. Immunol. 228:34–41. 10.1016/j.cellimm.2004.03.007 [DOI] [PubMed] [Google Scholar]
- 26.Laskin DL, Weinberger B, Laskin JD. 2001. Functional heterogeneity in liver and lung macrophages. J. Leukoc. Biol. 70:163–170 [PubMed] [Google Scholar]
- 27.Kirby AC, Coles MC, Kaye PM. 2009. Alveolar macrophages transport pathogens to lung draining lymph nodes. J. Immunol. 183:1983–1989. 10.4049/jimmunol.0901089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giambartolomei GH, Zwerdling A, Cassataro J, Bruno L, Fossati CA, Philipp MT. 2004. Lipoproteins, not lipopolysaccharide, are the key mediators of the proinflammatory response elicited by heat-killed Brucella abortus. J. Immunol. 173:4635–4642 [DOI] [PubMed] [Google Scholar]
- 29.Velasco J, Bengoechea JA, Brandenburg K, Lindner B, Seydel U, Gonzalez D, Zahringer U, Moreno E, Moriyón I. 2000. Brucella abortus and its closest phylogenetic relative, Ochrobactrum spp., differ in outer membrane permeability and cationic peptide resistance. Infect. Immun. 68:3210–3218. 10.1128/IAI.68.6.3210-3218.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fulton SA, Reba SM, Pai RK, Pennini M, Torres M, Harding CV, Boom WH. 2004. Inhibition of major histocompatibility complex II expression and antigen processing in murine alveolar macrophages by Mycobacterium bovis BCG and the 19-kilodalton mycobacterial lipoprotein. Infect. Immun. 72:2101–2110. 10.1128/IAI.72.4.2101-2110.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S. 1999. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189:371–380. 10.1084/jem.189.2.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Revelli DA, Boylan JA, Gherardini FC. 2012. A non-invasive intratracheal inoculation method for the study of pulmonary melioidosis. Front. Cell. Infect. Microbiol. 2:164. 10.3389/fcimb.2012.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volf J, Havlickova H, Hradecka H, Ondrackova P, Matiasovic J, Faldyna M, Rychlik I. 2010. Epidemiology and interaction of Salmonella enterica serovar Derby, Infantis and Typhimurium with porcine alveolar macrophages. Vet. Microbiol. 146:105–110. 10.1016/j.vetmic.2010.04.031 [DOI] [PubMed] [Google Scholar]
- 34.Pecora ND, Fulton SA, Reba SM, Drage MG, Simmons DP, Urankar-Nagy NJ, Boom WH, Harding CV. 2009. Mycobacterium bovis BCG decreases MHC-II expression in vivo on murine lung macrophages and dendritic cells during aerosol infection. Cell. Immunol. 254:94–104. 10.1016/j.cellimm.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrionuevo P, Delpino MV, Velasquez LN, Garcia Samartino C, Coria LM, Ibanez AE, Rodriguez ME, Cassataro J, Giambartolomei GH. 2011. Brucella abortus inhibits IFN-gamma-induced FcgammaRI expression and FcgammaRI-restricted phagocytosis via toll-like receptor 2 on human monocytes/macrophages. Microbes Infect. 13:239–250. 10.1016/j.micinf.2010.10.020 [DOI] [PubMed] [Google Scholar]
- 36.Chu RS, Askew D, Noss EH, Tobian A, Krieg AM, Harding CV. 1999. CpG oligodeoxynucleotides down-regulate macrophage class II MHC antigen processing. J. Immunol. 163:1188–1194 [PubMed] [Google Scholar]
- 37.Harding CV, Boom WH. 2010. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat. Rev. Microbiol. 8:296–307. 10.1038/nrmicro2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campos MA, Rosinha GM, Almeida IC, Salgueiro XS, Jarvis BW, Splitter GA, Qureshi N, Bruna-Romero O, Gazzinelli RT, Oliveira SC. 2004. Role of Toll-like receptor 4 in induction of cell-mediated immunity and resistance to Brucella abortus infection in mice. Infect. Immun. 72:176–186. 10.1128/IAI.72.1.176-186.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delpino MV, Barrionuevo P, Macedo GC, Oliveira SC, Di Genaro S, Scian R, Miraglia MC, Fossati CA, Baldi PC, Giambartolomei GH. 2012. Macrophage-elicited osteoclastogenesis in response to Brucella abortus infection requires TLR2/MyD88-dependent TNF-alpha production. J. Leukoc. Biol. 91:285–298. 10.1189/jlb.04111185 [DOI] [PubMed] [Google Scholar]
- 40.Fernandez-Lago L, Rodriguez-Tarazona E, Vizcaino N. 1999. Differential secretion of interleukin-12 (IL-12) subunits and heterodimeric IL-12p70 protein by CD-1 mice and murine macrophages in response to intracellular infection by Brucella abortus. J. Med. Microbiol. 48:1065–1073. 10.1099/00222615-48-12-1065 [DOI] [PubMed] [Google Scholar]
- 41.Gross A, Spiesser S, Terraza A, Rouot B, Caron E, Dornand J. 1998. Expression and bactericidal activity of nitric oxide synthase in Brucella suis-infected murine macrophages. Infect. Immun. 66:1309–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macedo GC, Magnani DM, Carvalho NB, Bruna-Romero O, Gazzinelli RT, Oliveira SC. 2008. Central role of MyD88-dependent dendritic cell maturation and proinflammatory cytokine production to control Brucella abortus infection. J. Immunol. 180:1080–1087 [DOI] [PubMed] [Google Scholar]
- 43.Zhan Y, Cheers C. 1995. Differential induction of macrophage-derived cytokines by live and dead intracellular bacteria in vitro. Infect. Immun. 63:720–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mense MG, Borschel RH, Wilhelmsen CL, Pitt ML, Hoover DL. 2004. Pathologic changes associated with brucellosis experimentally induced by aerosol exposure in rhesus macaques (Macaca mulatta). Am. J. Vet. Res. 65:644–652. 10.2460/ajvr.2004.65.644 [DOI] [PubMed] [Google Scholar]
- 45.Yingst SL, Huzella LM, Chuvala L, Wolcott M. 2010. A rhesus macaque (Macaca mulatta) model of aerosol-exposure brucellosis (Brucella suis): pathology and diagnostic implications. J. Med. Microbiol. 59:724–730. 10.1099/jmm.0.017285-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Covert J, Mathison AJ, Eskra L, Banai M, Splitter G. 2009. Brucella melitensis, B. neotomae and B. ovis elicit common and distinctive macrophage defense transcriptional responses. Exp. Biol. Med. (Maywood) 234:1450–1467. 10.3181/0904-RM-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.García Samartino C, Delpino MV, Pott Godoy C, Di Genaro MS, Pasquevich KA, Zwerdling A, Barrionuevo P, Mathieu P, Cassataro J, Pitossi F, Giambartolomei GH. 2010. Brucella abortus induces the secretion of proinflammatory mediators from glial cells leading to astrocyte apoptosis. Am. J. Pathol. 176:1323–1338. 10.2353/ajpath.2010.090503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhan Y, Liu Z, Cheers C. 1996. Tumor necrosis factor alpha and interleukin-12 contribute to resistance to the intracellular bacterium Brucella abortus by different mechanisms. Infect. Immun. 64:2782–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooper AM, Solache A, Khader SA. 2007. Interleukin-12 and tuberculosis: an old story revisited. Curr. Opin. Immunol. 19:441–447. 10.1016/j.coi.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss DS, Takeda K, Akira S, Zychlinsky A, Moreno E. 2005. MyD88, but Not Toll-Like Receptors 4 and 2, Is Required for Efficient Clearance of Brucella abortus. Infect. Immun. 73:5137–5143. 10.1128/IAI.73.8.5137-5143.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inoue K, Takano H, Yanagisawa R, Hirano S, Kobayashi T, Fujitani Y, Shimada A, Yoshikawa T. 2007. Effects of inhaled nanoparticles on acute lung injury induced by lipopolysaccharide in mice. Toxicology 238:99–110. 10.1016/j.tox.2007.05.022 [DOI] [PubMed] [Google Scholar]
- 52.Gehring AJ, Rojas RE, Canaday DH, Lakey DL, Harding CV, Boom WH. 2003. The Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits gamma interferon-regulated HLA-DR and Fc gamma R1 on human macrophages through Toll-like receptor 2. Infect. Immun. 71:4487–4497. 10.1128/IAI.71.8.4487-4497.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, Barnes PF, Rollinghoff M, Bolcskei PL, Wagner M, Akira S, Norgard MV, Belisle JT, Godowski PJ, Bloom BR, Modlin RL. 2001. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science 291:1544–1547. 10.1126/science.291.5508.1544 [DOI] [PubMed] [Google Scholar]
- 54.Pai RK, Pennini ME, Tobian AA, Canaday DH, Boom WH, Harding CV. 2004. Prolonged toll-like receptor signaling by Mycobacterium tuberculosis and its 19-kilodalton lipoprotein inhibits gamma interferon-induced regulation of selected genes in macrophages. Infect. Immun. 72:6603–6614. 10.1128/IAI.72.11.6603-6614.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]