Abstract
Hepcidin, the iron-regulatory hormone, is increased during infection or inflammation, causing hypoferremia. This response is thought to be a host defense mechanism that restricts iron availability to invading pathogens. It is not known if hepcidin is differentially induced by bacterial versus viral infections, whether the stimulation of pattern recognition receptors directly regulates hepcidin transcription, or which of the proposed signaling pathways are essential for hepcidin increase during infection. We analyzed hepcidin induction and its dependence on interleukin-6 (IL-6) in response to common bacterial or viral infections in mice or in response to a panel of pathogen-derived molecules (PAMPs) in mice and human primary hepatocytes. In wild-type (WT) mice, hepcidin mRNA was induced several hundred-fold both by a bacterial (Streptococcus pneumoniae) and a viral infection (influenza virus PR8) within 2 to 5 days. Treatment of mice and human primary hepatocytes with most Toll-like receptor ligands increased hepcidin mRNA within 6 h. Hepcidin induction by microbial stimuli was IL-6 dependent. IL-6 knockout mice failed to increase hepcidin in response to S. pneumoniae or influenza infection and had greatly diminished hepcidin response to PAMPs. In vitro, hepcidin induction by PAMPs in primary human hepatocytes was abolished by the addition of neutralizing IL-6 antibodies. Our results support the key role of IL-6 in hepcidin regulation in response to a variety of infectious and inflammatory stimuli.
INTRODUCTION
Iron is essential for microbial growth. During infections, microbes use multiple complex mechanisms to acquire iron from their host, while hosts resist infection by sequestering iron in forms and locations less accessible to microbes, thereby starving them of iron and slowing their multiplication within the host (1). The iron-regulatory hormone hepcidin is thought to be an important orchestrator of this host response.
Hepcidin, a 25-amino-acid peptide hormone secreted by hepatocytes, is the principal regulator of iron homeostasis (2). Hepcidin regulates the absorption and tissue distribution of iron in response to plasma iron concentration, iron stores, erythropoietic demand, and inflammation. Its molecular target, ferroportin, is the sole mammalian cellular iron exporter (3) and is expressed on the membranes of cells that deliver iron to the plasma: duodenal enterocytes, which absorb dietary iron, macrophages, which recycle iron from senescent erythrocytes, and hepatocytes, which serve as an iron storage site. Hepcidin acts by binding to ferroportin and inducing its internalization and degradation (4), thereby diminishing iron supply to the plasma and causing hypoferremia. Hepcidin production is greatly increased during infections, which is thought to decrease iron concentrations available to extracellular microbes.
Previous reports provided evidence that hepcidin is induced during various infections. Intraperitoneal Pseudomonas aeruginosa injection and subcutaneous group A streptococcus infections induced liver hepcidin mRNA in mice (5). C. albicans and influenza A virus infections increased hepcidin mRNA in peripheral blood mononuclear cells (PBMCs) and in mice (6). Hepcidin levels were also found to be increased in malarial infections in mice and humans (7, 8).
Several mechanisms have been proposed to increase hepcidin during infection and inflammation. The cytokines interleukin-6 (IL-6) (9, 10), IL-1 (11), and IL-22 (6) stimulate hepcidin transcription through STAT3 signaling (12–14). Type I interferons were also reported to increase hepcidin via STAT1 or STAT3 in vitro (15–17). Activin B was proposed to mediate inflammatory increase in hepcidin mRNA via SMAD1/5/8 signaling (18). These observations point to the importance of STAT as well as BMP/Smad pathways in the regulation of hepcidin during infections. It is not yet clear to what extent each of these pathways contribute to hepcidin mRNA response to diverse infections in vivo.
Innate immune responses serve as the first line of host defense against pathogens. Early responses to invading microbes are triggered by the interaction of pattern recognition receptors (PRR) with pathogen-specific microbial molecules, such as lipopolysaccharide, peptidoglycan, flagellin, and double-stranded RNA (dsRNA), commonly referred to as pathogen associated molecular patterns (PAMPs) (19). Recognition of PAMPs by PRRs induces signaling and transcriptional responses, including the production of inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), IL-1, IL-6, and type I interferons (IFN). Selected PAMPs were shown to stimulate hepcidin production in hepatic cells, peripheral blood mononuclear cells (PBMCs), or mice (6, 10, 20), but this response and its dependence on signaling pathways has not been systematically analyzed.
In this study, we investigated the hepcidin response and its IL-6 dependence using mouse models of common community-acquired bacterial and viral infections. We also examined IL-6 dependence of hepcidin induction by a large panel of microbial molecules in mice and in isolated human primary hepatocytes.
MATERIALS AND METHODS
Mice.
All animals were housed in UCLA vivaria under pathogen-free conditions throughout the experiment. All animal experiments were performed in accordance with NIH policies regarding the humane care and use of laboratory animals and were approved by the UCLA Animal Research Committee. Six-week-old wild-type (WT) C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). IL-6−/− (IL-6 knockout [KO]) mice on a C57BL/6 background were bred in our rodent facility (original stock was obtained from The Jackson Laboratory, Bar Harbor, ME, USA) (10) and were used for experiments at the same age as the WT mice. Only male mice were used to minimize the gender-related variability in hepcidin and iron parameters (21).
As standard mouse chow contains high iron content (∼300 ppm) and causes near-maximal hepcidin expression (10), mice at 6 weeks of age were placed on a low-iron diet (4 ppm; Harlan Teklad, Indianapolis, IN) for 2 weeks to lower endogenous hepcidin expression. This period of iron limitation leads to suppression of endogenous hepcidin, a small decrease in mean corpuscular volume (MCV), and no iron deficiency anemia (MCV [fl], 45.4 ± 0.8 [4 ppm] versus 46.5 ± 0.9 [standard diet], P = 0.01; hemoglobin [g/dl], 14.8 ± 0.6 [4 ppm] versus 14.7 ± 1.0 [standard diet], no significant difference; n = 12 per group; values represent means ± standard deviations). Interestingly, IL-6 knockout mice had a more variable suppression of hepcidin baseline on the 4-ppm Fe diet than WT mice for an unknown reason.
Bacterial and viral pathogens and their administration.
The type 3 Streptococcus pneumoniae (ATCC 6303 clinical isolate with capsular serotype 3) strain used in our studies was provided by Jane Deng (22). This serotype was chosen because it is virulent in mice and commonly causes human disease. Frozen bacterial stocks were grown in Todd-Hewitt broth (Sigma, St. Louis, MO) with 0.5% yeast extract at 37°C until log phase (optical density [OD], ∼0.3). The concentration of bacteria in broth was determined by absorbance at 600 nm and using a standard curve generated by known CFU concentrations. The bacterial culture then was centrifuged at 3,000 × g and diluted in sterile, endotoxin-free phosphate-buffered saline (PBS) to the desired concentration.
Frozen stocks of mouse-adapted influenza A virus PR8 (22) were thawed quickly and diluted in sterile, endotoxin-free PBS to the desired concentration.
Mice were anesthetized with isoflurane, followed by oropharyngeal aspiration of 100 μl sterile PBS containing either 1 × 104 or 5 × 104 CFU S. pneumoniae, 100 or 500 PFU of PR8, or PBS alone. Mice were suspended vertically by the upper incisors on a board with a 45° incline. The tongue was pulled with a blunted forceps gently from the mouth for maximal oropharyngeal area exposure, and 100 μl fluid was delivered to the glottic area with a pipette tip, resulting in the aspiration of the liquid. In preliminary experiments, the delivery of material to the lungs and not the stomach was confirmed using an indicator dye. For every S. pneumoniae experiment, a 100× dilution of the lowest dose (104 CFU) was plated on blood agar to ensure that microbes were viable and to confirm the administered CFU count. Furthermore, successful in vivo infection was confirmed by observing bacterial growth on blood agar plated with blood from control and treatment mice at the time of sacrifice. For all infected mice, animal weight was measured daily as another indicator of illness.
Mice were euthanized 2 or 5 days after infection. Liver samples were obtained for hepcidin mRNA measurements.
Human primary hepatocytes and Kupffer cells.
Fresh human primary hepatocytes (HH) and nonparenchymal cells were obtained from the Liver Tissue Procurement and Distribution System (Stephen Strom, University of Pittsburgh). Human hepatocytes were maintained in hepatocyte maintenance medium (HMM; Lonza, Walkersville, MD). Kupffer cells were isolated from the nonparenchymal fraction and maintained in Iscove's modified Dulbecco's medium (IMDM) plus 10% fetal calf serum (10). To prepare conditioned medium (CM), Kupffer cells were treated will Toll-like receptor (TLR) ligands for 24 h and supernatant was harvested.
Human hepatocytes were stimulated with PAMPs or with a 1/8 dilution of CM (12.5% final concentration) for 6 h, and cells were harvested for hepcidin mRNA measurements.
PAMPs and cytokines.
Agonists for TLRs and NOD-like receptors (NLRs) were purchased from InvivoGen (San Diego, CA) and are listed in Table 1.
TABLE 1.
List of PAMPs and their concentrations used in experiments
| TLR/NLR liganda | Target TLR/NLR | In vivo dose (μg/mouse) (reference) | In vitro dose (μg/ml) |
|---|---|---|---|
| Pam3CSK4 | TLR1/2 | 100 (32, 33) | 2 |
| FSL–1 | TLR2/6 | 20 (34) | 1 |
| LPS (E. coli 0111:B4) | TLR4 | 25 (35) | 1 |
| Flagellin (B. subtilis) | TLR5 | 30 (36) | 1 |
| Poly(I·C) HMW | TLR3 | 500 (37, 38) | 10 |
| Imiquimod (R837) | TLR7 | 25 (39) | 5 |
| ODN2006 | TLR9 | 100 (40, 41) | 5 |
| Tri-DAP | NOD1 | 80 (42) | 10 |
| MDP | NOD2 | 500 (43) | 10 |
HMW, high molecular weight.
For in vivo experiments, WT and IL-6 KO mice were injected intraperitoneally (i.p.) with compounds diluted in 100 μl sterile water at commonly used concentrations (Table 1). The mice were euthanized 6 or 24 h later.
For in vitro experiments, human hepatocytes and Kupffer cells were stimulated for 6 h with TLR/NLR agonists using concentrations listed in Table 1 and based on references 23 to 25. The treatment was performed in the presence or absence of anti-human IL-6 (1 μg/ml) and anti-human IL-6 receptor antibodies (5 μg/ml) (R&D Biosystems, Minneapolis, MN). Cells were preincubated with antibodies for 10 min prior to treatment with PAMPs. Cells were also treated with 10 ng/ml IL-6 or 25 ng/ml BMP-2 (R&D Biosystems) (26) to confirm their responsiveness to standard hepcidin stimuli.
Isolation of mRNA and quantitative reverse transcription-PCR (qRT-PCR).
Total cellular RNA from mouse livers and HH was extracted by TRIzol (Invitrogen) by following the manufacturer's protocol and reverse transcribed into cDNA using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Quantitative PCR (qPCR) was performed using Bio-Rad IQ SYBR green supermix (Bio-Rad). All values were normalized against β-actin (ΔCT = CT [actin] − CT [hepcidin]). Fold change in expression was calculated as 2ΔΔCT, where ΔΔCT = ΔCT (test sample) − ΔCT (control). When identical experiments were conducted on different days, each experiment had its separate saline control group and ΔΔCT was calculated using only contemporaneous controls.
The primer sequences for the genes examined were the following: human Hamp, forward, 5′-GACCAGTGGCTCTGTTTTCC-3′; reverse, 5′-AGATGGGGAAGTGGGTGTCT-3′; human β-actin, forward, 5′-ATCGTGCGTGACATTAAG-3′; reverse, 5′-ATTGCCAATGGTGATGAC-3′; mouse Hamp, forward, 5′-AAGCAGGGGCAGACATTGCGAT-3′; reverse, 5′-CAGGATGTGGCTCTAGGCTAT-3′; mouse β-actin, forward, 5′-ACCCACACTGTGCCCATCTA-3′; reverse, 5′-CACGCTCGGTCAGGATCTTC-3′.
Statistics.
SigmaPlot version 11.0 (Systat Software) was used for statistical analysis. Data are expressed as means ± standard deviation of the means for each group. Differences between groups were determined by one-way analysis of variance (ANOVA), and differences between two groups were determined by t test.
RESULTS
IL-6 is required for induction of hepcidin by Streptococcus pneumoniae infection.
As a model of bacterial infection, we used one of the most common bacterial pneumonias, that caused by Streptococcus pneumoniae. The specific doses used and time points for analysis were established in preliminary experiments where we monitored for signs of illness, such as weight loss, decreased mobility and grooming behavior, and mortality. We tested a range of doses, 1 × 103 to 1 × 106 CFU per mouse, and found that mortality was high, above 1 × 105 CFU, whereas clinical signs of infection were mild or absent at 1 × 103 CFU; thus, we selected 1 × 104 and 5 × 104 CFU for our studies. As clinical signs of infection were noted as early as 2 days and peak illness at 5 days, these time points were selected for analysis.
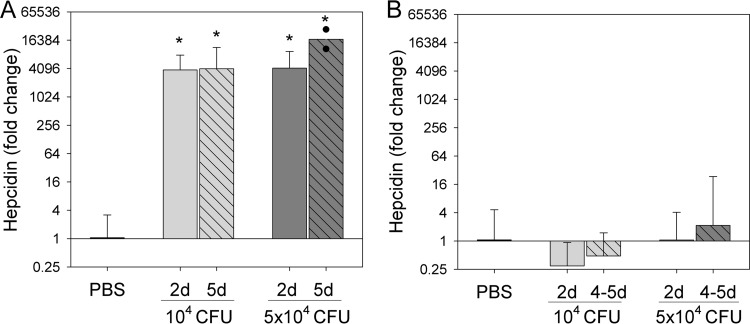
Oropharyngeal administration of both 1 × 104 and 5 × 104 CFU S. pneumoniae in WT mice caused significant and progressive weight loss up to day 5 compared to the PBS group (see Table S1 in the supplemental material). Both doses of bacteria also massively induced hepcidin mRNA in the liver at days 2 and 5 (10 to 15 ΔCT, or ∼1,000 to 15,000-fold; P < 0.001) (Fig. 1A). The fold increase in hepcidin during inflammation greatly depends on the level of hepcidin baseline expression. Mice in our study were placed on a low-iron diet for 2 weeks prior to the infection, which caused a significant reduction of hepcidin baseline and allowed us to demonstrate the large increase in hepcidin mRNA after infection. However, when mice are fed standard chow, the relatively high iron content of this diet (∼300 ppm Fe) results in high (near-maximal) hepcidin baseline expression so that inflammatory stimuli cause no or only a small increase in hepcidin expression (10, 18).
FIG 1.
IL-6 is required for induction of hepcidin by Streptococcus pneumoniae infection. WT (A) or IL-6 KO (B) mice were infected with 104 or 5 × 104 CFU of S. pneumoniae, and liver hepcidin mRNA was measured 2 and 5 days after infection. (A) WT mice. For the PBS group, n = 10 (vertical bar includes animals from both days 2 and 5 as their hepcidin expression was indistinguishable); 104-CFU group, n = 6 (day 2) and 4 (day 5); 5 × 104-CFU group, n = 7 (day 2) and 2 (day 5). Survival on day 5 was 4/8 mice for the 104-CFU group and 2/8 mice for the 5 × 104-CFU group. (B) IL-6 KO mice. PBS group, n = 12 (includes animals from both days 2 and 5); 104-CFU group, n = 6 (day 2) and 3 (days 4 to 5); 5 × 104-CFU group, n = 7 (day 2) and 7 (days 4 to 5). Survival on day 5 was 1/12 mice for the 104-CFU group (2 were euthanized on day 4) and 2/12 for the 5 × 104-CFU group (5 were euthanized on day 4). Because of the high mortality rate in IL-6 KO mice, hepcidin values from both days 4 and 5 were combined. Hepcidin expression is shown as fold change compared to the PBS group. Expression was calculated as 2ΔΔCT (where ΔΔCT = ΔCT [test sample] − ΔCT [contemporaneous saline control]). Vertical bars indicate means, and error bars indicate standard deviations. *, P < 0.001 by one-way ANOVA compared to the PBS group as the control.
Infected IL-6 KO mice experienced weight loss similar to that of WT mice by day 5 (P = 0.26 compared to the infected WT mouse weight loss). However, in contrast to WT mice, IL-6 KO mice infected with S. pneumoniae did not increase hepcidin mRNA (Fig. 1B).
By day 5, we observed 92% and 83% mortality in IL-6 KO mice infected with 104 and 5 × 104 CFU SP, respectively, compared to 50% and 75% for WT mice (proportions were not statistically different between IL-6 and WT mice by Fischer exact test).
IL-6 is required for the induction of hepcidin by influenza A virus (PR8) infection.
As a model of viral infection, we used pneumonia caused by the mouse-adapted influenza A virus (PR8) (22). The doses used in the study were established in preliminary experiments where a range of PR8 doses was tested (100, 500, 1,000 and 2,000 PFU). One thousand and 2,000 PFU caused high mortality, whereas 100 and 500 PFU per mouse resulted in weight loss and sickness behavior similar to the S. pneumoniae infection, although with a slightly lower rate of onset of clinical signs of infection. This is expected, as bacterial pneumonia in mice is known to have a more rapid onset than viral pneumonia (J. C. Deng, unpublished observations). Based on the preliminary studies, the 100- and 500-PFU doses were selected for investigating the hepcidin response to PR8 infection.
WT and IL-6 KO mice were infected oropharyngeally with PR8 or were administered PBS by the same route (control group). Animals were analyzed after 2 or 5 days, the same time course as that for the S. pneumoniae infection. By day 5, infection resulted in significant weight loss in both WT and IL-6 KO mice (see Table S1 in the supplemental material). Hepcidin mRNA expression in WT mice started increasing by day 2 and reached up to 1,000-fold higher levels than those of control animals.(Fig. 2A). In contrast, IL-6 KO mice infected with PR8 did not significantly induce hepcidin mRNA at any time point (Fig. 2B).
FIG 2.
IL-6 is required for the induction of hepcidin by influenza A virus (PR8) infection. WT (A) or IL-6 KO (B) mice were administered either PBS or PR8 virus (100 or 500 PFU) by the oropharyngeal route. Liver hepcidin mRNA was measured 2 and 5 days after infection and normalized to β-actin mRNA values. (A) WT mice. PBS controls, n = 15 (vertical bar combines animals from both days 2 and 5, as their hepcidin expression was indistinguishable); 100- and 500-PFU groups, n = 8 for each time point. (B) IL-6 KO mice. PBS controls, n = 17 (combines animals from days 2 and 5); 100-PFU group, n = 3 for each time point; 500-PFU group, n = 7 (day 2) and 11 (day 5). Hepcidin expression is shown as fold change compared to the PBS group. Expression was calculated as 2ΔΔCT, where ΔΔCT = ΔCT (test sample) − ΔCT (contemporaneous saline control). Vertical bars indicate means, and error bars indicate standard deviations. P < 0.001 (*) and P = 0.014 (**) by one-way ANOVA compared to the PBS group as the control.
Induction of hepcidin by PAMPs in vivo is strongly dependent on IL-6.
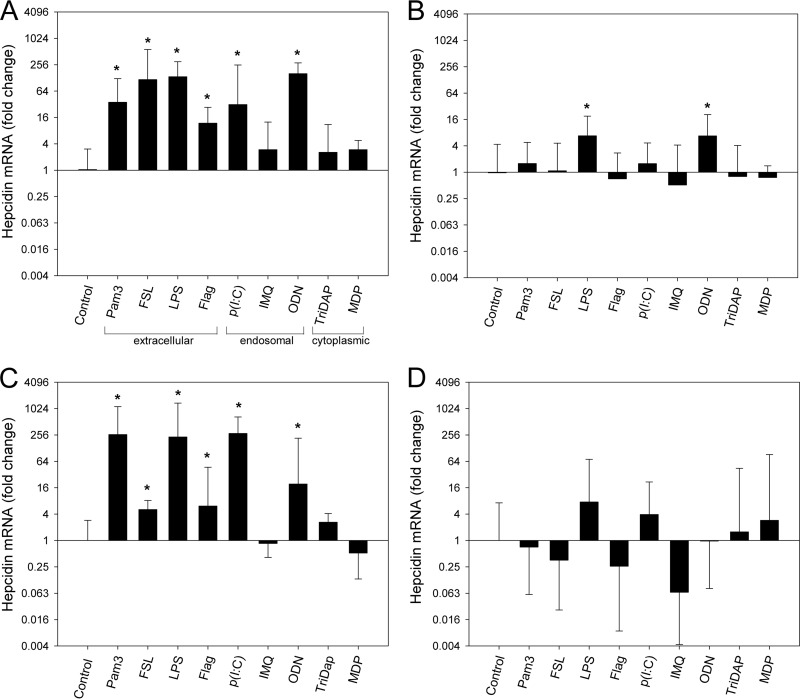
Bacteria and viruses cause inflammation as microbial molecules (PAMPs) are recognized by the host's pattern recognition receptors (PRRs), including TLRs and NLRs. To investigate which of these receptors induce hepcidin when stimulated and whether PRR-mediated hepcidin induction was dependent on IL-6, WT and IL-6 KO mice were injected i.p. with either endotoxin-free water (vehicle) or PAMPs stimulating the following receptors: TLR1/2 (ligand Pam-3), TLR2/6 (ligand FSL-1), TLR4 (ligand lipopolysaccharide [LPS]), TLR5 (ligand flagellin), TLR3 [ligand poly(I·C)], TLR7 (ligand imiquimod), TLR9 (ligand CpG oligonucleotide [ODN]), NOD1 (ligand l-alanyl-γ-d-glutamyl-meso-diaminopimelic acid [Tri-DAP]), and NOD2 (ligand muramyl dipeptide [MDP]). Concentrations used to inject mice are indicated in Table 1. Liver hepcidin mRNA expression was evaluated 6 or 24 h after injection.
In WT mice, hepcidin mRNA expression increased strongly in response to extracellularly sensed PAMPs, i.e., those stimulating TLR1, TLR2, TLR6, TLR4, and TLR5 (the 6-h time point is shown in Fig. 3A and 24-h time point in Fig. 3C). Hepcidin was also induced by stimulation of endosomal receptors TLR3 and TLR9, although TLR3 may also be expressed on the cell membrane (27). Hepcidin was not significantly increased by ligands stimulating endosomal TLR7 or cytoplasmic NOD1 and NOD2 (Fig. 3A and C).
FIG 3.
Induction of hepcidin by PAMPs in vivo requires IL-6. PAMPs were injected intraperitoneally at concentrations listed in Table 1. Graph A indicates where cellular sensing of specific PAMPs occurs. Hepcidin mRNA was quantified by qPCR and normalized to β-actin mRNA values, and the results are shown as fold change in expression compared to the control group (calculated as 2ΔΔCT, where ΔΔCT = ΔCT [test sample] − [ΔCT control]). (A) WT mice 6 h after PAMP injection. n = 4 mice per group. (B) IL-6 KO 6 h after PAMP injection. n = 5 mice for each PAMP and 9 for control. (C) WT mice 24 h after PAMP injection. n = 4 to 8 mice per group. (D) IL-6 KO mice 24 h after PAMP injection. n = 4 to 6 mice per group. Vertical bars and error bars represent the means and standard deviations. *, P < 0.05 by one-way ANOVA compared to the control group. Abbreviations: Pam3, Pam3CSK4; FSL, FSL-1; Flag, flagellin; p(I·C), poly(I·C); IMQ, imiquimod; MDP, muramyl dipeptide.
In IL-6 KO mice, PAMPs either failed to induce hepcidin (Fig. 3B and D, showing 6- and 24-h time points) or, in the case of LPS and ODN, hepcidin induction was significantly blunted compared to WT mice (P = 0.016 for LPS and 0.012 for ODN).
Hepcidin induction by PAMPs in primary human hepatocytes is dependent on IL-6.
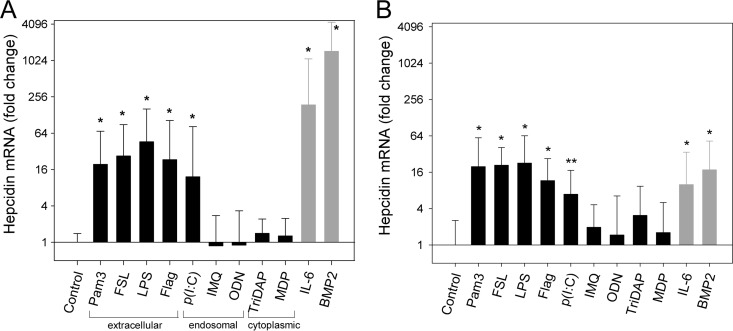
To examine whether the regulation of human hepcidin by PAMPs is similar to that of the mouse model, we asked whether microbial molecules induce hepcidin mRNA directly in primary human hepatocytes (HH). We stimulated HH for 6 h with the same PAMPs as those in the in vivo experiment and observed a similar pattern of hepcidin induction (Fig. 4A). Hepcidin mRNA expression increased by at least 16-fold by extracellularly recognized TLR agonists (those stimulating TLR1, TLR2, TLR6, TLR4, and TLR5) and by the agonist stimulating TLR3, a predominantly endosomal receptor that also may be expressed on the cell surface (27). Intracellularly recognized ligands (those stimulating endosomal TLR7 and TLR9 or cytoplasmic NOD1 and NOD2) failed to induce hepcidin significantly relative to the control (Fig. 4A).
FIG 4.
PAMPs directly and indirectly induce hepcidin expression in primary human hepatocytes. (A) Direct stimulation of human hepatocytes with PAMPs. HH were incubated overnight in serum-free medium and treated for 6 h with the indicated PAMPs, and hepcidin mRNA was analyzed by qPCR. PAMP concentrations are listed in Table 1. The graph indicates where cellular sensing of specific PAMPs occurs. Cells were also treated with known hepcidin inducers recombinant human IL-6 and BMP-2 (rhIL-6 and rhBMP-2, respectively) to confirm the ability of each human hepatocyte preparation to induce hepcidin mRNA (gray bars). Hepcidin mRNA was normalized to β-actin mRNA values, and the results are shown as fold change in expression compared to the control group (calculated as 2ΔΔCT, where ΔΔCT = ΔCT [test sample] − ΔCT [control]). Vertical bars represent means from 5 different hepatocyte preparations, each with two replicates, and error bars represent standard deviations. *, P < 0.001 (by one-way ANOVA with Holm Sidak compared to the control group). (B) Human hepatocytes treated with diluted conditioned medium (CM) from Kupffer cells stimulated with PAMPs. HH were incubated overnight in serum-free medium and treated in duplicate for 6 h with CM (12.5% final concentration), and hepcidin mRNA was analyzed by qPCR as described for panel A. Vertical bars represent the means and standard deviations from 3 different hepatocyte preparations. P < 0.001 (*) and P = 0.015 (**) by one way ANOVA with Holm Sidak compared to the control group. Abbreviations: Pam3, Pam3CSK4; FSL, FSL-1; Flag, flagellin; p(I·C), poly(I·C); IMQ, imiquimod; MDP, muramyl dipeptide.
In vivo, PAMPs would be expected to stimulate hepatocytes not only directly to produce hepcidin but also indirectly via stimulating autocrine/paracrine cytokine production (9). To model indirect stimulation of hepcidin in vitro, we first stimulated human Kupffer cells with various PAMPs for 24 h and then collected the conditioned media and stimulated HH with 12.5% conditioned media for 6 h. Hepcidin expression in HH treated with diluted conditioned media had a response pattern similar to that of direct stimulation of HH by PAMPs (Fig. 4B). Although the conditioned media still contained PAMPs (diluted to 12.5% of the original dose), hepcidin induction was likely dependent on the cytokines produced by Kupffer cells rather than direct stimulation by PAMPs, because we observed no significant increase in hepcidin expression in HH after stimulation by 12.5% nonconditioned media containing PAMPs only (data not shown).
We next examined the role of autocrine IL-6 as a mediator of hepcidin induction by PAMPs. The addition of neutralizing antibodies against IL-6 and IL-6 receptor to human hepatocytes led to a reduction in the hepcidin response to PAMPs, with a mean decrease of 17-fold for the hepcidin-inducing PAMPs (Fig. 5). As expected, neutralizing antibodies completely ablated the response to IL-6, and even the response to BMP-2 was partially decreased, likely because of the known synergistic effect between the IL-6 and BMP-2 pathways (6, 26). The addition of antibodies against BMP-2 did not affect the hepcidin mRNA response to TLR/NLR ligands (data not shown).
FIG 5.
Hepcidin induction by PAMPs in human hepatocytes is IL-6 dependent. HH were incubated overnight in serum-free medium and then treated for 6 h with either the indicated PAMPs (white bars) or PAMPs and neutralizing antibodies against IL-6 and IL-6 receptor (gray bars). Hepcidin mRNA was analyzed by qPCR and normalized to β-actin mRNA, and the results are shown as fold change in expression compared to the control (calculated as 2ΔΔCT, where ΔΔCT = ΔCT [test sample] − ΔCT [control]). Vertical bars represent means from 4 different hepatocyte cultures obtained from two donor livers. Individual data points are shown as open circles (PAMP treatment) or triangles (PAMP plus anti-IL-6/IL6R). Statistical analysis was done only for the PAMPs that induced hepcidin [Pam3, FSL-1, LPS, flagellin, and poly(I·C)], and hepcidin expression was analyzed with or without anti-IL-6/IL-6R for each of the two human livers. Hepcidin mRNA was significantly suppressed by the antibody treatment, with a geometric mean decrease of 17-fold (P < 10−5 by paired t test). Abbreviations: Pam3, Pam3CSK4; FSL, FSL-1; Flag, flagellin; p(I·C), poly(I·C); IMQ, imiquimod; MDP, muramyl dipeptide.
DISCUSSION
The iron-regulatory hormone hepcidin controls the absorption of dietary iron as well as the distribution of iron between intracellular stores and extracellular fluids, including plasma (2). Increased hepcidin concentrations and accompanying hypoferremia, observed during various infections, are thought to be a host defense mechanism that decreases iron available to microbial pathogens, thereby restricting their growth.
We previously demonstrated in a mouse model of turpentine-induced inflammation and with an in vitro LPS-stimulated human hepatocyte/macrophage model that IL-6 was required for hepcidin increase and also showed that IL-6 by itself rapidly induced hypoferremia in humans (10). However, it remained to be tested whether these observations were generalizable to other infectious or inflammatory stimuli.
In the present study, we, for the first time, systematically analyzed hepcidin regulation in response to bacterial and viral stimuli and a panel of pathogen-associated molecules, using both mouse models and isolated human liver cells. Severe infections with Streptococcus pneumoniae and influenza A virus induced hepcidin in mouse models several hundred-fold to several thousand-fold, indicating that the hepcidin response could be triggered by both bacterial and viral infections. Armitage et al. (6) previously showed a 2-fold increase in hepcidin 3 days after intranasal administration of 3.5 hemagglutinin units of PR8 virus. The much greater magnitude of hepcidin response by day 5 in our study could be related to a difference in virus dosage, administration route (oropharyngeal versus intranasal), and the timing of the measurements (latest measurement at 5 versus 3 days). Furthermore, our study used dietary preconditioning where animals were placed on a low-iron diet for 2 weeks to prevent the maximal stimulation of hepcidin production by the high iron content of standard mouse chow.
Importantly, using IL-6 knockout mice, we demonstrated that hepcidin induction in vivo in both S. pneumoniae and influenza A virus infection was completely dependent on IL-6. It remains to be seen whether such strong dependence of the in vivo hepcidin response on IL-6 is characteristic of severe infections with other common pathogens. The role of hepcidin-mediated hypoferremia in controlling different bacterial and viral infections remains to be determined.
We also analyzed the effect of stimulation of different TLRs and NLRs on hepcidin expression in vivo in mice and in vitro in primary human hepatocytes. Apart from infection, hepcidin regulation by PRRs may also be relevant in autoimmune diseases, where activation of certain PRRs occurs (28). A PRR-mediated increase in hepcidin may explain the development of hypoferremia and anemia observed in these disorders. In our study, intraperitoneal injections of those PAMPs that act on cell surface receptors and some of the intracellular receptors significantly increased hepatic hepcidin expression in mice, with 15- to 250-fold induction within 24 h. A similar pattern of hepcidin induction by extracellularly acting PAMPs was seen with isolated human hepatocytes, but in these cells most of the intracellularly acting PAMPs failed to increase hepcidin expression. In partially overlapping studies, Armitage and colleagues (6) analyzed hepcidin mRNA induction by selected PAMPs in human peripheral blood mononuclear cells (PBMCs). In these cells, which are relatively minor contributors to systemic hepcidin levels, hepcidin expression was also induced by several extracellularly acting PAMPS, about 2- to 8-fold, with flagellin as the most potent inducer. Using neutralizing antibodies in vitro, the hepcidin response to flagellin in peripheral mononuclear blood cells was also shown to be IL-6 dependent. Armitage et al. similarly reported that endosomally expressed TLRs were poor inducers of hepcidin in PBMCs.
We do not know why hepcidin was not increased in response to some intracellularly sensed PAMPs. The delivery of intracellular PAMPs (such as Nod1 and Nod2 ligands) is reported to occur either through invasion of the cytosol by intracellular pathogens or through other cellular uptake mechanisms (e.g., the type IV secretion system of Helicobacter pylori for a Nod1 ligand) (29). Thus, it is possible that the intracellular delivery of some PAMPs in our experiments was not efficient and resulted in poor activation of their receptors. Measurement of IL-6 in hepatocyte supernatants and mouse sera from a limited number of samples (data not shown) suggested that IL-6 production varied similarly to hepcidin expression: IL-6 was low after stimulation with those PAMPs that failed to increase hepcidin mRNA. We cannot, however, exclude the possibility that hepcidin mRNA are induced at longer time points, higher concentrations, or a different set of PAMPs that stimulate the same receptors. In accordance with the primary goal of this study, we focused on whether hepcidin regulation was IL-6 dependent for those PAMPs that increased hepcidin in our experiments. Both murine in vivo and human in vitro hepcidin induction was dependent on IL-6, as genetic ablation of IL-6 in vivo or its neutralization by antibodies in vitro largely prevented the hepcidin response.
Other cytokines implicated in inflammatory regulation of hepcidin include IL-1α and -β (11), IL-22 (6), and activin B (18). In the activin B study, IL-6 was not required for an approximately 4-fold increase in hepcidin mRNA in a murine model of LPS-induced inflammation 4 h after stimulus. In contrast, in our study hepcidin increase by LPS was significantly blunted in IL-6 KO mice. Our experiments differed in the duration of LPS stimulation (6 and 24 h versus 4 h), the type of LPS (Escherichia coli 0111:B4 versus 055:B5), where the 0111:B4 strain may be slightly more potent (30), and the dietary preconditioning of mice which makes hepcidin more responsive to stimuli. Because of the complexity of cytokine networks and likely synergies, illustrated by the ability of IL-1β to induce IL-6 (31) and synergistic interactions between activin B and IL-6 (18), our study does not rule out a significant contributory role for cytokines other than IL-6. A potential role for other cytokines is also suggested by the residual increase of hepcidin mRNA in IL-6 KO mice after treatment with LPS or ODN (Fig. 3B). Nevertheless, in mouse models of a common bacterial and a common viral infection, and with a representative panel of PAMPs, we now conclusively demonstrate the very strong IL-6 dependence of hepcidin induction in vivo. Cumulative evidence continues to support the key role of IL-6 in hepcidin regulation during diverse infections and inflammation. Therapeutic targeting of IL-6, its receptor, and signaling pathways may be useful for the treatment of anemia of inflammation associated with elevated hepcidin (2).
Supplementary Material
ACKNOWLEDGMENTS
We thank Leon Kautz for assistance with hematological analyses.
This work was supported by UCLA Today's and Tomorrow's Children Fund (to Y.B.) and NIH grant R01 DK082717 (to E.N.).
Footnotes
Published ahead of print 2 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00983-13.
REFERENCES
- 1.Drakesmith H, Prentice AM. 2012. Hepcidin and the iron-infection axis. Science 338:768–772. 10.1126/science.1224577 [DOI] [PubMed] [Google Scholar]
- 2.Ganz T, Nemeth E. 2011. Hepcidin and disorders of iron metabolism. Annu. Rev. Med. 62:347–360. 10.1146/annurev-med-050109-142444 [DOI] [PubMed] [Google Scholar]
- 3.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. 2005. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 1:191–200. 10.1016/j.cmet.2005.01.003 [DOI] [PubMed] [Google Scholar]
- 4.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. 2004. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093. 10.1126/science.1104742 [DOI] [PubMed] [Google Scholar]
- 5.Peyssonnaux C, Zinkernagel AS, Datta V, Lauth X, Johnson RS, Nizet V. 2006. TLR4-dependent hepcidin expression by myeloid cells in response to bacterial pathogens. Blood 107:3727–3732. 10.1182/blood-2005-06-2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, Ho LP, Townsend AR, Drakesmith H. 2011. Hepcidin regulation by innate immune and infectious stimuli. Blood 118:4129–4139. 10.1182/blood-2011-04-351957 [DOI] [PubMed] [Google Scholar]
- 7.Wang HZ, He YX, Yang CJ, Zhou W, Zou CG. 2011. Hepcidin is regulated during blood-stage malaria and plays a protective role in malaria infection. J. Immunol. 187:6410–6416. 10.4049/jimmunol.1101436 [DOI] [PubMed] [Google Scholar]
- 8.de Mast Q, Syafruddin D, Keijmel S, Riekerink TO, Deky O, Asih PB, Swinkels DW, van der Ven AJ. 2010. Increased serum hepcidin and alterations in blood iron parameters associated with asymptomatic P. falciparum and P. vivax malaria. Haematologicaae 95:1068–1074. 10.3324/haematol.2009.019331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. 2003. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 101:2461–2463. 10.1182/blood-2002-10-3235 [DOI] [PubMed] [Google Scholar]
- 10.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. 2004. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 113:1271–1276. 10.1172/JCI20945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee P, Peng H, Gelbart T, Wang L, Beutler E. 2005. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc. Natl. Acad. Sci. U. S. A. 102:1906–1910. 10.1073/pnas.0409808102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verga Falzacappa MV, Vujic SM, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. 2007. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood 109:353–358. 10.1182/blood-2006-07-033969 [DOI] [PubMed] [Google Scholar]
- 13.Wrighting DM, Andrews NC. 2006. Interleukin-6 induces hepcidin expression through STAT3. Blood 108:3204–3209. 10.1182/blood-2006-06-027631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pietrangelo A, Dierssen U, Valli L, Garuti C, Rump A, Corradini E, Ernst M, Klein C, Trautwein C. 2007. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology 132:294–300. 10.1053/j.gastro.2006.10.018 [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Rovin BH. 2010. Hepcidin expression by human monocytes in response to adhesion and pro-inflammatory cytokines. Biochim. Biophys. Acta 1800:1262–1267. 10.1016/j.bbagen.2010.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartolomei G, Cevik RE, Marcello A. 2011. Modulation of hepatitis C virus replication by iron and hepcidin in Huh7 hepatocytes. J. Gen. Virol. 92:2072–2081. 10.1099/vir.0.032706-0 [DOI] [PubMed] [Google Scholar]
- 17.Ryan JD, Altamura S, Devitt E, Mullins S, Lawless MW, Muckenthaler MU, Crowe J. 2012. Pegylated interferon-alpha induced hypoferremia is associated with the immediate response to treatment in hepatitis C. Hepatology 56:492–500. 10.1002/hep.25666 [DOI] [PubMed] [Google Scholar]
- 18.Besson-Fournier C, Latour C, Kautz L, Bertrand J, Ganz T, Roth MP, Coppin H. 2012. Induction of activin B by inflammatory stimuli up-regulates expression of the iron-regulatory peptide hepcidin through Smad1/5/8 signaling. Blood 120:431–439. 10.1182/blood-2012-02-411470 [DOI] [PubMed] [Google Scholar]
- 19.Kawai T, Akira S. 2011. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34:637–650. 10.1016/j.immuni.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 20.Layoun A, Santos MM. 2012. Bacterial cell wall constituents induce hepcidin expression in macrophages through MyD88 signaling. Inflammation 35:1500–1506. 10.1007/s10753-012-9463-4 [DOI] [PubMed] [Google Scholar]
- 21.Courselaud B, Troadec MB, Fruchon S, Ilyin G, Borot N, Leroyer P, Coppin H, Brissot P, Roth MP, Loreal O. 2004. Strain and gender modulate hepatic hepcidin 1 and 2 mRNA expression in mice. Blood Cells Mol. Dis. 32:283–289. 10.1016/j.bcmd.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 22.Shahangian A, Chow EK, Tian X, Kang JR, Ghaffari A, Liu SY, Belperio JA, Cheng G, Deng JC. 2009. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J. Clin. Investig. 119:1910–1920. 10.1172/JCI35412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J, Meng Z, Jiang M, Pei R, Trippler M, Broering R, Bucchi A, Sowa JP, Dittmer U, Yang D, Roggendorf M, Gerken G, Lu M, Schlaak JF. 2009. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology 49:1132–1140. 10.1002/hep.22751 [DOI] [PubMed] [Google Scholar]
- 24.Manni M, Ding W, Stohl LL, Granstein RD. 2011. Muramyl dipeptide induces Th17 polarization through activation of endothelial cells. J. Immunol. 186:3356–3363. 10.4049/jimmunol.1000847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Heel DA, Ghosh S, Butler M, Hunt K, Foxwell BM, Mengin-Lecreulx D, Playford RJ. 2005. Synergistic enhancement of Toll-like receptor responses by NOD1 activation. Eur. J. Immunol. 35:2471–2476. 10.1002/eji.200526296 [DOI] [PubMed] [Google Scholar]
- 26.Maes K, Nemeth E, Roodman GD, Huston A, Esteve F, Freytes C, Callander N, Katodritou E, Tussing-Humphreys L, Rivera S, Vanderkerken K, Lichtenstein A, Ganz T. 2010. In anemia of multiple myeloma, hepcidin is induced by increased bone morphogenetic protein 2. Blood 116:3635–3644. 10.1182/blood-2010-03-274571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunlevy F, McElvaney NG, Greene CM. 2010. TLR3 Sensing of Viral Infection. Open Infect. Dis. J. 4:1–10. 10.2174/1874279301004010001 [DOI] [Google Scholar]
- 28.Mills KH. 2011. TLR-dependent T cell activation in autoimmunity. Nat. Rev. Immunol. 11:807–822. 10.1038/nri3095 [DOI] [PubMed] [Google Scholar]
- 29.Franchi L, McDonald C, Kanneganti TD, Amer A, Nunez G. 2006. Nucleotide-binding oligomerization domain-like receptors: intracellular pattern recognition molecules for pathogen detection and host defense. J. Immunol. 177:3507–3513 [DOI] [PubMed] [Google Scholar]
- 30.Gaekwad J, Zhang Y, Zhang W, Reeves J, Wolfert MA, Boons GJ. 2010. Differential induction of innate immune responses by synthetic lipid a derivatives. J. Biol. Chem. 285:29375–29386. 10.1074/jbc.M110.115204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber A, Wasiliew P, Kracht M. 2010. Interleukin-1 (IL-1) pathway. Sci. Signal. 3:cm1. 10.1126/scisignal.3105cm1 [DOI] [PubMed] [Google Scholar]
- 32.Du X, Fleiss B, Li H, D'angelo B, Sun Y, Zhu C, Hagberg H, Levy O, Mallard C, Wang X. 2011. Systemic stimulation of TLR2 impairs neonatal mouse brain development. PLoS One 6:e19583. 10.1371/journal.pone.0019583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mullick AE, Tobias PS, Curtiss LK. 2005. Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Investig. 115:3149–3156. 10.1172/JCI25482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Funayama H, Huang L, Asada Y, Endo Y, Takada H. 2010. Enhanced induction of a histamine-forming enzyme, histidine decarboxylase, in mice primed with NOD1 or NOD2 ligand in response to various Toll-like receptor agonists. Innate Immun. 16:265–272. 10.1177/1753425909341070 [DOI] [PubMed] [Google Scholar]
- 35.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. 2009. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat. Genet. 41:478–481. 10.1038/ng.320 [DOI] [PubMed] [Google Scholar]
- 36.Vijay-Kumar M, Aitken JD, Sanders CJ, Frias A, Sloane VM, Xu J, Neish AS, Rojas M, Gewirtz AT. 2008. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J. Immunol. 180:8280–8285 [DOI] [PubMed] [Google Scholar]
- 37.Ilievski V, Hirsch E. 2010. Synergy between viral and bacterial toll-like receptors leads to amplification of inflammatory responses and preterm labor in the mouse. Biol. Reprod. 83:767–773. 10.1095/biolreprod.110.085464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong Z, Wei H, Sun R, Hu Z, Gao B, Tian Z. 2004. Involvement of natural killer cells in polyI:C-induced liver injury. J. Hepatol. 41:966–973. 10.1016/j.jhep.2004.08.021 [DOI] [PubMed] [Google Scholar]
- 39.Pawar RD, Ramanjaneyulu A, Kulkarni OP, Lech M, Segerer S, Anders HJ. 2007. Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J. Am. Soc. Nephrol. 18:1721–1731 [DOI] [PubMed] [Google Scholar]
- 40.Anders HJ, Vielhauer V, Eis V, Linde Y, Kretzler M, Perez de LG, Strutz F, Bauer S, Rutz M, Wagner H, Grone HJ, Schlondorff D. 2004. Activation of toll-like receptor-9 induces progression of renal disease in MRL-Fas(lpr) mice. FASEB J. 18:534–536. 10.1096/fj.03-0646fje [DOI] [PubMed] [Google Scholar]
- 41.Krysko DV, Kaczmarek A, Krysko O, Heyndrickx L, Woznicki J, Bogaert P, Cauwels A, Takahashi N, Magez S, Bachert C, Vandenabeele P. 2011. TLR-2 and TLR-9 are sensors of apoptosis in a mouse model of doxorubicin-induced acute inflammation. Cell Death Differ. 18:1316–1325. 10.1038/cdd.2011.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magalhaes JG, Philpott DJ, Nahori MA, Jehanno M, Fritz J, Le BL, Viala J, Hugot JP, Giovannini M, Bertin J, Lepoivre M, Mengin-Lecreulx D, Sansonetti PJ, Girardin SE. 2005. Murine Nod1 but not its human orthologue mediates innate immune detection of tracheal cytotoxin. EMBO Rep. 6:1201–1207. 10.1038/sj.embor.7400552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato T, Shikama Y, Shimauchi H, Endo Y, Takada H. 2011. Analgesic effects of chemically synthesized NOD1 and NOD2 agonists in mice. Innate Immun. 17:54–59. 10.1177/1753425909351904 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.