Abstract
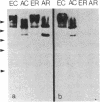
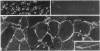
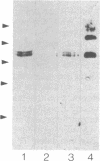
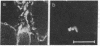
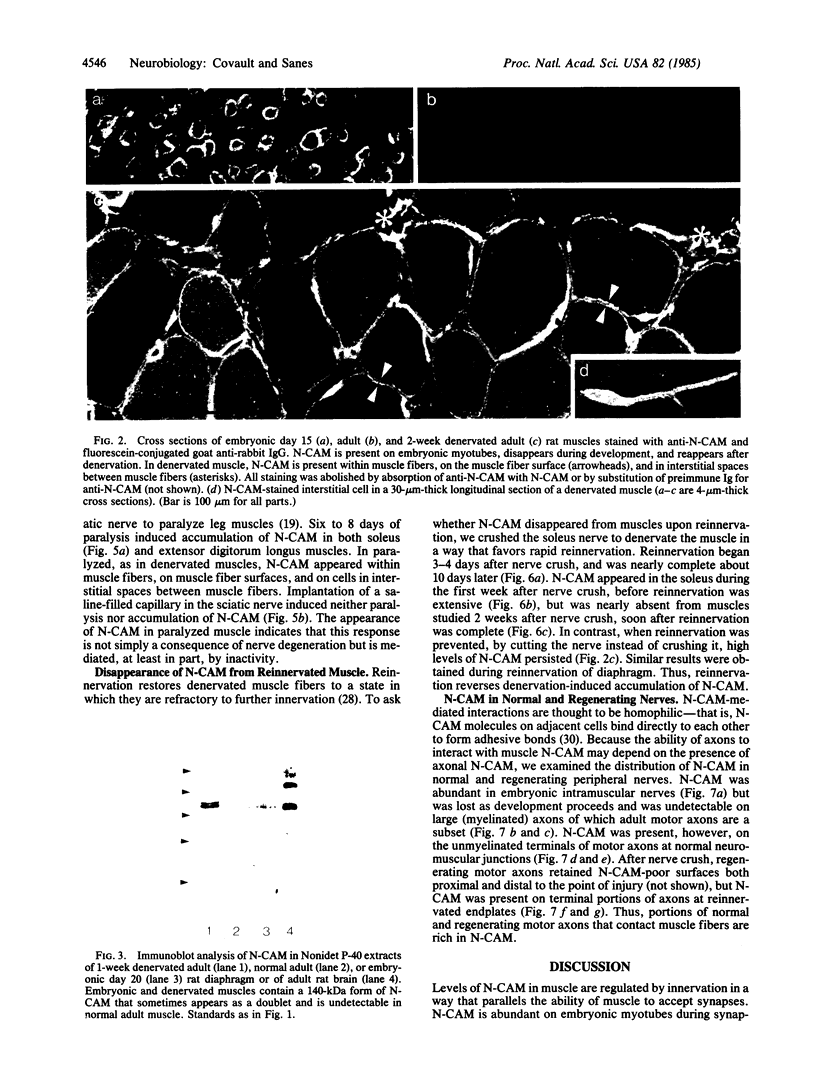
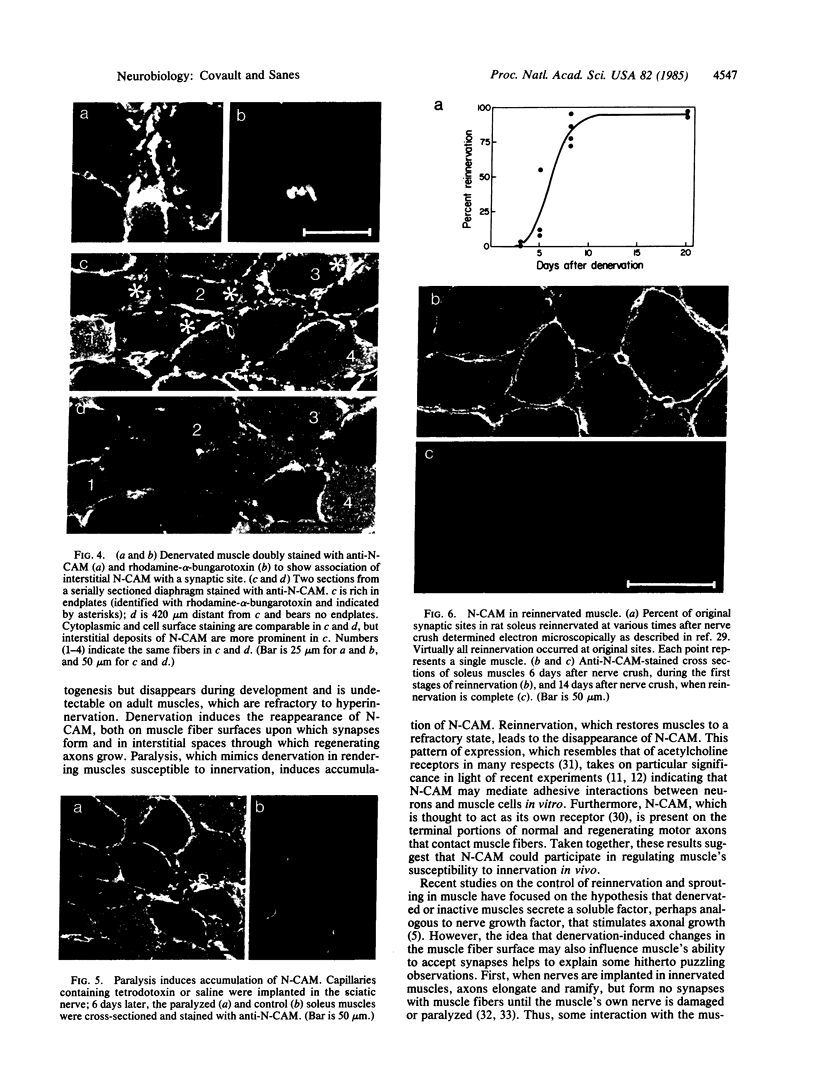
We have used immunofluorescence and immunoblotting methods to study the amount and distribution of the neural cell adhesion molecule (N-CAM) in rat skeletal muscle; this molecule is thought to mediate adhesion of neurons to cultured myotubes. N-CAM is present on the surface of embryonic myotubes, but it is lost as development proceeds and is nearly absent from adult muscle. However, denervation of adult muscle results in the reappearance of N-CAM. In denervated muscle, N-CAM is associated both with muscle fibers and with cells in interstitial spaces between fibers. The N-CAM in interstitial spaces is concentrated near denervated endplates, which are known to be preferential sites for reinnervation. Paralysis of innervated muscle, known to mimic denervation in many respects, also induces the accumulation of N-CAM. Axons that regenerate to reinnervate muscle bear N-CAM on their terminals, and reinnervation results in the disappearance of N-CAM from muscle. Denervation induces accumulation of N-CAM in mouse and chicken, as well as in rat muscles. Thus, the expression of N-CAM in muscle is regulated by the muscle's state of innervation. In that N-CAM-rich muscles (embryonic, denervated, and paralyzed) are known to be competent to accept synapses, while N-CAM-poor muscles (normal adult and reinnervated) are refractory to hyperinnervation, N-CAM might, in turn, participate in regulating muscle's susceptibility to innervation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken J. T. Growth of nerve implants in voluntary muscle. J Anat. 1950 Jan;84(Pt 1):38–49. [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Hopkins W. G., Keynes R. J. An assessment of the spread of the signal for terminal sprouting within and between muscles. Brain Res. 1981 Apr 6;210(1-2):145–151. doi: 10.1016/0006-8993(81)90891-x. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Hopkins W. G. Motor nerve sprouting. Annu Rev Neurosci. 1981;4:17–42. doi: 10.1146/annurev.ne.04.030181.000313. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Hoffman S., Chuong C. M., Thiery J. P., Brackenbury R., Gallin W. J., Grumet M., Greenberg M. E., Hemperly J. J., Cohen C. Structure and modulation of neural cell adhesion molecules in early and late embryogenesis. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):515–526. doi: 10.1101/sqb.1983.048.01.056. [DOI] [PubMed] [Google Scholar]
- Elsberg C. A. EXPERIMENTS ON MOTOR NERVE REGENERATION AND THE DIRECT NEUROTIZATION OF PARALYZED MUSCLES BY THEIR OWN AND BY FOREIGN NERVES. Science. 1917 Mar 30;45(1161):318–320. doi: 10.1126/science.45.1161.318. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Frank E., Jansen J. K., Lomo T., Westgaard R. H. The interaction between foreign and original motor nerves innervating the soleus muscle of rats. J Physiol. 1975 Jun;247(3):725–743. doi: 10.1113/jphysiol.1975.sp010954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier G. F., Lowey S. Distribution of myosin isoenzymes among skeletal muscle fiber types. J Cell Biol. 1979 Apr;81(1):10–25. doi: 10.1083/jcb.81.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarini G., Rougon G., Deagostini-Bazin H., Hirn M., Goridis C. Studies on the transmembrane disposition of the neural cell adhesion molecule N-CAM. A monoclonal antibody recognizing a cytoplasmic domain and evidence for the presence of phosphoserine residues. Eur J Biochem. 1984 Jul 2;142(1):57–64. doi: 10.1111/j.1432-1033.1984.tb08250.x. [DOI] [PubMed] [Google Scholar]
- Glicksman M. A., Sanes J. R. Differentiation of motor nerve terminals formed in the absence of muscle fibres. J Neurocytol. 1983 Aug;12(4):661–671. doi: 10.1007/BF01181529. [DOI] [PubMed] [Google Scholar]
- Goridis C., Deagostini-Bazin H., Hirn M., Hirsch M. R., Rougon G., Sadoul R., Langley O. K., Gombos G., Finne J. Neural surface antigens during nervous system development. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):527–537. doi: 10.1101/sqb.1983.048.01.057. [DOI] [PubMed] [Google Scholar]
- Grumet M., Rutishauser U., Edelman G. M. Neural cell adhesion molecule is on embryonic muscle cells and mediates adhesion to nerve cells in vitro. Nature. 1982 Feb 25;295(5851):693–695. doi: 10.1038/295693a0. [DOI] [PubMed] [Google Scholar]
- Gurney M. E. Suppression of sprouting at the neuromuscular junction by immune sera. Nature. 1984 Feb 9;307(5951):546–548. doi: 10.1038/307546a0. [DOI] [PubMed] [Google Scholar]
- Hoffman S., Sorkin B. C., White P. C., Brackenbury R., Mailhammer R., Rutishauser U., Cunningham B. A., Edelman G. M. Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem. 1982 Jul 10;257(13):7720–7729. [PubMed] [Google Scholar]
- Jansen J. K., Lomo T., Nicolaysen K., Westgaard R. H. Hyperinnervation of skeletal muscle fibers: dependence on muscle activity. Science. 1973 Aug 10;181(4099):559–561. doi: 10.1126/science.181.4099.559. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Korneliussen H., Sommerschild H. Ultrastructure of the new neuromuscular junctions formed during reinnervation of rat soleus muscle by a "foreign" nerve. Cell Tissue Res. 1976 Apr 9;167(4):439–452. doi: 10.1007/BF00215176. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemmon V., Staros E. B., Perry H. E., Gottlieb D. I. A monoclonal antibody which binds to the surface of chick brain cells and myotubes: cell selectivity and properties of the antigen. Brain Res. 1982 Mar;255(3):349–360. doi: 10.1016/0165-3806(82)90003-7. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970 Apr;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills R. G., Bray J. J. A slow-release technique for inducing prolonged paralysis by tetrodotoxin. Pflugers Arch. 1979 Dec;383(1):67–70. doi: 10.1007/BF00584476. [DOI] [PubMed] [Google Scholar]
- Murray M. A., Robbins N. Cell proliferation in denervated muscle: identity and origin of dividing cells. Neuroscience. 1982 Jul;7(7):1823–1833. doi: 10.1016/0306-4522(82)90040-9. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Rathjen F. G., Rutishauser U. Comparison of two cell surface molecules involved in neural cell adhesion. EMBO J. 1984 Feb;3(2):461–465. doi: 10.1002/j.1460-2075.1984.tb01828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin P., Axelrod D. Fluorescent tetramethyl rhodamine derivatives of alpha-bungarotoxin: preparation, separation, and characterization. Anal Biochem. 1977 Jun;80(2):585–592. doi: 10.1016/0003-2697(77)90682-0. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Grumet M., Edelman G. M. Neural cell adhesion molecule mediates initial interactions between spinal cord neurons and muscle cells in culture. J Cell Biol. 1983 Jul;97(1):145–152. doi: 10.1083/jcb.97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., Hoffman S., Edelman G. M. Binding properties of a cell adhesion molecule from neural tissue. Proc Natl Acad Sci U S A. 1982 Jan;79(2):685–689. doi: 10.1073/pnas.79.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U. Molecular and biological properties of a neural cell adhesion molecule. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):501–514. doi: 10.1101/sqb.1983.048.01.055. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Chiu A. Y. The basal lamina of the neuromuscular junction. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):667–678. doi: 10.1101/sqb.1983.048.01.070. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Hall Z. W. Antibodies that bind specifically to synaptic sites on muscle fiber basal lamina. J Cell Biol. 1979 Nov;83(2 Pt 1):357–370. doi: 10.1083/jcb.83.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Marshall L. M., McMahan U. J. Reinnervation of muscle fiber basal lamina after removal of myofibers. Differentiation of regenerating axons at original synaptic sites. J Cell Biol. 1978 Jul;78(1):176–198. doi: 10.1083/jcb.78.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R. More nerve growth factors? Nature. 1984 Feb 9;307(5951):500–500. doi: 10.1038/307500a0. [DOI] [PubMed] [Google Scholar]
- Slack J. R., Pockett S. Terminal sprouting of motoneurones is a local response to a local stimulus. Brain Res. 1981 Aug 3;217(2):368–374. doi: 10.1016/0006-8993(81)90013-5. [DOI] [PubMed] [Google Scholar]
- Thanos S., Bonhoeffer F., Rutishauser U. Fiber-fiber interaction and tectal cues influence the development of the chicken retinotectal projection. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1906–1910. doi: 10.1073/pnas.81.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte D., Gottlieb D. I. Time of appearance and tissue distribution of a cell surface antigen in early chick development. Brain Res. 1983 Jul;285(1):63–67. doi: 10.1016/0165-3806(83)90109-8. [DOI] [PubMed] [Google Scholar]
- Zak R., Grove D., Rabinowitz M. DNA synthesis in the rat diaphragm as an early response to denervation. Am J Physiol. 1969 Mar;216(3):647–654. doi: 10.1152/ajplegacy.1969.216.3.647. [DOI] [PubMed] [Google Scholar]