Abstract
Microsporum canis is a pathogenic fungus with worldwide distribution that causes tinea capitis in animals and humans. M. canis also causes invasive infection in immunocompromised patients. To defy pathogenic fungal infection, the host innate immune system is the first line of defense. As an important arm of innate immunity, the inflammasomes are intracellular multiprotein complexes that control the activation of caspase-1, which cleaves proinflammatory cytokine pro-interleukin-1β (IL-1β) into its mature form. To determine whether the inflammasome is involved in the host defense against M. canis infection, we challenged human monocytic THP-1 cells and mouse dendritic cells with a clinical strain of M. canis isolated from patients with tinea capitis. We found that M. canis infection triggered rapid secretion of IL-1β from both THP-1 cells and mouse dendritic cells. Moreover, by using gene-specific shRNA and competitive inhibitors, we determined that M. canis-induced IL-1β secretion was dependent on NLRP3. The pathways proposed for NLRP3 inflammasome activation, namely, cathepsin B activity, K+ efflux, and reactive oxygen species production, were all required for the inflammasome activation triggered by M. canis. Meanwhile, Syk, Dectin-1, and Card9 were found to be involved in M. canis-induced IL-1β secretion via regulation of pro-IL-1β transcription. More importantly, our data revealed that M. canis-induced production of IL-1β was dependent on the NLRP3 inflammasome in vivo. Together, this study unveils that the NLRP3 inflammasome exerts a critical role in host innate immune responses against M. canis infection, and our data suggest that diseases that result from M. canis infection might be controlled by regulating the activation of inflammasomes.
INTRODUCTION
The innate immune system is the first line of host defense against invading microbes. To counter microbial infection, the innate immune system employs a group of evolutionarily conserved pattern recognition receptors (PRRs) to recognize molecular patterns expressed by invading pathogens. The PRRs identified so far include membrane-bound Toll-like receptors (TLRs) and C-type lectin receptors (CLRs), as well as intracellular Nod-like receptors (NLRs), RNA-sensing RIG-I-like receptors (RLRs), and DNA sensors, such as the proteins DNA-dependent activator of IRFs (DAI) and absent in melanoma 2 (AIM2) (1, 2). Some of these PRRs, including a number of NLRs and DNA sensors, can assemble into a complex called the inflammasome (3). An inflammasome contains PRRs such as NLRP3 (NOD-like receptor family, pyrin domain-containing 3), NLRC4, or AIM2, adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD), and pro-caspase-1 (4). The assembly of the inflammasome results in caspase-1 activation and interleukin-1β (IL-1β) secretion, and the latter is an important cytokine required for host elimination of invading pathogens and for shaping adaptive immune responses (5–7).
Microsporum canis is a pathogenic fungus distributed worldwide and has led to outbreaks of dermatophytosis in many areas during the recent decades (8, 9). Infection of this fungus causes microsporosis, which is a type of tinea capitis characterized by severe itching of the scalp, red scaly papules around hair shafts, and hairs breaking off (10, 11). M. canis-caused tinea capitis is highly contagious and is readily transmitted between animals and humans via direct physical contact or through indirect contact with fungus-contaminated materials (12, 13). This disease mainly affects children, the elderly, and immunocompromised individuals, such as patients receiving immunosuppressive therapy or AIDS patients (14–18). The disorders caused by M. canis infection are not well controlled to date, and M. canis is still one of the most common dermatophytes to cause human tinea capitis in Europe (19, 20) as well as in South America (21, 22). Moreover, it is also the principal pathogen that causes tinea capitis in most areas of China according to recent reports (23, 24). Because of the growing popularity of keeping pets in Chinese cities, the incidence of disease caused by M. canis infection is rising quickly (24, 25).
However, the host immune responses to M. canis infection have not been well studied. Recent reports showed that a number of PRRs are involved in the host immune responses against fungal infections (26–30). The role of inflammasomes in host immune responses toward Candida albicans, Aspergillus fumigatus, and Cryptococcus neoformans has also been described (31–34). All these fungi activate the NLRP3 inflammasome (31–34), and NLRP10 is also important for antifungal immunity against Candida albicans (35). Until now, whether dermatophytes such as M. canis activate the NLRP3 inflammasome has not been studied. A recent report showed that M. canis induces production of proinflammatory cytokines, including IL-1β, from feline polymorphonuclear neutrophils, indicating a possible role for the inflammasome in the host immune response to M. canis infection (36).
In the present study, we found that M. canis induced secretion of IL-1β in vitro and in vivo, which was dependent on the NLRP3 inflammasome. M. canis-induced production of IL-1β required ROS production, Cathepsin B release and K+ efflux. Syk, Dectin-1, and Card9 were all involved in M. canis-induced IL-1β production by regulating the transcription of pro-IL-1β.
MATERIALS AND METHODS
Mice and reagents.
C57BL/6 wild-type (WT) mice were obtained from Shanghai Laboratory Animal Center (SLAC). Asc-deficient mice were provided by Vishva M. Dixit of Genentech, and Nlrp3-deficient mice were provided by Warren Strober from NIH. All animals were maintained at the specific-pathogen-free facility of SLAC. Animal care, use, and experimental procedures complied with national guidelines and were approved by the Animal Care and Use Committee at Institut Pasteur of Shanghai. Lipopolysaccharide (LPS; L3012), phorbol myristate acetate (PMA; P8139), AC-YVAD-CHO (A3707), CA-074 Me (C5857), diphenylene iodonium (DPI; D2926), N-acetyl-l-cysteine (NAC; A7250), and butylated hydroxyanisole (BHA; B1253) were purchased from Sigma-Aldrich. KCl (PB0440) was obtained from Bio Basic Inc.
Cell culture.
THP-1 cells were maintained in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 100 IU/ml penicillin, 1 mg/ml streptomycin, and 50 μM β-mercaptoethanol. THP-1-derived macrophages were generated by treating THP-1 cells with PMA at a concentration of 100 ng/ml for 3 h. The cells were then rested for 48 h before use. Mouse bone marrow-derived dendritic cells (BMDCs) were allowed to differentiate in this RPMI 1640 medium mixture with recombinant granulocyte-macrophage colony-stimulating factor (20 ng/ml; Peprotech). The cells were cultured in a humidified incubator with 5% CO2 at 37°C. On day 3, the cell culture medium was replenished, and then the cells were maintained in the incubator for another 3 days. We harvested the cells on day 6. The purity of CD11C+ cells was higher than 80% when tested by flow cytometry (data not shown). These cells were used for fungal challenge experiments.
M. canis culture and preparation.
The M. canis strain used in this study was a clinical strain isolated from patients with tinea capitis at The First Hospital of Xinjiang Medical University. The fungus was inoculated on potato dextrose agar medium (PDA; BD Biosciences) and cultivated for 7 days at 27°C. The culture was triturated and washed with sterile phosphate-buffered saline (PBS) 3 times and counted with a hemocytometer prior to infection of cells for experiments.
Real-time PCR.
Total RNA was extracted from the THP-1 cells by using TRIzol reagent (Invitrogen). Reverse transcription of mRNA and synthesis of cDNA was performed using TaqMan reverse transcription reagents (Applied Biosystems). Real-time PCR was performed using the SYBR green quantitative PCR (qPCR) master mix (Toyobo) and the 7900HT Fast real-time PCR system (Applied Biosystems). Relative quantification of genes was achieved via normalization against β-actin. The primers used were the following: for β-actin, 5′-AGTGTGACGTGGACATCCGCAAAG-3′ (forward), 5′-ATCCACATCTGCTGGAAGGTGGAC-3′ (reverse); for pro-IL-1β, 5′-CACGATGCACCTGTACGATCA-3′ (forward), 5′-GTTGCTCCATATCCTGTCCCT-3′ (reverse); for NLRP3, 5′-AAGGGCCATGGACTATTTCC-3′ (forward), 5′-GACTCCACCCGATGACAGTT-3′ (reverse); for caspase-8, 5′-AACTGTGTTTCCTACCGAAACCC-3′ (forward), 5′-AGGACATCGCTCTCTCAGGC-3′ (reverse); for AIM2, 5′-TGGCAAAACGTCTTCAGGAGG-3′ (forward), 5′-GATGCAGCAGGACTCATTTCA-3′ (reverse); for NLRP1, 5′-ATTCCAGTTTGTGCGAATCCA-3′ (forward), 5′-GTTCCTTGGGGAGTATTTCCAG-3′ (reverse); for Syk, 5′-CGTATGAGCCAGAACTTGCACC-3′ (forward), 5′-CTTTCGGTCCAGGTAAACCTCC-3′ (reverse); for Dectin-1, 5′-ACAATGCTGGCAACTGGGCTCT-3′ (forward), 5′-AGAGCCATGGTACCTCAGTCTG-3′ (reverse); for Card9, 5′-TCCGACCTGGAAGATGGCTCAC-3′ (forward), 5′-CAGAGCTGCAAAGGGCTGTTTC-3′ (reverse).
Quantification of cytokines by ELISA.
Supernatants of THP-1 cells were harvested, and IL-1β as well as IL-8 secretion were analyzed in an enzyme-linked immunosorbent assay (ELISA; BD Biosciences). From mouse BMDCs, the supernatants were harvested for IL-1β and IL-6 ELISA (eBioscience). All the procedures were performed according to the manufacturers' instructions.
Generation of THP-1 cells expressing shRNA.
shRNA vectors against human NLRP3, caspase-1, ASC, and their scramble vectors were gifts from Jurg Tschopp (37). Caspase-8, AIM2, NLRP1, Syk, Dectin-1, and Card9 shRNA vectors were constructed by cloning shRNA targeting respective genes into the PLKO.1 vector. Lentiviruses expressing hairpins against these genes were produced by transfecting shRNA vectors and the second-generation packaging plasmids pMD2-VSVG and pCMV-R8.91 (38) into 293T cells. The virus particles were harvested 24 h later and used for infection of THP-1 cells. At 48 h after initiation of infection, 5 μg/ml of puromycin was added to the culture medium to select positive clones. The silencing efficiency was analyzed by real-time PCR, and the most efficiently silenced cell clones were used for further studies. Targeting sequences selected were the following: caspase-8, GATGCGGAAGCTCTTCAGTTTCA; AIM2, GCCTGAACAGAAACAGATG; NLRP1, GAGGGAAGAATCTGAGGAA; Syk, CCTCATCAGGGAATATGTG; Dectin-1, GGATAGCTGTTGTTTCAGA; Card9, GGTCATCCGCAAACGGAAA.
Western blot analysis.
THP-1 cells or BMDCs were pelleted by centrifugation at 3,000 × g for 3 min. The cell pellets were incubated in lysis buffer containing 50 mM Tris (pH 7.5), 1% NP-40, 150 mM NaCl, and protease inhibitor cocktail. After centrifugation at 12,000 × g for 10 min at 4°C, the supernatants were harvested and mixed with SDS loading buffer for the detection of pro-IL-1β, NLRP3, ASC, pro-caspase-1, and β-actin via immunoblotting. For assay of mature caspase-1 and IL-1β assay, the cell culture supernatants were harvested and concentrated via the protein precipitation approach, with the harvested protein pellets mixed with SDS loading buffer for the detection of caspase-1 p10 and IL-1β p17. The protein loading buffer mixture was then heated at 100°C for 10 min and separated via SDS-PAGE. The proteins were transferred onto nitrocellulose membranes and blocked with 5% fat-free milk in 1× Tris-buffered saline containing 0.05% Tween 20 and then probed with the following primary antibodies: rabbit anti-human or mouse mature IL-1β and pro-IL-1β (sc-7884; Santa Cruz Biotechnology), mouse anti-human or mouse NLRP3 (ALX-804-881; Enzo Life Sciences), rabbit anti-human or mouse ASC (sc-22514-R; Santa Cruz Biotechnology), rabbit anti-human caspase-1 (sc-515; Santa Cruz Biotechnology), rabbit anti-mouse caspase-1 (sc-514; Santa Cruz Biotechnology), mouse anti-human β-actin (KM9001; Tianjin Sungene Biotech). After incubation with appropriate horseradish peroxidase-conjugated secondary antibodies, the immunoreactive bands were visualized using enhanced chemiluminescence reagent (Amersham).
ASC pyroptosome detection.
The procedure for ASC pyroptosome detection was carried out according to methods described in our previous work (39). Briefly, THP-1 cells were seeded in 6-well plates (1 × 106 cells per well) and treated with PBS, LPS (100 ng/ml), or M. canis (multiplicity of infection [MOI], 1) for 6 h. The cells were centrifuged at 3,000 × g for 3 min at 4°C. The cell pellets were resuspended in 0.2 ml of lysis buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 1% NP-40, and a protease inhibitor mixture and lysed by passage through a 21-gauge needle 10 times. The cell lysates were then centrifuged at 12,000 × g for 10 min at 4°C. The resultant insoluble cell debris was washed 3 times with PBS and resuspended in 500 μl of PBS. This insoluble cell debris was then cross-linked with freshly prepared disuccinimidyl suberate (DSS; 4 mM; Sigma-Aldrich) for 30 min at 37°C and pelleted by centrifugation at 6,000 × g for 10 min. This cross-linked cell debris was resuspended in 30 μl SDS loading buffer for Western blot detection of ASC oligomerization.
In vitro M. canis challenge.
To test if M. canis stimulated expression of IL-1β, normal THP-1 cells were treated with M. canis at various MOIs and the supernatants were harvested for IL-1β assay. In other cases, normal THP-1 cell, Scramble, or NLRP3/ASC/caspase-1-silenced THP-1 cells or BMDCs (1 × 105) were treated with M. canis (MOI, 1). The supernatants were harvested 6 h later for determination of IL-1β and IL-8 concentration by ELISA (BD Biosciences). For the Western blot assay, 1 × 106 THP-1 cells or BMDCs were treated with M. canis (MOI, 1), the culture supernatants, cell extracts, and cell pellets were collected to detect caspase-1 activity, expression of inflammasome components, or ASC pyroptosome formation, respectively.
PAS staining.
THP-1-derived macrophages were first plated on coverslips and challenged with M. canis for 6 h. The macrophages on the coverslips were then fixed with 95% ethanol for 10 min and treated with periodic acid-Schiff (PAS) solution for 20 min at room temperature. After rinsing with PBS 3 times, the coverslips were incubated with Schiff solution for 60 min at room temperature. The coverslips were then rinsed 10 times with PBS for 15 min and mounted with UltraCruz mounting medium (sc-24941; Santa Cruz Biotechnology) for observation under confocal microscopy (Zeiss).
Cell viability assay.
The cell viability after M. canis challenge was determined using MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] (Promega) in an assay according to the manufacturer's instructions. Briefly, the MTS solution was first allowed to melt at room temperature before use. Twenty microliters of MTS solution was mixed with 100 μl of culture medium in wells of a 96-well plate. The plate was maintained in an incubator with 5% CO2 at 37°C for 4 h. The reaction was stopped by combining the reaction mixture with 25 μl 10% SDS solution. Afterwards, the absorbance at 490 nm was recorded to evaluate the macrophage viability.
In vivo peritonitis induction with M. canis.
Mice were injected intraperitoneally (i.p.) with 2 × 107 M. canis in 200 μl PBS; control mice received the same volume of PBS. Mice were sacrificed 8 days after injection. Peritoneal lavage fluid (PLF) was harvested by rinsing the peritoneal cavity with 200 μl PBS containing 10% FBS. The harvested PLF was used for IL-1β detection by ELISA.
Statistical analysis.
Data were analyzed for statistical significance by a two-tailed Students' t test. A P value of ≤0.05 was considered statistically significant.
RESULTS
M. canis induces IL-1β secretion and inflammasome activation in human monocytes, macrophages, and mouse dendritic cells.
M. canis is a fungus that causes tinea capitis in humans. To test whether M. canis induces IL-1β secretion in human innate immune cells, we treated the human monocytic cell line THP-1 with a clinical strain of M. canis at different multiplicities of infection. LPS was used as a positive control to stimulate the secretion of IL-1β. THP-1 cells were incubated with PBS, PDA, LPS (100 ng/ml), or M. canis for 6 h. The cell culture supernatants were harvested for IL-1β measurement via ELISA. We observed a strong induction of IL-1β by M. canis in a dose-dependent manner; in contrast, PBS- or PDA-treated cells did not secret detectable IL-1β (Fig. 1A). To determine the kinetics of IL-1β induction by M. canis, THP-1 cells were incubated with M. canis (MOI, 1), and the supernatants were harvested at different time points. The results showed that M. canis induced a gradually increased level of IL-1β during the time course and peaked at 24 to 48 h after M. canis challenge (Fig. 1B).
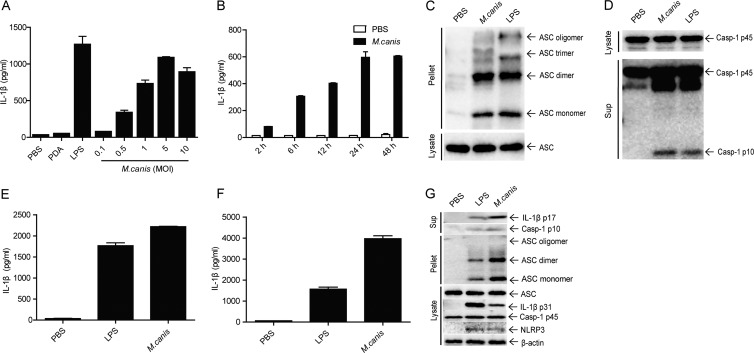
FIG 1.
M. canis-triggered IL-1β production and inflammasome activation in human monocytes, macrophages, and mouse dendritic cells. (A) THP-1 cells (1 × 105) were infected with M. canis at different doses (MOIs), and 24 h later the supernatants were harvested for IL-1β measurement by ELISA. PBS- or PDA-treated cells served as negative controls. (B) THP-1 cells (1 × 105) were treated with M. canis (MOI, 1), and supernatants were collected at different time points to detect IL-1β by ELISA. (C and D) THP-1 cells (1 × 106) were incubated with PBS, M. canis (MOI, 1), or LPS (100 ng/ml) for 6 h, the cell pellets were collected for ASC pyroptosome detection, and the culture supernatants were harvested for caspase-1 assay via Western blotting. (E) THP-1-derived macrophages (1 × 105) were treated with PBS, LPS (100 ng/ml), or M. canis (MOI, 1) for 6 h, and culture supernatants were collected for IL-1β detection via ELISA. (F) Mouse BMDCs (1 × 105) were stimulated with PBS, LPS (1,000 ng/ml), or M. canis (MOI, 1) for 6 h, and the culture supernatant was collected for IL-1β detection via ELISA. (G) Mouse BMDCs (1 × 106) were infected with M. canis (MOI, 1) or treated with PBS or LPS (1,000 ng/ml) for 6 h, and the culture supernatants were harvested for IL-1β immunoblotting. The cell pellets were used to detect ASC pyroptosome, and cell lysates were analyzed for detection of the expression of inflammasome components. Data in panels A, B, E, and F are means ± standard deviations from one out of three independent experiments. Data in panels C, D, and G are from one out of two independent experiments.
The induction of IL-1β indicated that the inflammasome might be activated upon M. canis challenge in THP-1 cells. Therefore, we checked the formation of ASC pyroptosome and the activation of caspase-1 in the infected cells, as these are the well-accepted criteria to monitor inflammasome activation (40). Indeed, we observed ASC oligomerization (Fig. 1C) and the appearance of the active form of caspase-1 (Fig. 1D) in M. canis-treated THP-1 cell pellets and culture supernatants, respectively. Taken together, these data indicated that M. canis induced IL-1β production, ASC pyroptosome formation, and caspase-1 activation in THP-1 cells. Moreover, we also observed a strong induction of IL-1β by M. canis in THP-1-derived macrophages (Fig. 1E).
To verify whether M. canis was able to induce inflammasome activation in mouse cells, we treated mouse BMDCs with PBS, LPS (1 μg/ml), or M. canis (MOI, 1) for 6 h, and the culture supernatants were harvested to detect IL-1β. As shown in Fig. 1F, M. canis treatment induced robust secretion of IL-1β in BMDCs. Furthermore, we also observed the formation of ASC pyroptosome and caspase-1 activation in M. canis-challenged BMDC pellets and culture supernatants, respectively (Fig. 1G). Interestingly, M. canis-induced IL-1β production and inflammasome activation were even stronger than those from LPS stimulation. Together, these data indicated that M. canis induced inflammasome activation in human monocytes, macrophages, and mouse dendritic cells.
M. canis-induced IL-1β secretion is dependent on caspase-1 activity.
Because caspase-1 is required for the production of mature IL-1β in myeloid cells, we then examined whether the M. canis-induced IL-1β production observed in the experiments described above was caspase-1 dependent. We first applied a specific inhibitor of caspase-1, AC-YVAD, to block caspase-1 activity in challenge experiments. The cells were pretreated with AC-YVAD at various concentrations for 0.5 hour, and then the cells were further treated with M. canis for 6 h. The results showed that M. canis-induced IL-1β production was remarkably inhibited in a dose-dependent manner after pretreatment with AC-YVAD (Fig. 2A), without affecting the production of IL-8, which was inflammasome independent (Fig. 2B). This indicated that caspase-1 activity is required for M. canis-induced IL-1β production. To confirm this finding, we employed shRNA-mediated knockdown of caspase-1 in THP-1 cells. The silencing of caspase-1 strongly decreased IL-1β production induced by M. canis in THP-1 cells (Fig. 2C), without affecting the production of IL-8 (Fig. 2D). As a specificity control, involvement of caspase-8 was examined, as this caspase has been reported to be important against various fungi challenges (41). However, in our experiments, caspase-8 silencing did not affect IL-1β production induced by M. canis (Fig. 2C). Caspase-8 silencing efficiency is shown in Fig. 2J. Therefore, M. canis-mediated IL-1β secretion in human monocytes was caspase-1 dependent.
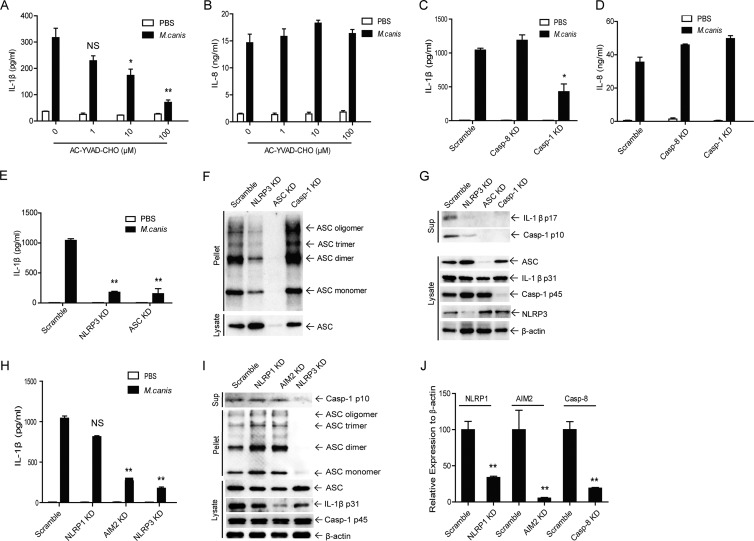
FIG 2.
The secretion of IL-1β induced by M. canis infection is dependent on caspase-1 and NLRP3 inflammasome activation in THP-1 cells. (A and B) THP-1 cells (1 × 105) were pretreated with the caspase-1 inhibitor AC-YVAD-CHO and then challenged with M. canis (MOI, 1) for 6 h. The supernatants were harvested for IL-1β and IL-8 ELISA. (C and D) Caspase-1 or caspase-8 knockdown cells or and Scramble THP-1 cells (1 × 105) were infected with M. canis (MOI, 1) for 6 h, and the culture supernatants were collected for IL-1β and IL-8 ELISA. (E, H, and I) NLRP3- (E), ASC- (E), NLRP1- (H), or AIM2-silenced (H) or Scramble THP-1 cells (1 × 105) were infected with M. canis (MOI, 1) for 6 h, and the culture supernatants were collected for IL-1β ELISA. (F, G, and I) Scramble, NLRP3-, ASC-, caspase-1- (F and G), or NLRP1- or AIM2-silenced (I) THP-1 cells (1 × 106) were treated with M. canis (MOI, 1) for 6 h, the culture supernatants were harvested for caspase-1 assay, and the cell pellets were used for ASC pyroptosome detection via Western blotting. (J) The silencing efficiencies of caspase-8, NLRP1, and AIM2 were well controlled. *, P < 0.05; **, P < 0.01. Data in panels A to E and H are means ± standard deviations from one out of three independent experiments. Data in panels F, G, and I are from one out of two independent experiments. Data in panel J are means ± standard deviations from one out of two independent experiments.
Secretion of IL-1β induced by M. canis is dependent on the NLRP3 inflammasome in THP-1 cells.
The NLRP3 inflammasome is the most studied inflammasome that is involved in host immune responses against various fungal infections. To test whether M. canis-induced IL-1β production and caspase-1 activation are dependent on NLRP3, we treated NLRP3- or ASC-silenced THP-1 cells with M. canis. The results showed that both NLRP3 and ASC silencing strongly decreased IL-1β production from THP-1 cells challenged with M. canis (Fig. 2E). These observations were further confirmed via immunoblotting for ASC oligomerization and caspase-1 activation. From these experiments, we noted that IL-1β release, caspase-1 activation, and ASC pyroptosome formation were all decreased in NLRP3- and ASC-silenced THP-1 cells (Fig. 2F and G). These results also showed the successful knockdown of caspase-1, ASC, and NLRP3 in THP-1 cells (Fig. 2G). As caspase-1 is downstream of ASC during inflammasome activation, ASC pyroptosome formation was not affected by caspase-1 silencing (Fig. 2G). Together, these data clearly demonstrated that M. canis-induced IL-1β production was dependent on caspase-1 and NLRP3 inflammasome activities.
As specificity controls, other inflammasomes were also checked for their possible contribution to IL-1β release and caspase-1 activation induced by M. canis. To this end, we treated NLRP1- or AIM2-silenced THP-1 cells with M. canis and determined IL-1β release. The silencing efficiencies of both knockdown cell lines were well controlled (Fig. 2J). As shown in Fig. 2H, silencing of NLRP1 did not affect the production of IL-1β induced by M. canis challenge. In contrast, we observed that M. canis-induced IL-1β production was significantly decreased by AIM2 silencing (Fig. 2H). To confirm the effect of AIM2 silencing on IL-1β secretion, we detected other parameters for inflammasome activation. We found that AIM2 silencing did not inhibit caspase-1 cleavage or ASC pyroptosome formation, but it resulted in decreased expression of pro-IL-1β upon M. canis infection (Fig. 2I). These data suggested a role for AIM2 in regulation of pro-IL-1β expression in M. canis-infected THP-1 cells without affecting inflammasome assembly.
M. canis-induced IL-1β secretion is dependent on the NLRP3 inflammasome in mouse BMDCs and in vivo.
In order to demonstrate whether M. canis-induced IL-1β production and caspase-1 activation in mouse BMDCs are also dependent on the NLRP3 inflammasome, we treated WT, Nlrp3-deficient, and Asc-deficient BMDCs with M. canis and determined IL-1β release. We found that IL-1β release was completely abolished in NLRP3- or Asc-deficient cells (Fig. 3A), while the release of IL-6 was not affected (Fig. 3B). These results were consistent with those from immunoblotting, wherein the released levels of IL-1β, the activation of caspase-1, and the formation of ASC pyroptosome were all completely diminished in NLRP3- or Asc-deficient cells (Fig. 3C). Therefore, similar with results in human monocytes, M. canis-induced IL-1β release and caspase-1 activation were also dependent on the NLRP3 inflammasome in BMDCs.
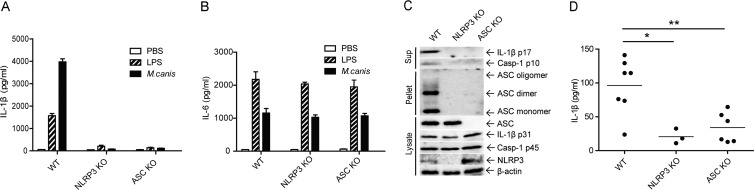
FIG 3.
M. canis-induced secretion of IL-1β is dependent on NLRP3 inflammasome activation in mouse BMDCs and in vivo. (A to C) BMDCs from WT, Nlrp3-deficient, or Asc-deficient mice were stimulated with PBS, LPS (1,000 ng/ml), or M. canis (MOI, 1) for 6 h, and the culture supernatants were collected to detect IL-1β by ELISA (A and B) and Western blotting (C). The cell pellets were used to detect ASC pyroptosome, and cell lysates were used to detect the expression of inflammasome components (C). (D) WT, Nlrp3-deficient, or Asc-deficient mice were injected intraperitoneally with 2 × 107 M. canis in 200 μl PBS; control mice received PBS. Mice were sacrificed 8 days after injection. PLF were harvested by rinsing the peritoneal cavity with 200 μl PBS containing 10% FBS. The harvested PLF were used for IL-1β detection by ELISA. *, P < 0.05; **, P < 0.01. Data in panels A and B are means ± standard deviations from one out of three independent experiments. Data in panels C and D are from one out of two independent experiments.
To examine whether M. canis induces an inflammatory response in vivo, we injected WT mice with 2 × 107 M. canis in 200 μl PBS. Eight days later, the mice were sacrificed and PLF were harvested. We observed an increase in IL-1β production in PLF (Fig. 3D). To evaluate whether the inflammasome was involved in the IL-1β increase during M. canis challenge, we injected WT, Nlrp3-deficient, and Asc-deficient mice with the fungus i.p. Our results showed that IL-1β secretion was significantly lower in Nlrp3-deficient and Asc-deficient mice (Fig. 3D). Therefore, M. canis induced in vivo production of IL-1β, and this was dependent on the NLRP3 inflammasome.
M. canis-induced inflammasome activation requires cathepsin B release, K+ efflux, and ROS production.
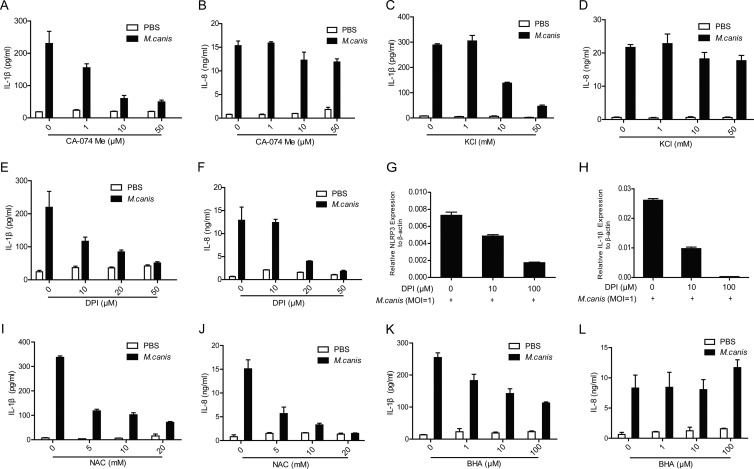
Detailed mechanisms leading to NLRP3 inflammasome activation are not yet fully understood. It is known that the NLRP3 inflammasome is activated upon challenge from a number of structurally different pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs). These stimuli lead to cathepsin B release, K+ efflux, and ROS production, which are all required for NLRP3 inflammasome activation (2). To test whether these factors are also involved in M. canis-induced NLRP3 inflammasome activation, we pretreated THP-1 cells with an inhibitor of cathepsin B release (CA-074 Me). We found that the cathepsin B inhibitor blocked M. canis-induced IL-1β in a dose-dependent manner (Fig. 4A) without affecting the production of IL-8 (Fig. 4B). We also found that the addition of K+ in cell culture medium inhibited M. canis-induced IL-1β production (Fig. 4C) without affecting the production of IL-8 either (Fig. 4D). To determine the role of ROS in M. canis-induced NLRP3 inflammasome activation, we pretreated THP-1 cells with DPI, a ROS inhibitor that block ROS activity, as shown in previous reports (42, 43) and our own experiments (44) without causing cell death. We then stimulated these cells with M. canis. We found that DPI impaired M. canis-induced IL-1β production in a dose-dependent manner (Fig. 4E). However, the addition of DPI also inhibited the production of IL-8 (Fig. 4F). Further experiments showed that DPI inhibited the synthesis of both NLRP3 and pro-IL-1β (Fig. 4G and H). Application of two other ROS inhibitors, NAC and BHA, also impaired M. canis-induced IL-1β production in a dose-dependent manner (Fig. 4I and K). Similar to DPI, the addition of NAC also inhibited the production of IL-8 (Fig. 4J). However, the addition of BHA did not affect the production of IL-8 (Fig. 4L). The differences behind the effects of these different ROS inhibitors await further analysis. These results suggested that cathepsin B release, K+ efflux, and ROS production were all involved in M. canis-induced IL-1β secretion via NLRP3.
FIG 4.
M. canis-induced inflammasome activation requires cathepsin B release, K+ efflux, and ROS production. THP-1 cells (1 × 105) were pretreated with CA-074 Me (A and B), KCl (C and D), DPI (E and F), NAC (I and J), and BHA (K and L) and then challenged with M. canis (MOI, 1) for 6 h. The supernatants were harvested for IL-1β and IL-8 detection by ELISA. (G and H) DPI-pretreated THP-1 cells (5 × 105) were stimulated with M. canis, and the cell lysates were harvested for mRNA extraction to detect NLRP3 and pro-IL-1β by qPCR. Data shown are means ± standard deviations from one out of three independent experiments.
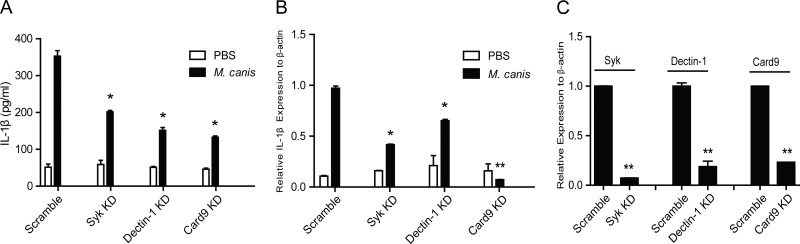
Syk, Dectin-1, and Card9 are involved in M. canis-induced IL-1β secretion.
According to recent reports, the C-type lectin receptor Dectin-1 is involved in C. albicans-induced inflammasome activation (41). The tyrosine kinase Syk is also important for fungus-induced IL-1β secretion and caspase-1 activation (33). The signaling molecule Card9 is involved in the regulation of pro-IL-1β (41). To test whether these molecules are involved in M. canis-induced inflammasome activation, we used a shRNA-mediated knockdown approach to downregulate these molecules in THP-1 cells. When treated with M. canis, Dectin-1 knockdown THP-1 cells secreted significantly decreased IL-1β than Scramble THP-1 cells (Fig. 5A). Similar results were obtained for Syk and Card9 knockdown THP-1 cells (Fig. 5A). Further experiments showed that M. canis-induced pro-IL-1β production was also downregulated in Dectin-1, Syk, and Card9 knockdown THP-1 cells (Fig. 5B). Figure 5C shows the knockdown efficiencies of these genes in THP-1 cells. Thus, these data indicated that Dectin-1, Syk, and Card9 were all involved in M. canis-induced IL-1β release via regulation of pro-IL-1β expression.
FIG 5.
Syk, Dectin-1, and Card9 are involved in the secretion of IL-1β induced by M. canis infection in THP-1-derived macrophages. Syk-, Dectin-1-, or Card9-silenced and Scramble THP-1-derived macrophages (1 × 105) were infected with M. canis (MOI, 1) for 6 h, the culture supernatants were collected for IL-1β ELISA (A), and the macrophages were harvested for pro-IL-1β assay via qPCR (B). (C) The knockdown efficiencies of Syk, Dectin-1, and Card9 were well controlled. *, P < 0.05; **, P < 0.01. Data in panels A, B, and C are means ± standard deviations from one out of three independent experiments.
A heat-sensitive component of M. canis is responsible for IL-1β synthesis and caspase-1 activation.
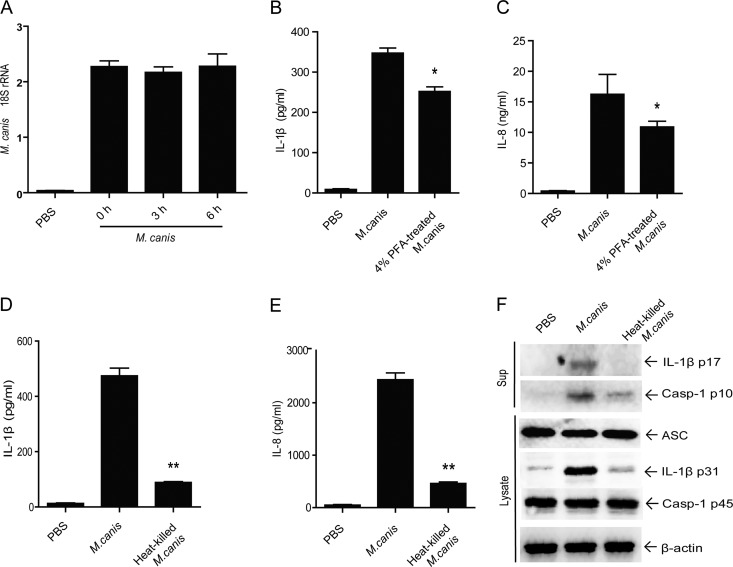
To demonstrate the component from M. canis that accounts for IL-1β induction from host cells, we first tested if the fungus was amplified during the process of THP-1 cell stimulation. By measuring the M. canis-specific 18S rRNA level, we found that M. canis did not multiply within 6 h (Fig. 6A). Moreover, we determined whether M. canis-induced IL-1β secretion was dependent on the germination or viability of the fungus within this 6-h incubation. To do that, we fixed M. canis by treatment with 4% paraformaldehyde (PFA) for 30 min at 25°C or inactivated the fungus by heating at 90°C for 30 min.
FIG 6.
Heat-sensitive components from M. canis contribute to pro-IL-1β induction and caspase-1 activation. (A) THP-1-derived macrophages (1 × 105) were treated with M. canis, the macrophages and fungus were harvested at various time points, total RNA was extracted, and M. canis 18S rRNA was detected using qPCR. THP-1 cells (1 × 105) were challenged with M. canis or 4% PFA-treated M. canis cells (25°C, 30 min) for 6 h, and the culture supernatants were harvested for IL-1β (B) and IL-8 (C) detection by ELISA. THP-1 cells (1 × 105) were challenged with M. canis or heat-killed M. canis (90°C, 30 min) for 6 h, and the culture supernatants were harvested for IL-1β (D) and IL-8 (E) detection by ELISA. (F) THP-1 cells (1 × 106) were stimulated with M. canis or heat-killed M. canis, and the cell lysates and supernatants were collected for pro-IL-1β and caspase-1 detection, respectively. *, P < 0.05; **, P < 0.01. Data in panel A are means ± standard deviations from one out of two independent experiments. Data in panels B, C, D, and E are mean ± standard deviations from one out of three independent experiments. Data in panel F are from one out of two independent experiments.
After PFA fixation, M. canis induced lower levels of IL-1β and IL-8 than did the live M. canis cells, but there was still clear secretion of these cytokines (Fig. 6B and C). After heating at 90°C for 30 min, the fungus could not form colonies on PDA slants (data not shown), indicating successful inactivation of the fungus. We then compared the effects of this heat-killed M. canis and live fungus on the ability to induce IL-1β secretion from THP-1 cells. The results showed that after the heat treatment, the fungus induced a strongly diminished level of IL-1β in comparison with live M. canis (Fig. 6D). Moreover, lower production of IL-8 triggered by the heat-inactivated M. canis was also noticed, indicating that the heat-sensitive components from M. canis affected NF-κB activation (Fig. 6E). To determine the exact role of the heat-sensitive components, we further detected IL-1β and caspase-1 activation via immunoblotting. Consistent with the ELISA results, much lower levels of mature IL-1β (p17) and activated caspase-1 (p10) secretion in cell culture supernatants were observed (Fig. 6F). In addition, a strongly decreased expression of pro-IL-1β in cell lysates was evident (Fig. 6F). These data suggested that a heat-sensitive component of M. canis contributed to IL-1β induction by promoting the synthesis of pro-IL-1β as well as the activation of caspase-1.
M. canis interacts with macrophages and does not trigger cell death.
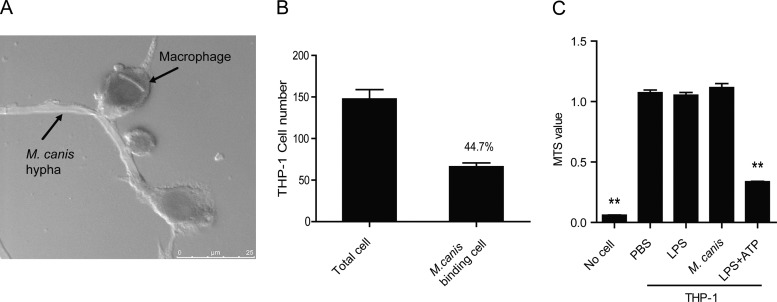
To further evaluate the interplay between M. canis and host cells during the fungal challenge, we observed the fungus-macrophage interaction via confocal microscopy. Our results showed that most of the fungus hyphae were bigger than macrophages. Figure 7A shows the general situation of the fungus-macrophage interaction, with several macrophages surrounding one fungal hypha. We randomly chose 5 view fields under microscopy and counted the macrophages that interacted with a fungal hypha. We found that about half of all the macrophages had interactions with the fungal hypha (Fig. 7B).
FIG 7.
M. canis interacts with host macrophages without inducing cell death. (A and B) THP-1-derived macrophages (1 × 105) were treated with M. canis, and the fungus-macrophage interaction was observed under confocal microscopy after PAS staining. (C) THP-1 cells (1 × 105) were challenged with PBS, LPS (100 ng/ml), or M. canis for 6 h, and the cellular mitochondrial dehydrogenase (MTS) activity was tested to measure cell viability. The combination of LPS (100 ng/ml; 6 h) and ATP (5 mM; 30 min) was used as a positive control for cell death. **, P < 0.01. Data in panel A are from one out of two independent experiments. Data in panels B and C are means ± standard deviations from one out of three independent experiments.
To demonstrate the effects of fungus challenge on the host cells, we detected mitochondrial dehydrogenase (MTS) activity from host macrophages during M. canis challenge. The results showed that M. canis challenge did not trigger a decrease of mitochondrial dehydrogenase within 6 h (Fig. 7C). As a control, LPS plus ATP, previously shown to be a stimulant of programmed cell death (45), induced a decrease of mitochondrial dehydrogenase (Fig. 7C). These data indicated that M. canis had interactions with host macrophages but did not induce cell death during the fungal challenge process.
DISCUSSION
In the present study, we investigated the host immune responses to the opportunistic pathogenic fungus M. canis, and we found that M. canis infection triggered IL-1β production and caspase-1 activation from human THP-1 cells as well as mouse BMDCs. Furthermore, our data showed that the NLRP3 inflammasome was required for M. canis-induced IL-1β secretion, and the three factors proposed to be important for NLRP3 inflammasome activation, namely, cathepsin B activity, ROS production, and K+ efflux, were all required for NLRP3 activation triggered by M. canis. Moreover, our results indicated that a heat-sensitive component was responsible for M. canis-induced IL-1β secretion.
The host innate immune system plays a critical role in combating infection from life-threatening fungal pathogens, such as C. albicans, C. neoformans, and A. fumigatus. All these fungi were recently reported to be sensed by the NLRP3 inflammasome and to trigger the secretion of IL-1β from both mouse and human cells (31–34). These data and our findings in the present study indicated that NLRP3 plays an important role in the host defense against fungal infection. Previous studies showed that fungal cell wall components, such as β-glucans, chitin, and mannans, are the major stimulants that trigger host innate immune responses (46, 47). Although we have not identified which component activates the inflammasome, our data suggested that the fungal component from M. canis responsible for the production of IL-1β is thermolabile. Further studies are needed to identify the heat-sensitive component that engages NLRP3-dependent IL-1β secretion upon M. canis infection.
NLRP3 is activated by a large variety of stimulants, including various PAMPs, DAMPs, and environmental irritants. The striking structural diversity of these stimuli argues against the idea that direct binding between NLRP3 and its known activators occurs (48). Certain upstream molecules may play the role of sensing these stimuli directly, and NLRP3 may serve as an adaptor, probably similar to the recent findings that revealed that Naip is upstream of NLRC4 for inflammasome activation upon certain bacterial infections (49, 50). Moreover, it has been reported that certain TLRs, such as TLR-2 and TLR-4, and C-type lectins, such as Dectin-1 (51) and Dectin-2 and Mincle (52, 53) are involved in recognition and clearance of fungal infections, as well as triggering proinflammatory cytokine responses (47, 54, 55). Further studies will be carried out to identify the direct upstream receptors that recognize M. canis.
Until now, the exact mechanisms that lead to NLRP3 inflammasome activation have not been fully understood. Accumulating literature suggests that the direct factor that engages NLRP3 inflammasome activation might be a certain intracellular perturbation, such as lysosome rupture, ROS production, or K+ efflux (56). Our data showed that cathepsin B, ROS production, and K+ efflux were all required for M. canis-triggered NLRP3 inflammasome activation, since the inhibition of these pathways resulted in significantly decreased secretion of IL-1β. A recent report showed that in mouse macrophages, ROS inhibitors such as DPI and NAC abolished IL-1β release through inhibition of the expression of NLRP3 and pro-IL-1β (57). Our results are consistent with these findings and showed that DPI and NAC strikingly inhibited NLRP3 and pro-IL-1β in human cells. According to previous reports (42, 43) and our own experiments (44), DPI efficiently inhibits ROS production without causing cell death; whether these two events are connected is still not clear. However, another ROS inhibitor, BHA, inhibited IL-1β secretion but not IL-8 production, speaking to a possible specific inhibition of caspase-1. For the time being, there are no better approaches other than use of inhibitors to study involvement of ROS in activation of the inflammasome. Future studies are still needed to clarify the connection between ROS inhibition and transcriptional inhibition of inflammasome components or caspase-1 activation by ROS inhibitors.
Besides IL-1β production and caspase-1 activation, ASC oligomerization is another means to monitor inflammasome activation. Interestingly, it was observed that an increased level of ASC oligomerization occurred in caspase-1-silenced cells (Fig. 2F). This phenomenon may be explained by a number of possibilities. First, previous studies showed that caspase-1 mediates the secretion of a number of unconventional proteins (58). As inflammasome components, including ASC, can be secreted into the culture supernatant (59), without caspase-1, the secretion of ASC monomers and/or oligomers might be prevented, resulting in more aggregates in the cell pellet. Second, a previous report showed that in the absence of caspase-1, ASC was able to activate caspase-8 upon Francisella infection and lead to apoptosis (60). Thus, it is possible that the oligomerization of ASC in caspase-1 knockdown THP-1 cells activates caspase-8 for apoptosis, which also involves strong ASC speck formation.
For mouse BMDC infection with M. canis, we applied LPS as a positive control. As a very potent activator of NF-κB signaling, LPS has a strong ability to prime the cells, which was indicated by the clearly higher level of pro-IL-1β and IL-6 in LPS-treated BMDCs than in M. canis-treated cells (Fig. 1G and 3B). Interestingly, we found that LPS induced a lower level of IL-1β than M. canis did (Fig. 1F). Besides the priming, the production of IL-1β requires a second activating signal (signal 2) in mouse cells. Our findings indicated that M. canis was able to give a stronger signal 2 than LPS in mouse BMDCs, but the detailed mechanism behind this difference needs further investigation.
In summary, our data suggest that the NLRP3 inflammasome functions as an important responder during M. canis infection. These findings are helpful for understanding the role of the inflammasome in fighting fungal infection. Meanwhile, our discovery suggests that M. canis infection might be controlled via regulation of the activation of the NLRP3 inflammasome.
ACKNOWLEDGMENTS
We thank Jurg Tschopp for providing shRNA constructs against human NLRP3, caspase-1, ASC. and Scramble. We are grateful to Warren Strober for sharing NLRP3-deficient mice and Vishva M. Dixit for providing Asc-deficient mice.
This work was supported by grants from the 100 Talent Program of the Chinese Academy of Sciences, Natural Science Foundation of China (81360237, 81160190, 91029707, 31170868, 812111134, 31100622), Shanghai Natural Science Foundation (11ZR1442600), SA-SIBS Scholarship Program, Chinese Post-Doctoral Science Foundation (20110490752), and the Post-Doctoral Research Foundation of Shanghai Institutes for Biological Sciences (2011KIP513), as well as the CAS/SAFEA International Partnership Program for Creative Research Teams.
Footnotes
Published ahead of print 9 December 2013
REFERENCES
- 1.Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140:805–820. 10.1016/j.cell.2010.01.022 [DOI] [PubMed] [Google Scholar]
- 2.Schroder K, Tschopp J. 2010. The inflammasomes. Cell 140:821–832. 10.1016/j.cell.2010.01.040 [DOI] [PubMed] [Google Scholar]
- 3.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. 2009. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10:241–247. 10.1038/ni.1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lupfer C, Kanneganti TD. 2013. Unsolved mysteries in NLR biology. Front. Immunol. 4:285. 10.3389/fimmu.2013.00285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vautier S, MacCallum DM, Brown GD. 2012. C-type lectin receptors and cytokines in fungal immunity. Cytokine 58:89–99. 10.1016/j.cyto.2011.08.031 [DOI] [PubMed] [Google Scholar]
- 6.Romani L. 2011. Immunity to fungal infections. Nat. Rev. Immunol. 11:275–288. 10.1038/nri2939 [DOI] [PubMed] [Google Scholar]
- 7.Dinarello CA, Simon A, van der Meer JW. 2012. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11:633–652. 10.1038/nrd3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andriasian SG. 1978. Epidemiological characteristics of the microsporosis caused by Microsporum canis. Zh. Mikrobiol. Epidemiol. Immunobiol. 1978(10):14–20 (In Russian.) [PubMed] [Google Scholar]
- 9.Merino FJ, del Pozo LJ, Campos A, Goberna F, Sanchez R, Osta P. 1991. Outbreak of dermatophytosis caused by Microsporum canis in a closed community in the province of Soria. Enferm. Infecc. Microbiol. Clin. 9:381–382 (In Spanish.) [PubMed] [Google Scholar]
- 10.Brillowska-Dabrowska A, Michalek E, Saunte DM, Nielsen SS, Arendrup MC. 2013. PCR test for Microsporum canis identification. Med. Mycol. 51:576–579. 10.3109/13693786.2012.755741 [DOI] [PubMed] [Google Scholar]
- 11.Sharma R, de Hoog S, Presber W, Graser Y. 2007. A virulent genotype of Microsporum canis is responsible for the majority of human infections. J. Med. Microbiol. 56:1377–1385. 10.1099/jmmm.0.47136-0 [DOI] [PubMed] [Google Scholar]
- 12.Yu J, Wan Z, Chen W, Wang W, Li R. 2004. Molecular typing study of the Microsporum canis strains isolated from an outbreak of tinea capitis in a school. Mycopathologia 157:37–41. 10.1023/B:MYCO.0000012221.66851.68 [DOI] [PubMed] [Google Scholar]
- 13.Vermout S, Baldo A, Tabart J, Losson B, Mignon B. 2008. Secreted dipeptidyl peptidases as potential virulence factors for Microsporum canis. FEMS Immunol. Med. Microbiol. 54:299–308. 10.1111/j,1574-695X.2008.00479.x [DOI] [PubMed] [Google Scholar]
- 14.Berg JC, Hamacher KL, Roberts GD. 2007. Pseudomycetoma caused by Microsporum canis in an immunosuppressed patient: a case report and review of the literature. J. Cutan. Pathol. 34:431–434. 10.1111/j.1600-0560.2006.00628.x [DOI] [PubMed] [Google Scholar]
- 15.Anemuller W, Baumgartner S, Brasch J. 2008. Atypical Microsporum canis variant in an immunosuppressed child. J. Dtsch. Dermatol. Ges. 6:473–475. 10.1111/j.1610-0387.2007.06458.x [DOI] [PubMed] [Google Scholar]
- 16.King D, Cheever LW, Hood A, Horn TD, Rinaldi MG, Merz WG. 1996. Primary invasive cutaneous Microsporum canis infections in immunocompromised patients. J. Clin. Microbiol. 34:460–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bournerias I, De Chauvin MF, Datry A, Chambrette I, Carriere J, Devidas A, Blanc F. 1996. Unusual Microsporum canis infections in adult HIV patients. J. Am. Acad. Dermatol. 35:808–810 [DOI] [PubMed] [Google Scholar]
- 18.Lowinger-Seoane M, Torres-Rodriguez JM, Madrenys-Brunet N, Aregall-Fuste S, Saballs P. 1992. Extensive dermatophytoses caused by Trichophyton mentagrophytes and Microsporum canis in a patient with AIDS. Mycopathologia 120:143–146. 10.1007/BF00436391 [DOI] [PubMed] [Google Scholar]
- 19.Binder B, Lackner HK, Poessl BD, Propst E, Weger W, Smolle J, Ginter-Hanselmayer G. 2011. Prevalence of tinea capitis in southeastern Austria between 1985 and 2008: up-to-date picture of the current situation. Mycoses 54:243–247. 10.1111/j.1439-0507.2009.01804.x [DOI] [PubMed] [Google Scholar]
- 20.del Boz J, Crespo V, Rivas-Ruiz F, de Troya M. 2011. A 30-year survey of paediatric tinea capitis in southern Spain. J. Eur. Acad. Dermatol. Venereol. 25:170–174. 10.1111/j.1468-3083.2010.03733.x [DOI] [PubMed] [Google Scholar]
- 21.Costa M, Passos XS, Hasimoto e Souza LK, Miranda AT, Lemos Jde A, Oliveira JG, Jr, Silva Mdo R. 2002. Epidemiology and etiology of dermatophytosis in Goiania, GO, Brazil. Rev. Soc. Bras. Med. Trop. 35:19–22 (In Portugese) http://dx.doi.org/10.1590/S0037-86822002000100004 [DOI] [PubMed] [Google Scholar]
- 22.Fernandes NC, Akiti T, Barreiros MG. 2001. Dermatophytoses in children: study of 137 cases. Rev. Inst. Med. Trop. Sao Paulo 43:83–85. 10.1590/S0036-46652001000200006 [DOI] [PubMed] [Google Scholar]
- 23.Yin B, Xiao Y, Ran Y, Kang D, Dai Y, Lama J. 2013. Microsporum canis infection in three familial cases with tinea capitis and tinea corporis. Mycopathologia 176:259–265. 10.1007/s11046-013-9685-5 [DOI] [PubMed] [Google Scholar]
- 24.Zhu M, Li L, Wang J, Zhang C, Kang K, Zhang Q. 2010. Tinea capitis in southeastern China: a 16-year survey. Mycopathologia 169:235–239. 10.1007/s11046-009-9260-2 [DOI] [PubMed] [Google Scholar]
- 25.Li C, Liu W. 2011. Epidemiology of tinea capitis among children in China in recent years: a retrospective analysis. Chinese J. Mycol. 6:77–82. 10.3969/j.issn.1673-3827.2011.02.003 [DOI] [Google Scholar]
- 26.Hontelez S, Sanecka A, Netea MG, van Spriel AB, Adema GJ. 2012. Molecular view on PRR cross-talk in antifungal immunity. Cell Microbiol. 14:467–474. 10.1111/j.1462-5822.2012.01748.x [DOI] [PubMed] [Google Scholar]
- 27.Roeder A, Kirschning CJ, Rupec RA, Schaller M, Weindl G, Korting HC. 2004. Toll-like receptors as key mediators in innate antifungal immunity. Med. Mycol. 42:485–498. 10.1080/13693780400011112 [DOI] [PubMed] [Google Scholar]
- 28.Cunha C, Di Ianni M, Bozza S, Giovannini G, Zagarella S, Zelante T, D'Angelo C, Pierini A, Pitzurra L, Falzetti F, Carotti A, Perruccio K, Latge JP, Rodrigues F, Velardi A, Aversa F, Romani L, Carvalho A. 2010. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood 116:5394–5402. 10.1182/blood-2010-04-279307 [DOI] [PubMed] [Google Scholar]
- 29.Hardison SE, Brown GD. 2012. C-type lectin receptors orchestrate antifungal immunity. Nat. Immunol. 13:817–822. 10.1038/ni.2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lionakis MS, Holland SM. 2013. CARD9: at the intersection of mucosal and systemic antifungal immunity. Blood 121:2377–2378. 10.1182/blood-2013-01-480434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Said-Sadier N, Padilla E, Langsley G, Ojcius DM. 2010. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS One 5(4):e10008. 10.1371/journal.pone.0010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. 2009. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5:487–497. 10.1016/j.chom.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. 2009. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459:433–436. 10.1038/nature07965 [DOI] [PubMed] [Google Scholar]
- 34.Lei G, Chen M, Li H, Niu JL, Wu S, Mao L, Lu A, Wang H, Chen W, Xu B, Leng Q, Xu C, Yang G, An L, Zhu LP, Meng G. 2013. Biofilm from a clinical strain of Cryptococcus neoformans activates the NLRP3 inflammasome. Cell Res. 23:965–968. 10.1038/cr.2013.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joly S, Eisenbarth SC, Olivier AK, Williams A, Kaplan DH, Cassel SL, Flavell RA, Sutterwala FS. 2012. Cutting edge: Nlrp10 is essential for protective antifungal adaptive immunity against Candida albicans. J. Immunol. 189:4713–4717. 10.4049/jimmunol.1201715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cambier L, Mathy A, Baldo A, Bagut ET, Tabart J, Antoine N, Mignon B. 2013. Feline polymorphonuclear neutrophils produce pro-inflammatory cytokines following exposure to Microsporum canis. Vet. Microbiol. 162:800–805. 10.1016/j.vetmic.2012.10.016 [DOI] [PubMed] [Google Scholar]
- 37.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. 2007. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14:1583–1589. 10.1038/sj.cdd.4402195 [DOI] [PubMed] [Google Scholar]
- 38.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263–267. 10.1126/science.272.5259.263 [DOI] [PubMed] [Google Scholar]
- 39.Mao K, Chen S, Chen M, Ma Y, Wang Y, Huang B, He Z, Zeng Y, Hu Y, Sun S, Li J, Wu X, Wang X, Strober W, Chen C, Meng G, Sun B. 2013. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res. 23:201–212. 10.1038/cr.2013.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandes-Alnemri T, Alnemri ES. 2008. Assembly, purification, and assay of the activity of the ASC pyroptosome. Methods Enzymol. 442:251–270. 10.1016/S0076-6879(08)01413-4 [DOI] [PubMed] [Google Scholar]
- 41.Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TB. 2012. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat. Immunol. 13:246–254. 10.1038/ni.2222 [DOI] [PubMed] [Google Scholar]
- 42.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. 2008. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl. Acad. Sci. U. S. A. 105:9035–9040. 10.1073/pnas.0803933105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. 2007. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 282:2871–2879. 10.1074/jbc.M608083200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Y, Li H, Chen W, Yao X, Xing Y, Wang X, Zhong J, Meng G. 2013. Mycoplasma hyorhinis activates the NLRP3 inflammasome and promotes migration and invasion of gastric cancer cells. PLoS One 8(11):e77955. 10.1371/journal.pone.0077955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng LM, Zychlinsky A, Liu CC, Ojcius DM, Young JD. 1991. Extracellular ATP as a trigger for apoptosis or programmed cell death. J. Cell Biol. 112:279–288. 10.1083/jcb.112.2.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kankkunen P, Teirila L, Rintahaka J, Alenius H, Wolff H, Matikainen S. 2010. (1,3)-β-Glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J. Immunol. 184:6335–6342. 10.4049/jimmunol.0903019 [DOI] [PubMed] [Google Scholar]
- 47.Hohl TM, Van Epps HL, Rivera A, Morgan LA, Chen PL, Feldmesser M, Pamer EG. 2005. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 1(3):e30. 10.1371/journal.ppat.0010030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng G, Strober W. 2010. New insights into the nature of autoinflammatory diseases from mice with Nlrp3 mutations. Eur. J. Immunol. 40:649–653. 10.1002/eji.200940191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kofoed EM, Vance RE. 2011. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477:592–595. 10.1038/nature10394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477:596–600. 10.1038/nature10510 [DOI] [PubMed] [Google Scholar]
- 51.Gersuk GM, Underhill DM, Zhu L, Marr KA. 2006. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J. Immunol. 176:3717–3724 http://www.jimmunol.org/content/176/3717.long [DOI] [PubMed] [Google Scholar]
- 52.Bugarcic A, Hitchens K, Beckhouse AG, Wells CA, Ashman RB, Blanchard H. 2008. Human and mouse macrophage-inducible C-type lectin (Mincle) bind Candida albicans. Glycobiology 18:679–685. 10.1093/glycob/cwn046. [DOI] [PubMed] [Google Scholar]
- 53.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. 2008. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat. Immunol. 9:1179–1188. 10.1038/ni.1651 [DOI] [PubMed] [Google Scholar]
- 54.Bretz C, Gersuk G, Knoblaugh S, Chaudhary N, Randolph-Habecker J, Hackman RC, Staab J, Marr KA. 2008. MyD88 signaling contributes to early pulmonary responses to Aspergillus fumigatus. Infect. Immun. 76:952–958. 10.1128/IAI.00927-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD, Steele C. 2009. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 182:4938–4946. 10.4049/jimmunol.0804250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schroder K, Zhou R, Tschopp J. 2010. The NLRP3 inflammasome: a sensor for metabolic danger? Science 327:296–300. 10.1126/science.1184003 [DOI] [PubMed] [Google Scholar]
- 57.Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V. 2011. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 187:613–617. 10.4049/jimmunol.1100613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keller M, Ruegg A, Werner S, Beer HD. 2008. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132:818–831. 10.1016/j.cell.2007.12.040 [DOI] [PubMed] [Google Scholar]
- 59.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. 2004. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430:213–218. 10.1038/nature02664 [DOI] [PubMed] [Google Scholar]
- 60.Pierini R, Juruj C, Perret M, Jones CL, Mangeot P, Weiss DS, Henry T. 2012. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 19:1709–1721. 10.1038/cdd.2012.51 [DOI] [PMC free article] [PubMed] [Google Scholar]