Abstract
Evidence from clinical trials of malaria vaccine candidates suggests that both cell-mediated and humoral immunity to pre-erythrocytic parasite stages can provide protection against infection. Novel pre-erythrocytic antibody (Ab) targets could be key to improving vaccine formulations, which are currently based on targeting antigens such as the circumsporozoite protein (CSP). However, methods to assess the effects of sporozoite-specific Abs on pre-erythrocytic infection in vivo remain underdeveloped. Here, we combined passive transfer of monoclonal Abs (MAbs) or immune serum with a luciferase-expressing Plasmodium yoelii sporozoite challenge to assess Ab-mediated inhibition of liver infection in mice. Passive transfer of a P. yoelii CSP MAb showed inhibition of liver infection when mice were challenged with sporozoites either intravenously or by infectious mosquito bite. However, inhibition was most potent for the mosquito bite challenge, leading to a more significant reduction of liver-stage burden and even a lack of progression to blood-stage parasitemia. This suggests that Abs provide effective protection against a natural infection. Inhibition of liver infection was also achieved by passive transfer of immune serum from whole-parasite-immunized mice. Furthermore, we demonstrated that passive transfer of a MAb against P. falciparum CSP inhibited liver-stage infection in a humanized mouse/P. falciparum challenge model. Together, these models constitute unique and sensitive in vivo methods to assess serum-transferable protection against Plasmodium sporozoite challenge.
INTRODUCTION
Malaria is a mosquito-borne disease caused by Plasmodium parasites, estimated to infect up to 289 million people annually, with nearly 1 million of those, mostly children, succumbing to death from the disease (43). The parasite life cycle progresses from sporozoite inoculation at the site of mosquito bite in the skin through the circulation to the liver, where each parasite multiplies as a liver stage within a single hepatocyte. Following liver-stage development, the parasite progresses to the blood-stage infection, which is the cause of all malaria-associated morbidity and mortality. Unfortunately, immunity that develops in response to natural parasite exposure is nonsterile and no fully protective malaria vaccine is currently available. Only one vaccine candidate to date, RTS,S, has progressed to phase III clinical trials. RTS,S is a subunit vaccine targeting the pre-erythrocytic antigen circumsporozoite protein (CSP) and showed promising preclinical and early clinical results (1). However, data from trials in areas of malaria endemicity show variable and short-lived vaccine efficacy depending on age group and transmission intensity (2, 3). Individuals that were protected showed higher levels of anti-CSP IgG and CD4+ T cell responses, indicating a role for both humoral and cell-mediated immunity in this vaccination model (3, 4).
In contrast, the only experimental vaccination to provide complete protection in humans to date has been via mosquito bite administration of radiation-attenuated sporozoites (RAS) (5) and, more recently, by intravenous (i.v.) administration of cryopreserved RAS (6) as well as infectious mosquito bite under antimalarial drug cover (7, 8). RAS infect the liver, suffer growth arrest, and fail to progress to blood-stage infection—allowing recognition of the pre-erythrocytic parasite by the immune system while avoiding clinical infection (9). High levels of protection can also be achieved experimentally in mice using genetically attenuated parasites (GAP) that can progress further through liver-stage development than RAS but also fail to develop to the blood stage (10, 11). While whole-sporozoite immunization strategies face manufacturing and delivery challenges, they constitute extremely useful models to elucidate mechanisms of pre-erythrocytic immunity and to identify parasite antigen targets for protection.
Cell-mediated immunity has been thought of as essential and even as the sole arm of the immune system necessary for protection against pre-erythrocytic infection via whole-sporozoite immunization. These conclusions arose from numerous studies in rodent models using immunization with RAS, where depletion of CD8+ T cells completely ablated protection from infectious sporozoite challenge in contrast to the maintenance of protection in animals lacking Abs or CD4+ T cells (12–15). However, those studies all utilized challenge by i.v. injection of sporozoites, which might not lend itself to observation of Ab function against sporozoite infection by the natural route (16).
Following mosquito bite, the sporozoite first migrates in the dermal tissue and then enters the circulation by traversing the endothelium of skin capillaries (17). Once it arrives in the liver, the sporozoite crosses the sinusoidal cell lining, completing its path to the liver (18, 19). Thus, an i.v. sporozoite challenge model does not assess Ab activity against the skin traversal phase. Antibodies have been shown to immobilize sporozoites in the dermis and even at the mosquito proboscis—both limiting the chances of a sporozoite successfully reaching the liver (20, 21). Recent data from clinical trials investigating immunization with novel DNA/adenoviral vaccine constructs, RTS,S, and RAS all support the idea of a critical role for these Ab-mediated mechanisms, as protection often correlates with humoral immunity, despite the generation of robust cell-mediated immune responses (4, 22–25).
Future vaccines will need to incorporate a strategy to evoke long-lasting Ab responses as well as T cell-mediated cellular immunity. To date, only a few sporozoite Ab targets have been investigated and identification of new target antigens should be a key priority in the near term (26). For example, recent work has identified a number of putative surface-exposed proteins on the sporozoite (27), posing the possibility of novel potential target antigens to block sporozoite infection via Abs. Passive immunization with Abs allows analysis of Ab efficacy without the effects of cell-mediated immunity. In mice, P. yoelii has served as one of the standard rodent malaria models in which to study both subunit vaccines and whole-sporozoite immunizations as well as passive transfer of Abs for protection against pre-erythrocytic malaria (15, 28–31). However, the tools with which to quantitatively assess the effects of these Abs have remained limited and relatively unchanged in recent decades.
Here, we set out to determine if bioluminescent imaging of liver-stage burden could be used to evaluate humoral immunity by passive transfer of MAbs or serum from GAP-immunized mice and under what conditions this assay is most sensitive. By using a new MAb against the P. yoelii CSP repeat region, we found that passive transfer of the MAb was effective at reducing liver infection after sporozoite challenge as measured by both quantitative PCR (qPCR) and bioluminescent imaging. Sporozoite challenge by mosquito bite was found to be more sensitive to MAb blocking than i.v. challenge, especially when passive transfer was performed 24 h prior to challenge, and ultimately was able to confer sterile protection (absence of blood-stage parasitemia). Passive transfer of serum from animals immunized with either early- or late-liver-stage-arresting GAPs (32, 33) was also effective at reducing liver infection as measured by bioluminescent imaging.
Finally, utilizing a recently developed, robust human liver-chimeric mouse model that supports infection with luciferase-expressing P. falciparum sporozoites (34, 35), we demonstrate Ab-mediated inhibition of P. falciparum liver infection.
MATERIALS AND METHODS
Mice.
Female Swiss Webster (SW) mice were purchased from Harlan Laboratories. Female C57BL/6 and BALB/cJ mice, 6 to 8 weeks of age, were purchased from Jackson Laboratories. FRG huHep mice were purchased from the Yecuris Corporation. All studies were performed according to the regulations of the Institutional Animal Care and Use Committee (IACUC). Approval was obtained from the Seattle BioMed Experimental Animal Ethical Committee (OLAW assurance no. A3640-01).
Parasite growth and sporozoite isolation.
Six-to-eight-week-old female SW mice were injected with blood from P. yoelii green fluorescent protein-luc (GFP-luc) or P. yoelii 17XNL wild-type (WT)-infected mice to begin the growth cycle. The infected mice were used to feed female Anopheles stephensi mosquitoes after gametocyte exflagellation was observed. P. falciparum GFP-luc parasites were maintained as previously described (34). Briefly, in vitro P. falciparum NF54HT–GFP-luc blood-stage cultures were maintained in RPMI 1640 (25 mM HEPES, 2 mM l-glutamine) supplemented with 50 μM hypoxanthine and 10% A+ human serum in an atmosphere of 5% CO2, 5% O2, and 90% N2. Gametocyte cultures were initiated at 5% hematocrit and 0.8 to 1% parasitemia (mixed stages) and maintained for up to 17 days with daily medium changes. Non-blood-fed adult female mosquitoes at 3 to 7 days postemergence were fed on gametocyte cultures. For both P. yoelii and P. falciparum parasites, 10 days after the blood meal, 15 to 20 mosquitoes were dissected to evaluate midgut oocyst formation. On days 14 through 17 post-infectious-blood meal, salivary gland sporozoites were isolated from the mosquitoes for experimentation.
MAbs to P. yoelii CSP.
A mouse was immunized with 100,000 P. yoelii RAS and boosted twice at 30-day intervals. Four days after the last boost, the mouse was sacrificed, the spleen was removed and dissociated, and a fusion to SP-2 cells was performed following standard protocols (44). Production of Abs specific for P. yoelii sporozoites by individual hybridoma cultures was assessed by immunofluorescence using air-dried sporozoites and enzyme-linked immunosorbent assays (ELISAs) using peptides from different regions of the circumsporozoite protein. Hybridoma 2F6 produced an Ab that was specific for the QQPP repeat region of P. yoelii CSP. Hybridoma clone 2A10 specific for P. falciparum CSP was obtained from MR4. ProMab Biotechnologies, Inc., performed antibody production and purification from each hybridoma.
Infection assays.
BALB/cJ and C57BL/6 mice were infected with P. yoelii GFP-luc or WT P. yoelii salivary gland sporozoites either by i.v. tail vein injection or by infectious mosquito bite. For i.v. injections, salivary gland sporozoites were enumerated and resuspended in 200 μl RPMI medium prior to injection of the indicated number of sporozoites per mouse as described in the figure legends. For mosquito bite infections, animals were anesthetized with a mixture of ketamine and xylazine and placed on a feeding cage containing 15 infected mosquitoes. Mosquitoes were allowed to feed on the mice for 10 min. Mice were lifted every minute and rotated among feeding cages every 2 min to ensure that all mice were equally exposed to infected mosquito bites. A sample of at least 20 mosquitoes from each feed was dissected, and salivary gland average sporozoite counts, with a lower cutoff value of an average of 10,000 sporozoites per mosquito for a mosquito feed, were assessed to ensure sufficient mosquito infection.
Real-time in vivo imaging of liver-stage development in whole bodies of live mice.
Luciferase activity in mice was visualized through imaging of whole bodies using an in vivo imaging system (IVIS) (Caliper Life Sciences) according to the specifications previously described (36). For C57BL/6 and FRG huHep mice, their abdomens were shaved prior to imaging to minimize the absorption of light by the pigmented fur. Mice were intraperitoneally injected with 100 μl of RediJect d-luciferin (PerkinElmer) prior to being anesthetized using isoflurane anesthesia (XGI-8; Caliper Life Sciences). Animals were kept anesthetized during bioluminescent imaging, which was performed within 5 to 10 min after the injection of d-luciferin. Imaging was acquired with a 10-cm-diameter field of view (FOV), a medium binning factor, and an exposure time of 2 min. Quantitative analysis of bioluminescence was performed by measuring the luminescence signal intensity utilizing the region of interest (ROI) settings of Living Image 3.0 software (XGI-8; Caliper Life Sciences). Copies of ROIs were placed around the abdominal area at the location of the liver for each mouse, and ROI measurements were expressed as total flux values (in photons/second [p/s]). Background flux was determined by placing ROIs over the pelvis of each mouse where luminescence should be absent at 48 h postinfection.
Passive Ab transfer.
BALB/cJ mice were injected i.v. with MAb to P. yoelii CSP (clone 2F6), FRG huHep mice were injected i.v. with MAb to P. falciparum CSP (clone 2A10), and both strains of mice were injected with control mouse IgG (mIgG) (Sigma) as specified in the figure legends. Thirty minutes or 24 h following injection of MAb, mice were infected with GFP-luc-expressing sporozoites by i.v. injection or by the bites of 15 infected mosquitoes as described above. Bioluminescence was measured 48 h following infection for P. yoelii and 6 days following infection for P. falciparum.
qPCR.
Total RNA was extracted from P. yoelii GFP-luc-infected livers using TRIzol reagent (Invitrogen) and treated with Turbo DNase (Ambion). Synthesis of cDNA was performed using a SuperScript III Platinum two-step quantitative reverse transcription-PCR (qRT-PCR) kit according to the instructions of the manufacturer (Invitrogen). Primers used for amplification of 18S from cDNA were 18S-fwd (GGGGATTGGTTTTGACGTTTTTGCG) and 18S-rev (AAGCATTAAATAAAGCGAATACATCCTTAT). Murine GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was amplified from cDNA using the gapdh-fwd (CCTCAACTACATGGTTTACAT) and gapdh-rev (GCTCCTGGAAGATGGTGATG) primers. All PCR amplification cycles were performed at 95°C for 30 s for DNA denaturation and at 60°C for 4 min for primer annealing and DNA strand extension. For semiquantitative PCR (semi-qPCR), a standard curve was generated using 1:4 dilutions of a reference cDNA sample for PCR amplification of all target PCR products. Experimental samples were compared to this standard curve to give relative abundances of transcript. All signals were normalized to the average abundance of transcript from a reference sample as designated in the figure legends.
Serum passive transfer.
For serum passive transfer, groups of 10 C57BL/6 mice were immunized i.v. with 5 × 104 P. yoelii fabb/f− or P. yoelii sap1− (32, 33) salivary gland sporozoites twice at 4-week intervals. Two weeks after the second immunization, mice were bled via the retro-orbital plexus and blood was collected into BD Microtainer serum separation tubes and allowed to clot at room temperature for 30 min before being subjected to spinning at 10,000 × g in a tabletop centrifuge for 90 s to separate the serum. All samples from the same experimental group of mice were pooled, and 300-μl volumes of serum from immunized mice or serum from naive C57BL/6 mice were injected i.v. 24 h prior to subsequent sporozoite challenge.
Statistical analysis.
Analysis of differences between treatment groups was performed using one-way analysis of variance (ANOVA) and Dunnett's posttest, where relevant comparisons were made between the groups of control (mIgG-treated) mice and experimental (MAb-treated) mice. Tukey's posttest was used for multiple comparisons where indicated, and Student's t test was used for comparisons between two groups as indicated in the figure legends. All analyses were carried out using GraphPad Prism software. Statistical significance is indicated in the figures as follows: *, 0.05 ≥ P > 0.01; **, 0.01 ≥ P > 0.001; ***, 0.001 ≥ P > 0.0001; ****, P ≤ 0.0001; NS, nonsignificant (P ≥ 0.05).
RESULTS
Passive transfer of a P. yoelii CSP MAb reduces P. yoelii liver infection in mice following i.v. sporozoite and mosquito bite challenge.
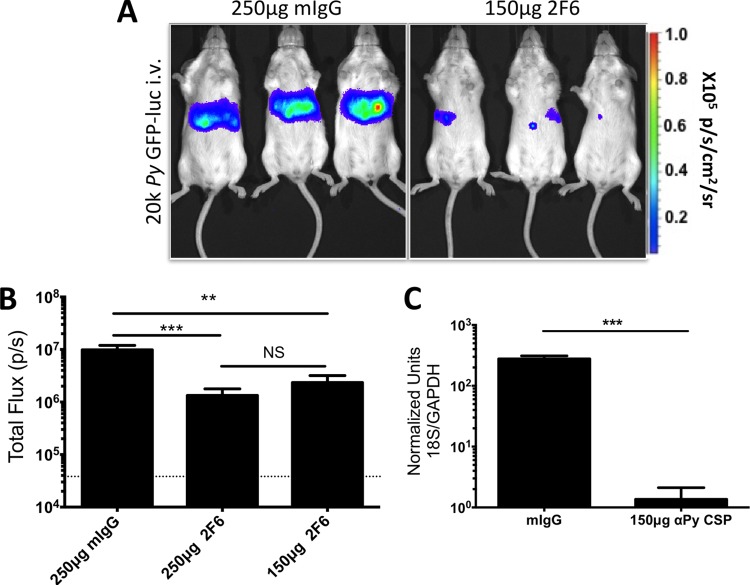
The novel 2F6 MAb against the P. yoelii CSP QQPP repeats blocked sporozoite host cell invasion and traversal in vitro (see Fig. S1 in the supplemental material). We thus determined if Ab-mediated reduction of liver infection could be demonstrated in vivo by passive transfer of MAb. The P. yoelii GFP-luc parasite expresses luciferase throughout the life cycle, allows noninvasive quantification of parasite burden in the liver using bioluminescent imaging (36–38), and was used for these studies. To test if blocking of infection by MAb can be detected in this manner, we first passively transferred either 250 μg or 150 μg of 2F6 MAb (or mIgG as a control) into BALB/cJ mice via i.v. injection 30 min prior to i.v. challenge with 20,000 P. yoelii GFP-luc sporozoites. At 48 h after challenge, when liver-stage development is almost complete, mice were injected with luciferin substrate and bioluminescent imaging was used for assessing liver-stage burden. Both doses of MAb significantly reduced liver-stage burden in challenged mice, and there was no statistically significant difference between the results seen with the 250-μg (7-fold) and 150-μg (4-fold) doses, suggesting saturation at both doses (Fig. 1A and B). This effect was confirmed by qPCR, which was used to measure parasite rRNA in whole infected livers of a subset of mice immediately after bioluminescent imaging (Fig. 1C). Although passive transfer of the 2F6 MAb achieved a significant reduction of liver-stage burden, all mice went on to develop blood-stage parasitemia (data not shown).
FIG 1.
Sporozoite-specific Ab reduces liver infection in the i.v. P. yoelii (Py) sporozoite challenge model. BALB/c mice (n = 6 to 8 total over two independent experiments) were i.v. injected with 250 μg of mIgG (n = 7 total) or 250 μg (n = 8 total) or 150 μg (n = 6 total) of MAb 2F6 30 min prior to receiving an i.v. challenge of 20,000 P. yoelii GFP-luc salivary gland sporozoites. At 48 h postchallenge, liver-stage parasite burden was quantified by bioluminescent imaging. (A and B) Representative images of mice injected with 250 μg mIgG and 150 μg 2F6 MAb are shown in panel A, with quantification of total flux shown in panel B. (C) Immediately after imaging, mice were sacrificed and liver samples were collected for qPCR quantification of liver-stage burden. Graphs show mean total flux values ± standard deviations (SD), with mean background luminescence indicated by the horizontal dotted line. Asterisks indicate a P value < 0.05 as determined by one-way ANOVA in panel B and Student's t test in panel C. Degrees of significance are indicated as follows: **, 0.01 ≥ P > 0.001; ***, 0.001 ≥ P > 0.0001; NS, nonsignificant (P ≥ 0.05).
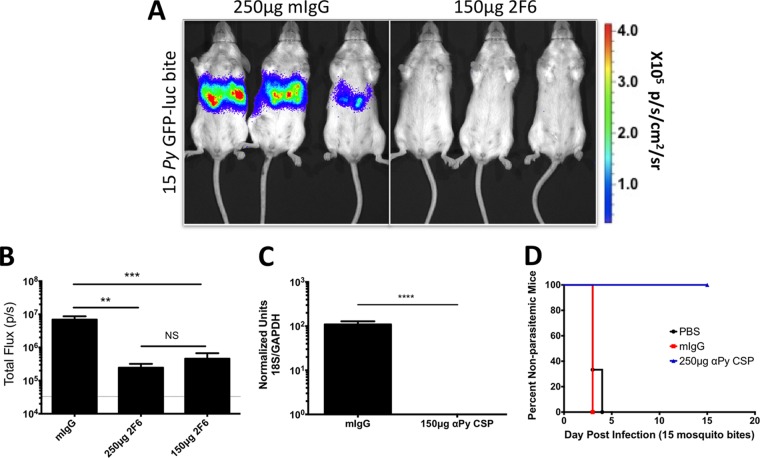
To determine assay sensitivity and Ab efficacy in the more biologically relevant mosquito bite challenge model, BALB/cJ mice were i.v. injected with 250 μg or 150 μg of 2F6 MAb 30 min prior to challenge by the bites of 15 P. yoelii GFP-luc-infected mosquitoes. Liver-stage burden was determined by bioluminescent imaging 48 h later. Both the dose of 250 μg and the dose of 150 μg significantly reduced liver-stage burden as measured by bioluminescence and qPCR (Fig. 2A to C). Interestingly, passive transfer of 250 μg of MAb completely protected all mice, as indicated by the absence of blood-stage parasitemia after mosquito bite challenge (Fig. 2D). This stands out in contrast to passive transfer and i.v. sporozoite challenge, in which all mice became blood-stage patent. Thus, bioluminescent imaging is able to detect a reduction in liver-stage infection following passive transfer of Abs and subsequent i.v. sporozoite challenge or mosquito bite challenge.
FIG 2.
Liver infection is decreased after sporozoite-specific Ab transfer prior to P. yoelii sporozoite challenge by mosquito bite. BALB/c mice (n = 8 to 9 mice/group total over 2 independent experiments) were i.v. injected with 250 μg of mIgG (n = 9 total) or 250 μg (n = 8 total) or 150 μg (n = 8 total) of MAb 2F6 30 min prior to receiving a challenge with bites from 15 P. yoelii GFP-luc-infected mosquitoes. At 48 h later, liver-stage parasite burden was assessed via bioluminescent imaging. (A and B) Representative images of bioluminescence are shown in panel A, with quantification of total flux shown in panel B. (C) Immediately after imaging, a subset of mice were sacrificed and liver samples were collected for qPCR quantification of liver-stage burden. (D) Mice were also tracked for blood-stage parasitemia by Giemsa-stained thin blood smear analysis. Graphs show mean total flux ± SD, with mean background luminescence indicated by the dotted lines on the y axis. Asterisks indicate a P value < 0.05 as determined by one-way ANOVA in panel B and Student's t test in panel C. Degrees of significance are indicated as follows: **, 0.01 ≥ P > 0.001; ***, 0.001 ≥ P > 0.0001; ****, P ≤ 0.0001; NS, nonsignificant (P ≥ 0.05).
Mosquito bite sporozoite challenge is more susceptible to MAb-mediated inhibition of infection.
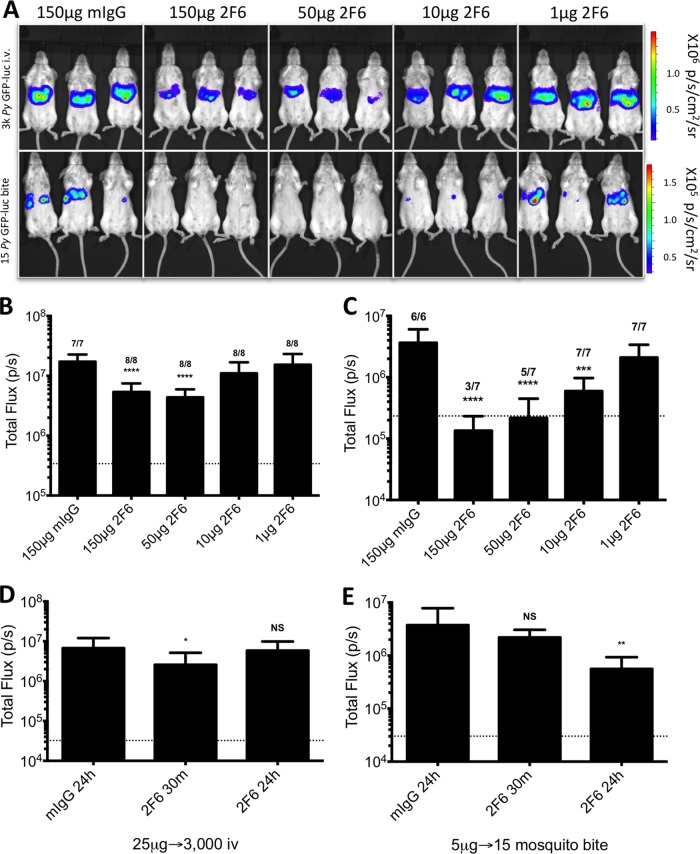
Future screening of MAbs in this system will require the ability to distinguish between Abs with a range of efficacies in blocking sporozoite infection. Therefore, we determined the lower Ab dose limits of our protocol and determined the optimal passive transfer/challenge method to most sensitively detect inhibition of sporozoite infection. BALB/cJ mice were passively immunized with 150 μg, 50 μg, 10 μg, or 1 μg of 2F6 or 150 μg of mIgG as a control 30 min prior to challenge either with 3,000 P. yoelii GFP-luc sporozoites injected i.v. or by the bites of 15 P. yoelii GFP-luc-infected mosquitoes. The reduced sporozoite dose was chosen as it results in a consistent i.v. sporozoite challenge with less than a 2-fold difference in liver-stage burden compared to challenge by bites of 15 mosquitoes (see Fig. S2 in the supplemental material). This more equivalent infection rate allowed us to make direct comparisons between mosquito bite and i.v. challenge (36, 39). Passive transfer of 2F6 MAb significantly reduced liver infection at 150 μg and 50 μg per mouse 3.2- and 3.9-fold compared to mIgG, respectively, for the i.v. challenge, but the results were not significantly different from each other (Fig. 3A and B). However, immunization with 10 μg and 1 μg per mouse did not affect liver infection levels as measured by bioluminescent imaging (Fig. 3A and B). Yet administration of the same Ab doses prior to mosquito bite challenge showed a much higher level of inhibition than i.v. challenge, with 27-, 17-, and 6-fold decreases in liver-stage burden at 150 μg, 50 μg, and 10 μg, respectively (Fig. 3A and C). Furthermore, passive transfer of 150 μg and 50 μg of MAb was able to prevent the onset of blood-stage parasitemia in some mosquito bite-infected mice, whereas none of the doses administered prior to i.v. challenge prevented onset of blood-stage parasitemia in any mice (Fig. 3B and C). Based on these experiments, we conclude that bioluminescence imaging is able to quantitatively assess inhibition of liver infection mediated by administration of decreasing MAb doses. Furthermore, sporozoite challenge via mosquito bite is more sensitive to MAb inhibition than i.v. sporozoite challenge at a given dose of MAb.
FIG 3.
Passive transfer of MAb reveals distinct differences in Ab blocking between i.v. challenge and mosquito bite challenge. BALB/c mice were i.v. injected with the indicated doses of 2F6 MAb 30 min prior to i.v. challenge with 3,000 P. yoelii GFP-luc sporozoites (A [top panel] and B; n = 7 mice/group total for 150 μg mIgG and n = 8 total for all other groups, over two independent experiments) or challenge by the bites of 15 P. yoelii GFP-luc-infected mosquitoes (A [bottom panel] and C; n = 6 mice/group total for 150 μg mIgG and n = 7 total for all other groups, over two independent experiments). At 48 h later, liver-stage parasite burden was assessed via bioluminescent imaging. Representative images for each group are shown in panel A, with quantification of total flux for each group shown in panels B and C. (B and C) The numbers above the columns indicate numbers of mice that became blood-stage patent over both experiments. (D and E) Mice (n = 6 to 9 mice/group total over two independent experiments) were administered 2F6 Ab either 24 h or 30 min prior to P. yoelii GFP-luc challenge. Mice were given 25 μg of MAb 2F6 (n = 8 for mIgG; n = 9 for 2F6 for 30 m; n = 8 for 2F6 for 24 h [in total for two independent experiments]) and challenged with 3,000 P. yoelii GFP-luc sporozoites (D) or were given 5 μg of MAb 2F6 (n = 7 for mIgG; n = 6 for 2F6 for 30 min; n = 8 for 2F6 for 24 h [in total over two independent experiments]) prior to bite challenges with 15 P. yoelii GFP-luc-infected mosquitoes (E). Graphs show mean total flux ± SD, with mean background luminescence indicated by the horizontal dotted line. Asterisks indicate a P value < 0.05 as determined by one-way ANOVA with Dunnett's posttest comparing groups to the mIgG group in panel B. Comparisons shown in panels D and E were carried out by Student's t test, with asterisks indicating a P value < 0.05. Degrees of significance are indicated as follows: *, 0.05 ≥ P > 0.01; **, 0.01 ≥ P > 0.001; ***, 0.001 ≥ P > 0.0001; ****, P ≤ 0.0001; NS, nonsignificant (P ≥ 0.05).
We next wanted to determine if the timing of MAb passive transfer could also impact Ab efficacy. For this, we compared the abilities of the anti-CSP MAb to inhibit liver infection when transferred at 30 min and at 24 h prior to sporozoite challenge by i.v. and mosquito bite. A dose that rendered minimal inhibition (25 μg/mouse for i.v. challenge or 5 μg/mouse for mosquito bite challenge) was used so that we might discriminate between doses that would result in low/no inhibition when administered at one time point and improved inhibition when administered at another. When mice were i.v. challenged with 3,000 P. yoelii GFP-luc sporozoites, administration of 25 μg MAb 2F6 was approximately 2.3-fold more effective when transferred at 30 min prior to challenge than it was when transferred at 24 h prior to challenge (Fig. 3D), a time point at which the regimen was virtually ineffective compared to the results seen with control (mIgG-injected) mice. Contrasting results were observed for a mosquito bite challenge. Administration of 5 μg of MAb 2F6 per mouse 24 h prior to a bite challenge with 15 mosquitoes significantly reduced liver-stage burden by 6.7-fold whereas administration 30 min prior to challenge resulted in a nonsignificant reduction of only 1.7-fold (Fig. 3E). Therefore, optimal Ab-mediated blocking of sporozoite infection requires Ab administration shortly before an i.v. challenge, whereas longer incubation times produce greater protection in a mosquito bite challenge model.
Passive transfer of immune serum before sporozoite challenge reduces P. yoelii liver infection.
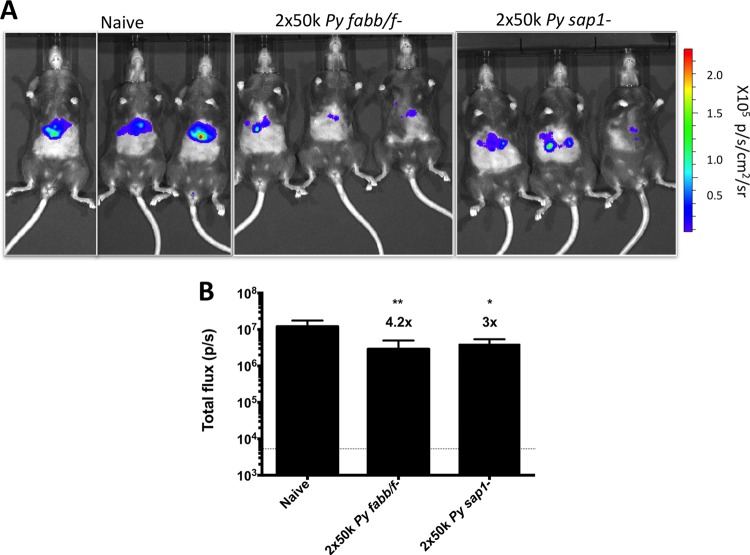
As an alternative to MAb passive transfer, we wanted to determine if this assay could be used to measure inhibition of liver infection by passive transfer of serum from mice immunized with GAPs. This could prove useful for the comparison of humoral responses between multiple-antigen subunit vaccines as well as whole-parasite vaccine candidates. Therefore, we collected serum from C57BL/6 mice that were immunized twice with GAPs deficient in either FabB/F (late-liver-stage arresting, P. yoelii fabb/f−) or SAP1 (early-liver-stage arresting, P. yoelii sap1−) genes (32, 33) at a dose of 50,000 sporozoites 4 weeks apart. Levels of serum IgG against CSP, as measured by ELISA, were similar for these two groups, while serum IgG levels against whole-sporozoite lysate were greater in P. yoelii fabb/f−-immunized mice (see Fig. S3 in the supplemental material). Naive C57BL/6 mice were injected i.v. with 300 μl of pooled serum from one of these immunization groups or 300 μl of pooled serum from naive C57BL/6 mice 24 h prior to a mosquito bite challenge performed using 15 P. yoelii GFP-luc-infected mosquitoes. Bioluminescent imaging of mice 48 h later was used to measure liver-stage burden. Passive transfer utilizing the sera from GAP-immunized mice significantly reduced liver infection by 4.2- and 3-fold with serum from P. yoelii fabb/f−-immunized mice and P. yoelii sap1−-immunized mice, respectively (Fig. 4A). This demonstrates that the experimental model can be used to assess functional humoral responses where Abs are elicited against multiple or complex antigens using GAP immunization.
FIG 4.
Passive transfer of serum from GAP-immunized mice reduces liver infection. C57BL/6 mice (n = 3 mice/group) were i.v. injected with 300 μl of serum from mice immunized with 2 × 50,000 P. yoelii fabb/f− or P. yoelii sap1− GAP sporozoites. Twenty-four hours later, mice were challenged by mosquito bite with 15 P. yoelii GFP-luc-infected mosquitoes. Liver-stage burden was determined by bioluminescent imaging 48 h postchallenge. Images of mice are shown in panel A, with quantification of total flux shown in panel B. The horizontal dotted line in panel B indicates mean background luminescence. Graphs show total mean flux ± SD. Asterisks indicate P value < 0.05 as determined by one-way ANOVA and Dunnett's posttest comparing groups to naive serum recipient mice. Degrees of significance are indicated as follows: *, 0.05 ≥ P > 0.01; **, 0.01 ≥ P > 0.001.
Passive transfer of a P. falciparum CSP MAb reduces P. falciparum liver infection in the humanized FRG huHep mouse model.
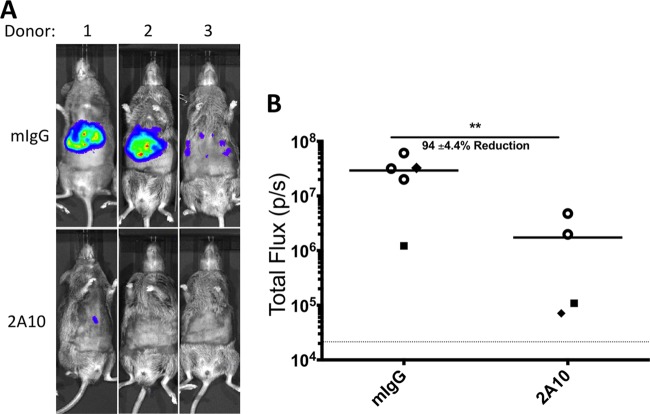
While rodent malaria infections in mice are a useful surrogate model for screening parasite antigen targets, it will be imperative for vaccine development efforts to test Ab effectiveness against human-infective P. falciparum sporozoites. We utilized the human liver-chimeric FRG huHep mouse, which is susceptible to P. falciparum sporozoite infection and supports complete liver-stage development in conjunction with a luciferase-expressing P. falciparum line (P. falciparum GFP-luc), to test passive-transfer Ab inhibition of infection (34, 35). The anti-CSP MAb 2A10 was chosen for this proof-of-concept experiment because it has been shown to reduce sporozoite motility, traversal, and invasion in vitro (40). We used FRG huHep mice repopulated with human hepatocytes from three different donors (designated donors 1, 2, and 3). Two “donor 1” mice and one mouse each for donor 2 and donor 3 received 500 μg of P. falciparum CSP MAb (2A10) 30 min prior to i.v. injection of 2 million P. falciparum GFP-luc sporozoites. Five mice (three donor 1 mice, one donor 2 mouse, and one donor 3 mouse) were used as mIgG-injected controls. Liver burden was measured by bioluminescence imaging 6 days after sporozoite injection (at peak liver-stage luciferase activity). Susceptibility to P. falciparum infection varied by donor (see Fig. S4 in the supplemental material); therefore, the results from each animal that received MAb 2A10 were compared only to those from the corresponding donor-matched mIgG control (or to the averages of the results from the three donor 1 control mice). Passive transfer of 500 μg 2A10 MAb blocked infection by 87% to 99% compared to control (Fig. 5). Therefore, the FRG huHep mouse model provides a promising in vivo method for assessing Ab-mediated protection against P. falciparum sporozoite challenge.
FIG 5.
Passive transfer of the P. falciparum CSP-specific MAb 2A10 reduces liver infection in P. falciparum sporozoite-challenged FRG huHep mice. FRG huHep mice (n = 3 for hepatocyte donor 1 mIgG; n = 2 for donor 1 2A10; n = 1 each for donor 2 and donor 3) were given 500 μg of either mIgG or 2A10 MAb 30 min prior to i.v. challenge with 2 × 106 P. falciparum GFP-luc parasites. Six days postchallenge, liver-stage parasite burden was assessed by bioluminescent imaging as shown by representative images in panel A. Quantification of total flux is shown in panel B, with each shape representing a different donor (○, donor 1; ⬥, donor 2; ■, donor 3), and the average reduction of the values for all 2A10-treated groups compared to mIgG controls is shown. The graph shows mean total flux ± SD, with mean background luminescence indicated by the horizontal dotted line. Asterisks indicate P value < 0.05 as determined by Student's t test, comparing all mIgG-treated mice and 2A10-treated mice. The degree of significance is indicated as follows: **, 0.01 ≥ P > 0.001.
DISCUSSION
Humoral immune responses play an important role in blocking initial infection of the mammalian host by the malaria parasite sporozoite, and yet tools and methods to evaluate the impact of humoral immunity on pre-erythrocytic parasite infection have remained insufficiently developed. In rodent malaria models, assessment of blood-stage patency and qPCR measurement of liver-stage burden after sporozoite challenge—which is most frequently conducted following i.v. administration of sporozoites—are the current methods used to determine vaccine-induced protection. Patency is a stringent yet binary readout, and delay of patency promotes inaccuracy in assessing the impact of interventions conferring less than complete, sterile protection. While the measurement of liver-stage burden by qPCR is more precise, it requires terminal sacrifice of the animal; thus, the same animal cannot be assessed for the concomitant transition to blood-stage patency. Here, we show that in vivo bioluminescence imaging can be used in the P. yoelii rodent malaria model to assess humoral protective immunity to pre-erythrocytic infection. Using the well-characterized CSP as a target, we show that this assay performs well in determining the efficacy of a MAb in a passive-transfer model. Bioluminescence not only affords measurements of a spectrum of reductions in liver-stage burden following passive transfer of MAbs or immune serum but also allows evaluation of this effect on the onset of subsequent blood-stage infection in the same animal. Given the sensitivity with respect to measurement of various degrees of liver-stage burden, it is now possible to compare various humoral blocking efficacies side-by-side in a manner that is not possible by measuring time to patency and level of parasitemia alone, with the added benefit that it does not require sacrifice of the animal. However, as our results show, bioluminescent imaging is not sensitive enough to predict sterile immunity when there is very low liver-stage burden.
Nevertheless, we have shown that this system reveals and discriminates differential levels of efficacy of Ab-mediated reduction of sporozoite infection of the liver after mosquito bite or i.v. sporozoite challenge. Importantly, sporozoite challenge by mosquito bite is much more sensitive to Ab blocking than i.v. sporozoite challenge at similar levels of liver infection in control animals. This is important to consider when evaluating new targets of humoral immunity, as an Ab response that can significantly block sporozoite activities prior to entry into a blood vessel is not detected when i.v. sporozoite challenge is used. This is particularly important if an antibody is used at low concentrations for transfer, if it is less efficient at blocking than anti-CSP antibodies, or if the target is exposed to Abs only prior to sporozoite entry into a skin capillary. The time taken for the sporozoite to traverse the dermis affords a greater opportunity for the sporozoite to be immobilized by Abs, subsequently preventing sporozoite entry into the bloodstream and transport to the liver. Whether this is due to increased preinfection contact time (21, 41), greater efficacy of opsonization in the skin, some other skin-dependent effect on Ab-mediated sporozoite neutralization, or a combination of these remains unknown but is worthy of further investigation. Our data showing that administration of Ab 24 h prior to challenge results in greater Ab efficacy against sporozoite challenge by mosquito bite but not i.v. challenge further highlights the likely importance of Abs localizing in the skin. Whereas the majority of injected Abs are in the circulation shortly after injection, 24 h later Abs are presumably distributed throughout the tissues—including the skin—and thus are most effective against sporozoites delivered by mosquito bite. These data are in agreement with a series of studies by Vanderberg et al., which detailed sporozoite motility in the skin in the presence or absence of anti-sporozoite Abs. These and other studies show that sporozoite exit from the skin can take some time (21, 41) and that sporozoites are immobilized by Abs present in the skin (20, 42). Furthermore, more-recent findings have also determined that in the presence of anti-sporozoite Abs, fewer sporozoites are actually injected from infected mosquitoes due to the formation of immune complexes at the mosquito proboscis (21). These data and our results, taken together with the fact that mosquito bite is a more relevant challenge model for protection against natural infection, show that it is important to evaluate the role of humoral immunity by a challenge that does not bypass the skin. Intradermal injection may prove useful, as it is easier to standardize the injection dose, but this would exclude the effect that immune complex formation at the mosquito proboscis has on Ab-mediated protection.
Finally, in vivo assessment of the effect of Abs on P. falciparum sporozoite infection was impossible until recently. Here we have shown that a combination of P. falciparum GFP-luc parasites (34) and the FRG huHep liver-chimeric mouse model (35) can be used effectively to evaluate sporozoite-blocking activities in vivo. Reduction of liver infection to nearly background levels was achieved by passive transfer of anti-P. falciparum CSP MAb to FRG huHep mice reconstituted with donor hepatocytes of both high and low susceptibilities to infection. Given that the various infection rates of mice repopulated with hepatocytes from different donors could affect evaluation of antibody-mediated blocking of sporozoite infection, it will be important to assess consistency of inhibition with a given Ab both within a donor and between different donors. Yet, despite differences in infection rates among mice repopulated with hepatocytes from different donors, we saw a consistent reduction of liver infection after passive transfer of 2A10 MAb both within a single donor group of mice and between mice repopulated from different donors. Additionally, infection rates of mice corresponding to the same donor (donor 1) were very consistent. Therefore, the use of defined hepatocyte sources that are permissive to infection might be important for future evaluations of novel antibodies. These results were obtained following an i.v. challenge, and it is important to further develop this model using P. falciparum sporozoite challenge by mosquito bite. In the future, it is anticipated that the FRG huHep liver-chimeric mouse/P. falciparum sporozoite challenge model will allow passive transfer of serum from immunized individuals from clinical trials to evaluate humoral immunity to pre-erythrocytic candidate vaccines and the assessment of MAbs to relevant sporozoite targets.
Supplementary Material
ACKNOWLEDGMENTS
We thank Michael Baldwin for his invaluable assistance in animal work and sporozoite preparation and the Center for Mosquito Production and Malaria Infection Research at Seattle BioMed.
This work was funded by grant R01AI095178 from the National Institutes of Health.
Footnotes
Published ahead of print 9 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01249-13.
REFERENCES
- 1.Casares S, Brumeanu TD, Richie TL. 2010. The RTS,S malaria vaccine. Vaccine 28:4880–4894. 10.1016/j.vaccine.2010.05.033 [DOI] [PubMed] [Google Scholar]
- 2.Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmuller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Machevo S, Acacio S, Bulo H, Sigauque B, Macete E, Alonso P, Abdulla S, Salim N, Minja R, Mpina M, Ahmed S, Ali AM, Mtoro AT, Hamad AS, Mutani P, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Bihoun B, Guiraud I, Kabore B, Sombie O, Guiguemde RT, Ouedraogo JB, Hamel MJ, Kariuki S, Oneko M, Odero C, Otieno K, Awino N, McMorrow M, Muturi-Kioi V, Laserson KF, Slutsker L, et al. 2012. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 367:2284–2295. 10.1056/NEJMoa1208394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olotu A, Fegan G, Wambua J, Nyangweso G, Awuondo KO, Leach A, Lievens M, Leboulleux D, Njuguna P, Peshu N, Marsh K, Bejon P. 2013. Four-year efficacy of RTS,S/AS01E and its interaction with malaria exposure. N. Engl. J. Med. 368:1111–1120. 10.1056/NEJMoa1207564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, Schwenk R, Nielsen RA, Debebe Z, Pinelis E, Juompan L, Williams J, Dowler M, Stewart VA, Wirtz RA, Dubois MC, Lievens M, Cohen J, Ballou WR, Heppner DG., Jr 2009. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J. Infect. Dis. 200:337–346. 10.1086/600120 [DOI] [PubMed] [Google Scholar]
- 5.Clyde DF, McCarthy VC, Miller RM, Hornick RB. 1973. Specificity of protection of man immunized against sporozoite-induced falciparum malaria. Am. J. Med. Sci. 266:398–403. 10.1097/00000441-197312000-00001 [DOI] [PubMed] [Google Scholar]
- 6.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, Richman A, Chakravarty S, Manoj A, Velmurugan S, Li M, Ruben AJ, Li T, Eappen AG, Stafford RE, Plummer SH, Hendel CS, Novik L, Costner PJ, Mendoza FH, Saunders JG, Nason MC, Richardson JH, Murphy J, Davidson SA, Richie TL, Sedegah M, Sutamihardja A, Fahle GA, Lyke KE, Laurens MB, Roederer M, Tewari K, Epstein JE, Sim BK, Ledgerwood JE, Graham BS, Hoffman SL, VRC 312 Study Team 8 August 2013. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 10.1126/science.1241800 [DOI] [PubMed] [Google Scholar]
- 7.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, van de Vegte-Bolmer M, van Schaijk B, Teelen K, Arens T, Spaarman L, de Mast Q, Roeffen W, Snounou G, Renia L, van der Ven A, Hermsen CC, Sauerwein R. 2009. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 361:468–477. 10.1056/NEJMoa0805832 [DOI] [PubMed] [Google Scholar]
- 8.Roestenberg M, Teirlinck AC, McCall MB, Teelen K, Makamdop KN, Wiersma J, Arens T, Beckers P, van Gemert G, van de Vegte-Bolmer M, van der Ven AJ, Luty AJ, Hermsen CC, Sauerwein RW. 2011. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 377:1770–1776. 10.1016/S0140-6736(11)60360-7 [DOI] [PubMed] [Google Scholar]
- 9.Luke TC, Hoffman SL. 2003. Rationale and plans for developing a non-replicating, metabolically active, radiation-attenuated Plasmodium falciparum sporozoite vaccine. J. Exp. Biol. 206:3803–3808. 10.1242/jeb.00644 [DOI] [PubMed] [Google Scholar]
- 10.Butler NS, Schmidt NW, Vaughan AM, Aly AS, Kappe SH, Harty JT. 2011. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe 9:451–462. 10.1016/j.chom.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan SM, Janse CJ, Kappe SH, Mikolajczak SA. 2012. Genetic engineering of attenuated malaria parasites for vaccination. Curr. Opin. Biotechnol. 23:908–916. 10.1016/j.copbio.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 12.Chen DH, Tigelaar RE, Weinbaum FI. 1977. Immunity to sporozoite-induced malaria infeciton in mice. I. The effect of immunization of T and B cell-deficient mice. J. Immunol. 118:1322–1327 [PubMed] [Google Scholar]
- 13.Weiss WR, Sedegah M, Beaudoin RL, Miller LH, Good MF. 1988. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc. Natl. Acad. Sci. U. S. A. 85:573–576. 10.1073/pnas.85.2.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egan JE, Weber JL, Ballou WR, Hollingdale MR, Majarian WR, Gordon DM, Maloy WL, Hoffman SL, Wirtz RA, Schneider I. 1987. Efficacy of murine malaria sporozoite vaccines: implications for human vaccine development. Science 236:453–456. 10.1126/science.3551073 [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues M, Nussenzweig RS, Zavala F. 1993. The relative contribution of antibodies, CD4+ and CD8+ T cells to sporozoite-induced protection against malaria. Immunology 80:1–5 [PMC free article] [PubMed] [Google Scholar]
- 16.Vanderberg J, Mueller AK, Heiss K, Goetz K, Matuschewski K, Deckert M, Schluter D. 2007. Assessment of antibody protection against malaria sporozoites must be done by mosquito injection of sporozoites. Am. J. Pathol. 171:1405–1406; author reply, 1406. 10.2353/ajpath.2007.070661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinnis P, Zavala F. 2008. The skin stage of malaria infection: biology and relevance to the malaria vaccine effort. Future Microbiol. 3:275–278. 10.2217/17460913.3.3.275 [DOI] [PubMed] [Google Scholar]
- 18.Coppi A, Tewari R, Bishop JR, Bennett BL, Lawrence R, Esko JD, Billker O, Sinnis P. 2007. Heparan sulfate proteoglycans provide a signal to Plasmodium sporozoites to stop migrating and productively invade host cells. Cell Host Microbe 2:316–327. 10.1016/j.chom.2007.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amino R, Giovannini D, Thiberge S, Gueirard P, Boisson B, Dubremetz JF, Prevost MC, Ishino T, Yuda M, Menard R. 2008. Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe 3:88–96. 10.1016/j.chom.2007.12.007 [DOI] [PubMed] [Google Scholar]
- 20.Vanderberg JP, Frevert U. 2004. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int. J. Parasitol. 34:991–996. 10.1016/j.ijpara.2004.05.005 [DOI] [PubMed] [Google Scholar]
- 21.Kebaier C, Voza T, Vanderberg J. 2009. Kinetics of mosquito-injected Plasmodium sporozoites in mice: fewer sporozoites are injected into sporozoite-immunized mice. PLoS Pathog. 5:e1000399. 10.1371/journal.ppat.1000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, Gunasekera A, Chakravarty S, James ER, Sedegah M, Richman A, Velmurugan S, Reyes S, Li M, Tucker K, Ahumada A, Ruben AJ, Li T, Stafford R, Eappen AG, Tamminga C, Bennett JW, Ockenhouse CF, Murphy JR, Komisar J, Thomas N, Loyevsky M, Birkett A, Plowe CV, Loucq C, Edelman R, Richie TL, Seder RA, Hoffman SL. 2011. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science 334:475–480. 10.1126/science.1211548 [DOI] [PubMed] [Google Scholar]
- 23.Sheehy SH, Duncan CJ, Elias SC, Choudhary P, Biswas S, Halstead FD, Collins KA, Edwards NJ, Douglas AD, Anagnostou NA, Ewer KJ, Havelock T, Mahungu T, Bliss CM, Miura K, Poulton ID, Lillie PJ, Antrobus RD, Berrie E, Moyle S, Gantlett K, Colloca S, Cortese R, Long CA, Sinden RE, Gilbert SC, Lawrie AM, Doherty T, Faust SN, Nicosia A, Hill AV, Draper SJ. 2012. ChAd63-MVA-vectored blood-stage malaria vaccines targeting MSP1 and AMA1: assessment of efficacy against mosquito bite challenge in humans. Mol. Ther. 20:2355–2368. 10.1038/mt.2012.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuang I, Sedegah M, Cicatelli S, Spring M, Polhemus M, Tamminga C, Patterson N, Guerrero M, Bennett JW, McGrath S, Ganeshan H, Belmonte M, Farooq F, Abot E, Banania JG, Huang J, Newcomer R, Rein L, Litilit D, Richie NO, Wood C, Murphy J, Sauerwein R, Hermsen CC, McCoy AJ, Kamau E, Cummings J, Komisar J, Sutamihardja A, Shi M, Epstein JE, Maiolatesi S, Tosh D, Limbach K, Angov E, Bergmann-Leitner E, Bruder JT, Doolan DL, King CR, Carucci D, Dutta S, Soisson L, Diggs C, Hollingdale MR, Ockenhouse CF, Richie TL. 2013. DNA prime/adenovirus boost malaria vaccine encoding P. falciparum CSP and AMA1 induces sterile protection associated with cell-mediated immunity. PLoS One 8:e55571. 10.1371/journal.pone.0055571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White MT, Bejon P, Olotu A, Griffin JT, Riley EM, Kester KE, Ockenhouse CF, Ghani AC. 2013. The relationship between RTS,S vaccine-induced antibodies, CD4(+) T cell responses and protection against Plasmodium falciparum infection. PLoS One 8:e61395. 10.1371/journal.pone.0061395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crompton PD, Pierce SK, Miller LH. 2010. Advances and challenges in malaria vaccine development. J. Clin. Invest. 120:4168–4178. 10.1172/JCI44423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindner SE, Swearingen KE, Harupa A, Vaughan AM, Sinnis P, Moritz RL, Kappe SH. 2013. Total and putative surface proteomics of malaria parasite salivary gland sporozoites. Mol. Cell. Proteomics 12:1127–1143. 10.1074/mcp.M112.024505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang R, Smith JD, Kappe SH. 2009. Advances and challenges in malaria vaccine development. Expert Rev. Mol. Med. 11:e39. 10.1017/S1462399409001318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charoenvit Y, Mellouk S, Cole C, Bechara R, Leef MF, Sedegah M, Yuan LF, Robey FA, Beaudoin RL, Hoffman SL. 1991. Monoclonal, but not polyclonal, antibodies protect against Plasmodium yoelii sporozoites. J. Immunol. 146:1020–1025 [PubMed] [Google Scholar]
- 30.Ak M, Bower JH, Hoffman SL, Sedegah M, Lees A, Carter M, Beaudoin RL, Charoenvit Y. 1993. Monoclonal antibodies of three different immunoglobulin G isotypes produced by immunization with a synthetic peptide or native protein protect mice against challenge with Plasmodium yoelii sporozoites. Infect. Immun. 61:2493–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brahimi K, Badell E, Sauzet JP, BenMohamed L, Daubersies P, Guerin-Marchand C, Snounou G, Druilhe P. 2001. Human antibodies against Plasmodium falciparum liver-stage antigen 3 cross-react with Plasmodium yoelii preerythrocytic-stage epitopes and inhibit sporozoite invasion in vitro and in vivo. Infect. Immun. 69:3845–3852. 10.1128/IAI.69.6.3845-3952.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaughan AM, O'Neill MT, Tarun AS, Camargo N, Phuong TM, Aly AS, Cowman AF, Kappe SH. 2009. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell. Microbiol. 11:506–520. 10.1111/j.1462-5822.2008.01270.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aly AS, Lindner SE, MacKellar DC, Peng X, Kappe SH. 2011. SAP1 is a critical post-transcriptional regulator of infectivity in malaria parasite sporozoite stages. Mol. Microbiol. 79:929–939. 10.1111/j.1365-2958.2010.07497.x [DOI] [PubMed] [Google Scholar]
- 34.Vaughan AM, Mikolajczak SA, Camargo N, Lakshmanan V, Kennedy M, Lindner SE, Miller JL, Hume JC, Kappe SH. 2012. A transgenic Plasmodium falciparum NF54 strain that expresses GFP-luciferase throughout the parasite life cycle. Mol. Biochem. Parasitol. 186:143–147. 10.1016/j.molbiopara.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 35.Vaughan AM, Mikolajczak SA, Wilson EM, Grompe M, Kaushansky A, Camargo N, Bial J, Ploss A, Kappe SH. 2012. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J. Clin. Invest. 122:3618–3628. 10.1172/JCI62684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller JL, Murray S, Vaughan AM, Harupa A, Sack B, Baldwin M, Crispe IN, Kappe SH. 2013. Quantitative bioluminescent imaging of pre-erythrocytic malaria parasite infection using luciferase-expressing Plasmodium yoelii. PLoS One 8:e60820. 10.1371/journal.pone.0060820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ploemen I, Behet M, Nganou-Makamdop K, van Gemert GJ, Bijker E, Hermsen C, Sauerwein R. 2011. Evaluation of immunity against malaria using luciferase-expressing Plasmodium berghei parasites. Malaria J. 10:350. 10.1186/1475-2875-10-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Portugal S, Carret C, Recker M, Armitage AE, Goncalves LA, Epiphanio S, Sullivan D, Roy C, Newbold CI, Drakesmith H, Mota MM. 2011. Host-mediated regulation of superinfection in malaria. Nat. Med. 17:732–737. 10.1038/nm.2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medica DL, Sinnis P. 2005. Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected anopheline mosquitoes. Infect. Immun. 73:4363–4369. 10.1128/IAI.73.7.4363-4369.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaushansky A, Rezakhani N, Mann H, Kappe SH. 2012. Development of a quantitative flow cytometry-based assay to assess infection by Plasmodium falciparum sporozoites. Mol. Biochem. Parasitol. 183:100–103. 10.1016/j.molbiopara.2012.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin SC, Vanderberg JP, Terzakis JA. 1982. Direct infection of hepatocytes by sporozoites of Plasmodium berghei. J. Protozool. 29:448–454. 10.1111/j.1550-7408.1982.tb05431.x [DOI] [PubMed] [Google Scholar]
- 42.Yamauchi LM, Coppi A, Snounou G, Sinnis P. 2007. Plasmodium sporozoites trickle out of the injection site. Cell. Microbiol. 9:1215–1222. 10.1111/j.1462-5822.2006.00861.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization 2012. World malaria report 2012. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/world_malaria_report_2012/report/en/index.html [Google Scholar]
- 44.Harlow E, Lane D. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.