Abstract
Leptospirosis is a potentially fatal zoonosis transmitted by reservoir host animals that harbor leptospires in their renal tubules and shed the bacteria in their urine. Leptospira interrogans serovar Copenhageni transmitted from Rattus norvegicus to humans is the most prevalent cause of urban leptospirosis. We examined L. interrogans LigA, domains 7 to 13 (LigA7-13), as an oral vaccine delivered by Escherichia coli as a lipidated, membrane-associated protein. The efficacy of the vaccine was evaluated in a susceptible hamster model in terms of the humoral immune response and survival from leptospiral challenge. Four weeks of oral administration of live E. coli expressing LigA7-13 improved survival from intraperitoneal (i.p.) and intradermal (i.d.) challenge by L. interrogans serovar Copenhageni strain Fiocruz L1-130 in Golden Syrian hamsters. Immunization with E. coli expressing LigA7-13 resulted in a systemic antibody response, and a significant LigA7-13 IgG level after the first 2 weeks of immunization was completely predictive of survival 28 days after challenge. As in previous LigA vaccine studies, all immunized hamsters that survived infection had renal leptospiral colonization and histopathological changes. In summary, an oral LigA-based vaccine improved survival from leptospiral challenge by either the i.p. or i.d. route.
INTRODUCTION
Leptospirosis is caused by pathogenic spirochetes belonging to the genus Leptospira and is the most widespread zoonosis in the world (1). Pathogenic leptospires are maintained in urban and sylvatic environments by colonization of the renal tubules of reservoir host animals, principally rodents. Humans are infected through skin abrasions or mucous membranes exposed to water contaminated by animal urine (1, 2). Severe leptospirosis involves multiorgan failure, including pulmonary hemorrhage, jaundice, and hepatic and renal dysfunction (3, 4), with a mortality rate of >10% in many settings. Leptospirosis has emerged as a major public health problem, with more than 500,000 severe human cases worldwide each year (1, 5, 6). Leptospira interrogans serovar Copenhageni is commonly isolated from the urban rat (Rattus sp.) and has been associated with human leptospirosis in an urban slum (7).
Killed whole-cell leptospiral vaccines for prevention of human leptospirosis are available in some countries, including France, Cuba, and Japan (8–10). Vaccine side effects, including both systemic and local reactions, are a concern, and efficacy is limited to the serovar(s) included in the vaccine (10). Parenteral immunization programs are difficult to administer in economically impoverished areas that lack a medical infrastructure. Because of the problems with existing vaccines and the projected increased incidence of leptospirosis, there is an urgent need for development of novel, low-cost strategies for the prevention of leptospirosis.
An alternative approach to prevention of leptospirosis is oral immunization. Orally delivered vaccines have several advantages over other routes of antigen delivery, including convenience, cost-effectiveness, and, most importantly, induction of both local and systemic immune responses (11). Oral immunization has been shown to provide protection against a variety of bacterial pathogens, including Vibrio cholerae, Salmonella enterica serovar Typhi, and Borrelia burgdorferi (12–15). Protection of mice against B. burgdorferi infection by oral immunization has been achieved using either Lactobacillus plantarum (12) or Escherichia coli (15) as delivery vehicles for the immunogen, OspA. Inclusion of the 16-amino-acid lipoprotein signal peptide of OspA tags the protein for translocation across the cytoplasmic membrane and subsequent lipidation. Lipidation is known to function as an adjuvant (16–18), and the immune response to oral immunization with OspA has been shown to be modulated by its lipidation (19).
Leptospiral surface-exposed outer membrane proteins (OMPs) that are expressed during infection of the mammalian host and mediate host tissue interactions (20, 21) are potential targets of a protective immune response. LigA is an outer membrane protein exposed on and released from the leptospiral surface (22, 23). Several lines of evidence suggest that ligA is upregulated during infection of the mammalian host, including induction of expression by physiologic osmolarity (23) and temperature (24) and an early humoral immune response to the Lig proteins during infection (25). Several groups have reported that immunization with the LigA unique region, consisting of domains 7 to 13 (LigA7-13), confers protection from intraperitoneal (i.p.) challenge in the hamster model of leptospirosis (26–30). In this study, we examined whether oral immunization with immunoglobulin-like domains 7 to 13 of LigA results in an immune response that is protective against intradermal (i.d.) challenge, a more biologically relevant challenge route than the standard intraperitoneal challenge route.
MATERIALS AND METHODS
Leptospiral strain and cultivation.
L. interrogans serovar Copenhageni strain Fiocruz L1-130, originally isolated from the bloodstream of a leptospirosis patient in Brazil (7), was grown in Ellinghausen-McCullough-Johnson-Harris (EMJH) medium (31, 32) supplemented with 1% rabbit serum (Rockland, Gilbertsville, PA) and 100 μg/ml 5-fluorouracil at 30°C in a shaker incubator. Leptospires (≤5 passages) were enumerated by dark-field microscopy as described by Miller (33). Hamster tissues were cultured in semisolid Probumin vaccine-grade solution (Millipore) containing 0.2% Bacto agar (BD, Franklin Lakes, NJ) and 100 μg/ml 5-fluorouracil in a stationary incubator at 30°C and were examined for growth for ≥1 month.
Plasmid construction and characterization of expressed antigens.
The nucleotide sequence encoding immunoglobulin-like domains 7 to 13 (amino acid residues 631 to 1224) of LigA of L. interrogans serovar Copenhageni strain Fiocruz L1-130 (34) was synthesized using codons optimized for expression in E. coli and fused to the nucleotide sequence encoding the leader peptide and first six amino acids of outer surface protein A (ospA) from Borrelia burgdorferi (Blue Heron Biotech). Recombinant ligA7-13 was cloned into the expression vector pET9c at its NdeI and BamHI sites and then transformed into E. coli BL21(DE3) pLysS. E. coli containing pET9c-LigA7-13 was cultivated in a shaking incubator at 37°C in TBY medium (10 g of tryptone, 5 g of yeast extract, 5 g of NaCl, and 1 g of glucose per liter) supplemented with 50 μg/ml kanamycin at 37°C. At an optical density at 600 nm (OD600) of ∼0.6, cells were induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Apex) for 2 h. Protein expression was examined by Coomassie blue staining and Western blotting using LigA-specific antiserum. Proteins were separated on a 4 to 12% gradient NuPAGE Bis Tris precast gel (Life Technologies) and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore) by semidry transfer. After being blocked overnight at 4°C with 5% skim milk (MP Biomedical) in phosphate-buffered saline (PBS; pH 7.4) and 0.05% Tween 80 (Thermo Scientific), LigA7-13 was detected using a 1:1,000 dilution of LigA rabbit polyclonal antiserum (22) followed by a 1:5,000 dilution of horseradish peroxidase-conjugated donkey anti-rabbit immunoglobulin G (Amersham) and enhanced chemiluminescence (SuperSignal West Pico, Pierce).
[14C]Palmitate radiolabeling of recombinant LigA7-13.
A 15-ml TBY medium sample containing E. coli BL21(DE3) pLysS containing either pET9c-LigA7-13 or pET9c alone in the exponential phase of growth was intrinsically labeled by the addition of 1 mM IPTG and 25 μCi (1.85 MBq) [U-14C]palmitate (GE Amersham), followed by further incubation in a shaker incubator at 37°C for 1 h. After SDS-PAGE using 10% Tris-glycine gels (Lonza), gels were stained with Bio-Safe Coomassie G-250 stain (Bio-Rad), dried, and exposed to BioMax MS film (Kodak) in a BioMax TranScreen low-energy intensifying screen (Kodak) or transferred to a PVDF membrane for Western blotting as described above, except that blots were developed with an ECL Western blotting substrate (Pierce).
E. coli cell fractionation.
E. coli containing pET9c-LigA7-13 was cultivated in TBY medium supplemented with 50 μg/ml kanamycin at 37°C, 225 rpm. At an OD600 of 0.6, cells were induced with 1 mM IPTG for 3 h and grown to an OD600 of ∼1. The cells were harvested by centrifugation at 20,000 × g for 10 min at 4°C and were washed 3 times with ice-cold phosphate-buffered salt solution (PBSM; Gibco, Grand Island, NY). The pellet was resuspended in ice-cold PBS supplemented with protease inhibitor cocktail (cOmplete EDTA-free; Roche Diagnostics GmBH, Germany) to an OD600 = 1. E. coli cells were disrupted with a French press (Thermo Electron Corporation, Milford, MA) and centrifuged at 20,000 × g for 10 min at 4°C to isolate the cytosol fraction (supernatant) from the cell envelope (pellet). The pellet was resuspended in 1 ml of ice-cold PBS–2% Triton X-114 (Sigma-Aldrich, St. Louis, MO) (vol/vol) and incubated at 0°C for 1 h with frequent gentle agitation. Phase separation was performed by warming the suspension for 30 min in a 37°C water bath, followed by centrifugation at 13,000 × g for 15 min at 25°C. The aqueous and detergent phases were collected, separated from each other, and washed 3 times as previously described (35). Briefly, the aqueous phase was washed by adding 10% Triton X-114 to a final concentration of 2%. The aqueous phase was rewarmed for 30 min in a 37°C water bath and centrifuged at 13,000 × g for 15 min at 25°C. The detergent phase was washed by diluting it to 1 ml in ice-cold PBS followed by rewarming for 30 min in a 37°C water bath and a centrifugation at 13,000 × g for 15 min at 25°C. The supernatant (SN), cell envelope (CE), cell envelope/aqueous (CE/AQ) phase, and cell envelope/detergent (CE/DET) phase were analyzed on a 10% denaturing polyacrylamide gel and electrotransferred to a PVDF membrane (Millipore, Billerica, MA). Western blotting was performed with LigA polyclonal rabbit antibody.
Preparation of oral vaccine.
E. coli containing pET9c-LigA7-13 or pET9c alone was cultivated in TBY medium supplemented with 50 μg/ml kanamycin at 37°C. At an OD600 of 0.6, cells were induced with 1 mM IPTG (Apex) and grown to an OD600 of ∼1. Cells from 2 liters of culture were harvested by centrifugation at 4,000 × g for 10 min at 4°C and resuspended in either 20 or 10 ml of 20% glycerol–phosphate-buffered salt solution (Gibco, Grand Island, NY) for experiment 1 or 2, respectively. Cell suspensions were promptly frozen and stored at −80°C until use. Quantification of LigA7-13 concentrations was performed by densitometry of Western blots of serial dilutions of E. coli and purified LigA7-13 protein with Image J (36). Purified, recombinant LigA7-13 protein was prepared as described previously (30).
Ethics statement and endpoint criteria.
All animals were routinely cared for according to the guidelines prescribed by the National Institutes of Health Guide to Laboratory Animal Care. Procedures involving hamsters were approved by the Veterans Affairs Greater Los Angeles Healthcare System Institutional Animal Care and Use Committee. Hamsters were weighed daily and observed for endpoint criteria, including loss of appetite, gait or breathing difficulty, prostration, ruffled fur, or weight loss of ≥10% of maximum weight. Animals that met any of the endpoint criteria were euthanized by isoflurane inhalation followed by thoracotomy.
Determination of median endpoint dose (ED50).
Eleven-week-old female Golden Syrian hamsters (Harlan) in groups of 4 were inoculated with 100 μl of EMJH culture medium containing serial 10-fold dilutions of L. interrogans serovar Copenhageni, from 101 to 105 bacteria, by the intraperitoneal (i.p.) or intradermal (i.d.) routes. Hamsters were monitored daily until they met endpoint criteria. Animals that did not meet endpoint criteria were sacrificed on day 28 after challenge. ED50 was determined using the method of Reed and Muench (37).
Oral immunization of hamsters.
Four-week-old female Golden Syrian hamsters (Harlan) in groups of 8 were immunized by oral gavage using a protocol similar to that previously described for OspA immunization of mice (12): 2 weeks of daily oral immunization, 1 week of rest, 1 week of daily oral immunization, 1 week of rest, and 1 week of daily oral immunization. Oral immunization was performed Monday through Friday. Two independent experiments were performed. In experiment 1, hamsters were immunized daily with 0.5 ml of LigA7-13 oral vaccine for a total of 37 mg of protein in 4 weeks or an equivalent number of E. coli cells containing the pET9c empty vector. In experiment 2, hamsters received 1 ml/day of the vaccine for a total of 148 mg of protein or an equivalent number of E. coli cells containing the pET9c empty vector.
Leptospiral challenge.
Hamsters were challenged 1 week after the last week of immunization. A total of 103 or 102 leptospires were inoculated intraperitoneally or intradermally in experiment 1 or 2, respectively. Hamsters were monitored daily until they met endpoint criteria or for 28 days. At the time of sacrifice, pulverized kidney tissue was diluted 1:100 into semisolid Probumin vaccine-grade solution (Millipore) and incubated at 30°C. Kidneys were also collected in formalin for histopathology and into cryotubes and immediately stored at −80°C for quantitative PCR.
ELISA.
Antibody responses in hamsters were determined by enzyme-linked immunosorbent assay (ELISA) as previously described (30) with the following modifications: ELISA wells were coated with 100 μl of 2 μg/ml of recombinant purified LigA7-13 and blocked with Pierce protein-free blocking buffer (Thermo Scientific). Peroxidase-conjugated goat anti-hamster IgG (catalog number 107-035-142; Jackson ImmunoResearch Laboratories) was used as the secondary antibody (1:5,000). ELISA reactions were developed with 3,3′,5,5′-tetramethylbenzidine (1-Step Ultra TMB-ELISA; Thermo Scientific). Plates were read with a Bio-Rad 550 microplate reader at 450 nm. The critical limit was defined as the value of the mean level of the control-immunized group plus 1.65 standard deviations (SD).
Histopathology.
Processing tissues for histopathology involved formalin fixation, paraffin embedding, sectioning, and staining with either hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS) in a Dako automated slide processor. Blinded scoring of kidney sections used a scale of 0 to 15 for the extent of histopathology, ranging from normal to severe, based on the degree of histopathology. Each section was assigned a score of 0 to 3 for each of the following five criteria: (i) the extent of hyaline cast deposition in the renal tubules, (ii) interstitial inflammation as indicated by invasion of polymorphonuclear leukocytes, (iii) tubular damage as indicated by thinning and atrophy of renal tubular cells, (iv) glomerular damage as indicated by contraction of the glomerular tuft and Bowman's space dilatation, and (v) the size and number of capsular concavities or depressions.
Quantitative PCR (qPCR).
DNA from kidneys was extracted with the DNeasy blood and tissue kit according to the manufacturer's instructions (Qiagen, Valencia, CA), except that an elution volume of 100 μl was used. The purified DNA was stored at −80°C until use. The concentration of leptospires was quantified using the Bio-Rad iQ5 real-time system (Bio-Rad, Hercules, CA) using the iTaq universal probes supermix (Bio-Rad). The lipL32 gene was amplified using primers LipL32-45F and LipL32-286R and detected using probe LipL32-189P, as previously described (38).
The PCR mixture contained 10 μM each primer, 5 μM the specific probe, and 5 μl of DNA in a total volume of 25 μl. The amplification protocol consisted of 10 min at 95°C, followed by 40 cycles of amplification (95°C for 15 s and 60°C for 1 min). A negative result was assigned where no amplification occurred or if the threshold cycle (CT) was greater than 36 (38). Real-time PCR was performed in duplicate for each sample. Results were expressed as the number of leptospires/mg of tissue used for DNA extraction.
Statistical analysis.
Data were represented as means ± standard deviations. Statistical analyses were performed using the Student t test or one-way analysis of variance (ANOVA) followed by a Tukey posttest, with P values of <0.05 considered statistically significant. The number of asterisks indicates the significance level: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Comparison of survival curves was performed using the Mantel-Cox log rank test.
RESULTS
Quantification of LigA7-13 expression by E. coli.
Serial dilutions of E. coli expressing LigA7-13 were compared by Western blotting with known amounts of recombinant LigA7-13 protein to quantify expression. Based on the amounts of LigA7-13 protein expressed, we estimate that hamsters received oral immunization with 37 mg and 148 mg of total LigA7-13 in experiments 1 and 2, respectively.
Localization and lipidation of recombinant LigA7-13.
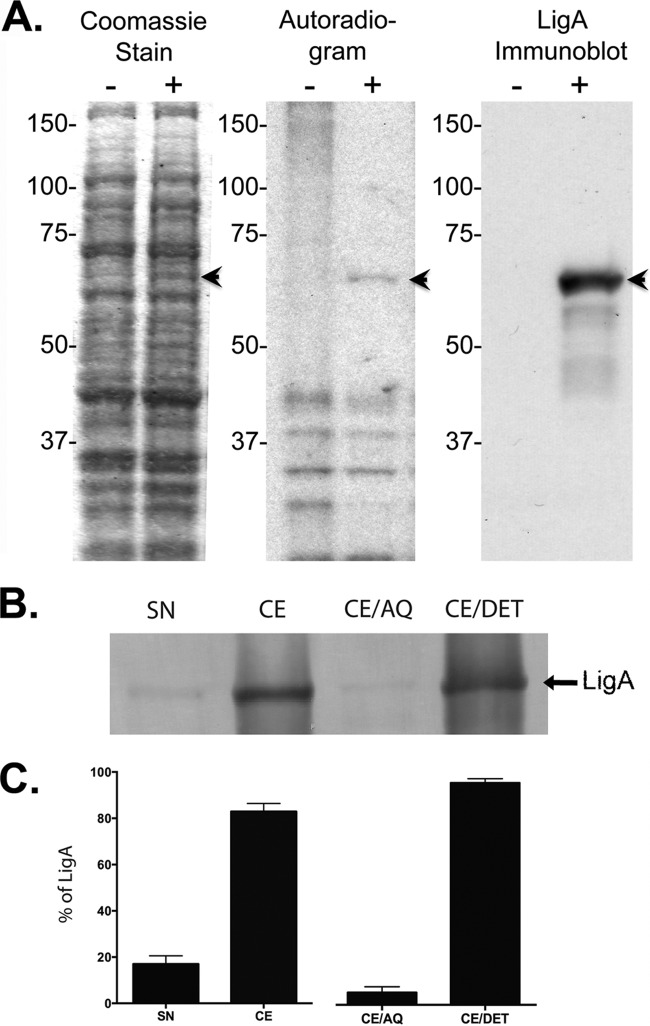
Comparison of E. coli containing pET9c versus pET9c-LigA7-13 induced with IPTG in the presence of [14C]palmitate revealed a unique 62-kDa band in the Coomassie blue-stained gel, the autoradiogram, and LigA Western blot (Fig. 1A). LigA Western blots of cytosol and cell envelope fractions revealed the protein primarily in the cell envelope fraction (Fig. 1B and C). When the cell envelope fraction was further fractionated by Triton X-114 solubilization and phase partitioning, LigA7-13 was found primarily in the Triton X-114 hydrophobic, detergent phase (Fig. 1B and C), consistent with a lipidated protein anchored to the membrane.
FIG 1.
Lipidation and localization of LigA7-13 in E. coli. Inclusion of the OspA lipoprotein signal peptide resulted in LigA7-13 lipidation and localization to the E. coli cell envelope fraction. (A) E. coli strains containing either pET9c alone (−) or pET9c-LigA7-13 (+) in the log phase of growth were incubated for 1 h in [14C]palmitate and separated by polyacrylamide gel electrophoresis. Bands (arrowheads) unique to E. coli containing pET9c-LigA7-13 were observed by staining with Coomassie blue, autoradiography, and Western blotting at the molecular weight corresponding to the predicted size of LigA7-13. (B) pET9c-LigA7-13-expressing E. coli was disrupted with a French press. The supernatant (SN) and the pellet (cell envelope [CE]) were collected. The cell envelope was then incubated with Triton X-114 and partitioned into aqueous (CE/AQ) and detergent (CE/DET) phases. Fractions were analyzed on a 10% SDS-PAGE gel and tested by immunoblotting with LigA-monospecific polyclonal rabbit antibody. (C) Protein in the supernatant, cell envelope, aqueous phase, and detergent phase was quantified by densitometry using Alpha Imager (Alpha Innotech, San Leandro, CA).
Comparison of the ED50 for different challenge routes.
The ED50 is the dose required for 50% of animals to meet endpoint criteria. We determined the ED50 for the intraperitoneal (i.p.) and intradermal (i.d.) challenge routes. As shown in Table 1, all four hamsters met endpoint criteria within 7 to 13 days after challenge for all doses administered by the i.d. route. Three of four hamsters challenged by the i.p. route with either 101, 102, 103, or 104 leptospires met endpoint criteria within 6 to 11 days. The time from challenge to when animals met endpoint criteria varied according to the challenge dose, with animals meeting the endpoint criteria sooner after higher challenge doses. The ED50 for the i.d. route of infection was estimated to be <10 leptospires and to be ∼20 leptospires for the i.p. route.
TABLE 1.
Comparison of the median endpoint doses for different challenge routesa
| Dose | No. of sacrificed animals/no. of infected animals (days of endpoint) |
|
|---|---|---|
| i.p. | i.d. | |
| 105 | 4/4 (6, 6, 8, 10) | 4/4 (7, 7, 9, 9) |
| 104 | 3/4 (7, 7, 8) | 4/4 (8, 9, 9, 9) |
| 103 | 3/4 (10, 11, 13) | 4/4 (8, 10, 11, 12) |
| 102 | 3/4 (9, 9, 9) | 4/4 (9, 11, 12, 13) |
| 101 | 3/4 (9, 9, 13) | 4/4 (10, 11, 12, 13) |
| ED50 (no. of leptospires) | ±20 | <10 |
Hamsters were infected with serial 10-fold dilutions (105 to 101) of L. interrogans serovar Copenhageni strain Fiocruz L1-130, using i.p. and i.d. routes of infection. Animals were observed and weighed daily for 28 days. They were sacrificed when a 10% decrease of the body weight was reached or at 28 days postinfection. These results were used to estimate the ED50 by the method of Reed and Muench (37), yielding <10 leptospires for the i.d. route and ∼20 leptospires for the i.p. route.
Humoral response to oral immunization in hamsters.
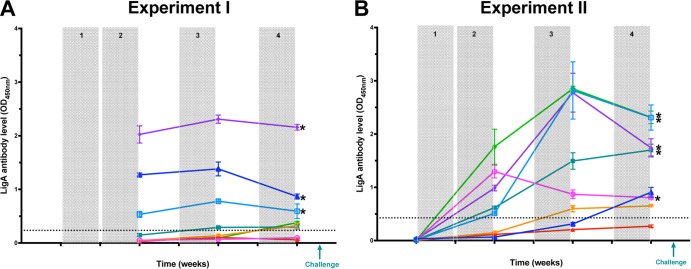
Hamsters were immunized by oral gavage with E. coli expressing LigA7-13 or E. coli containing pET9c alone. The LigA7-13 IgG response was measured by ELISA. We defined the critical limit as the value of the mean level of the ELISA result in the control-immunized animals plus 1.65 SD (experiment 1, 0.236; experiment 2, 0.427). In experiment 1, oral immunization with 37 mg of LigA7-13 resulted in a significant antibody response in 3/8 and 6/8 animals after 2 and 4 weeks of immunization, respectively (Fig. 2A). In experiment 2, oral immunization with 148 mg of LigA7-13 resulted in a significant antibody response in 5/8 and 7/8 animals after 2 and 4 weeks of immunization (Fig. 2B).
FIG 2.
IgG response to oral immunization with E. coli expressing LigA7-13. Hamsters were immunized orally with E. coli expressing LigA7-13 (LigA7-13-immunized group) or with E. coli containing the empty vector (control-immunized group). The immunization protocol included an initial immunization of 2 weeks (shaded), followed by 1 week of rest, a third week of immunization for the first boost (shaded), 1 week of rest, and a fourth week of immunization for the second boost (shaded). Serum samples were collected at different time points during oral immunization with total doses of either 37 mg in experiment 1 (A) or 148 mg in experiment 2 (B) of LigA7-13. Hamsters were challenged the week following the last week of immunization using either the i.p. route (experiment 1) or i.d. route (experiment 2) of inoculation. LigA7-13 antibody levels were measured in triplicate by ELISA. Geometric mean endpoint and standard deviation are shown for each animal in both experiments (one color per animal). The dotted line shows the critical limit, defined as the value of the mean level of the control-immunized group plus 1.65 SD (experiment 1 = 0.236; experiment 2 = 0.427). Asterisks indicate animals that survived challenge.
Effects of LigA7-13 oral immunization on survival after leptospiral challenge.
Two independent experiments were performed (Table 2). In experiment 1, involving i.p. challenge with 103 leptospires (≥50-fold over ED50), all control-immunized animals met the endpoint criteria within 8 to 10 days, whereas 5/8 LigA7-13-immunized animals met endpoint criteria (i.e., 37.5% survival) ≥10 days postinfection (Fig. 3A; survival curves statistically different, P < 0.0001). In experiment 2, hamsters were immunized with 4-fold more LigA7-13 than in the first experiment (148 mg total) and challenged i.d. with 102 leptospires (>10-fold over ED50). All control-immunized animals (100%) met endpoint criteria, whereas LigA7-13 immunization resulted in only 3/8 animals (62.5% survival) meeting endpoint criteria (Fig. 3B; survival curves significantly different, P < 0.02). No animals in either study died spontaneously.
TABLE 2.
Comparison of oral vaccine experiments
| Characteristic | Value for experiment: |
P value | |
|---|---|---|---|
| 1 (EcLigA7-13 10/i.p.) | 2 (EcLigA7-13 40/i.d.) | ||
| LigA7-13 dose (mg) | 37 | 148 | |
| Challenge route | i.p. | i.d. | |
| Challenge dose (no. of leptospires) | 1,000 | 100 | |
| Challenge dose (no. of leptospires)/ED50 | 50 | 10 | |
| No. of control-immunized animals | 8 | 8 | |
| No. of LigA7-13-immunized animals | 8 | 8 | |
| % survival for control immunized | 0 | 0 | |
| % survival for LigA7-13 immunized | 37.5 | 62.5 | |
| ELISA Lc (OD450)a | 0.236 | 0.427 | |
| Mean LigA antibody levels (OD450) (all animals) after: | |||
| 2nd wk | 0.539 | 0.688 | NSb |
| 3rd wk | 0.432 | 1.491 | <0.05 |
| 4th wk | 0.499 | 1.337 | <0.03 |
| Mean LigA antibody levels (OD450) (survivors) after: | |||
| 2nd wk | 1.244 | 1.035 | NS |
| 3rd wk | 1.066 | 2.162 | NS |
| 4th wk | 1.077 | 1.774 | NS |
The critical limit (Lc) is defined as the signal that indicates that the target is present (rejection of the null hypothesis): 1.65 SD over the mean of background signals.
NS, not significant.
FIG 3.
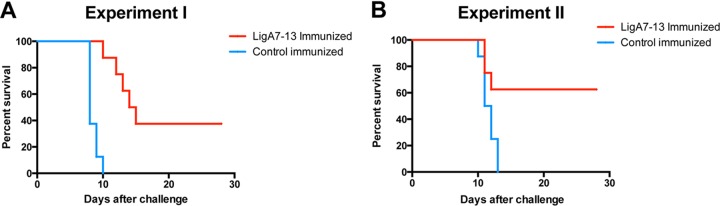
Survival of hamsters immunized with E. coli expressing LigA7-13. Hamsters in groups of 8 were immunized with E. coli expressing LigA7-13 (in red) or E. coli containing the empty vector (i.e., control-immunized group, in blue) and challenged with L. interrogans serovar Copenhageni strain Fiocruz L1-130. Hamsters were challenged with 103 bacteria by the i.p. route in experiment 1 (A) and with 102 bacteria by the i.d. route in experiment 2 (B). Animals were observed and weighed daily for 28 days. Animals were sacrificed when they met endpoint criteria, which included a 10% decrease of the body weight, or at 28 days postinfection. None of the control-immunized animals survived. In contrast, 3 of 8 (37.5%) LigA7-13-immunized animals survived in experiment 1, and 5 of 8 (62.5%) LigA7-13-immunized animals survived in experiment 2. In both experiments, survival curves are significantly different between LigA7-13-immunized and control-immunized hamsters (experiment 1, P < 0.0001; experiment 2, P = 0.0174).
Relationship between LigA7-13 antibody level and survival.
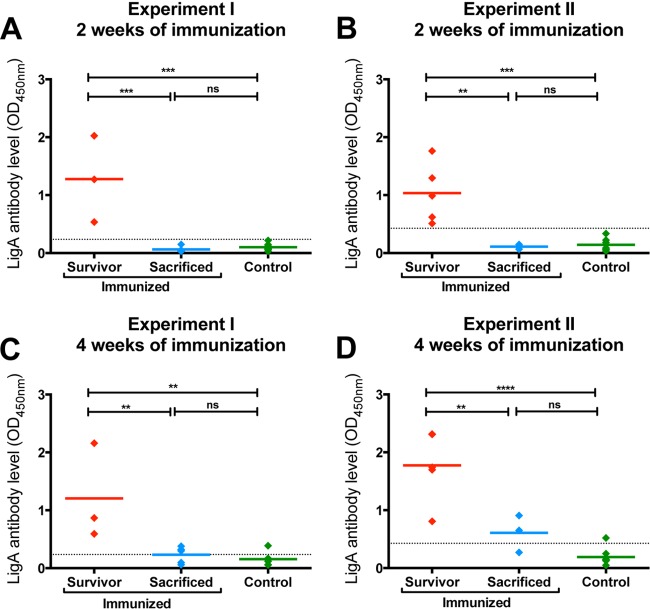
LigA7-13 antibody levels were measured by ELISA in serum collected from hamsters after 2 and 4 weeks of oral immunization. Levels in animals meeting endpoint criteria were compared with those in animals that did not meet endpoint criteria. In both experiments, LigA7-13-immunized animals that survived had higher antibody levels after 2 weeks of immunization than either control-immunized animals (experiment 1, P = 0.0009; experiment 2, P = 0.0005) or LigA7-13-immunized animals that met endpoint criteria (sacrificed) (experiment 1, P = 0.0085; experiment 2, P = 0.0231) (Fig. 4A and B). Similar results were observed after 4 weeks of immunization: LigA7-13-immunized survivors had higher antibody levels than either control-immunized animals (experiment 1, P = 0.0040; experiment 2, P < 0.0001) or LigA7-13-immunized animals that met the endpoint criteria (experiment 1, P = 0.0361; experiment 2, P = 0.0249) (Fig. 4C and D). In each case, there was no difference in the antibody levels of LigA7-13-immunized animals that met the endpoint criteria and control immunized hamsters (P < 0.05). All animals that had a significant LigA7-13 antibody response after 2 weeks of immunization survived to 28 days. In contrast, animals that did not achieve a significant antibody level met endpoint criteria early. These data suggest that the LigA7-13 IgG level after 2 weeks of immunization may be a surrogate marker for vaccine efficacy, i.e., only animals which develop significant antibody levels to LigA7-13 after 2 weeks of immunization will survive until day 28 after challenge without meeting endpoint criteria.
FIG 4.
Relationship between LigA7-13 antibody level and survival. LigA IgG antibody levels after two (A and B) and four (C and D) weeks of immunization were analyzed depending on the immunization and survival status of the animals. After both 2 and 4 weeks of oral immunization, LigA7-13-immunized hamsters that survived (avoided endpoint criteria) had higher antibody levels than either immunized hamsters that met endpoint criteria sooner (sacrificed) or control-immunized hamsters. LigA7-13 antibody levels represent the mean of ELISAs performed in triplicate. The number of asterisks indicates the significance level: **, P < 0.01; ***, P < 0.001. The letters “ns” indicate nonsignificance (P > 0.05). The dotted line shows the critical limit, defined as the value of the mean level of the control-immunized group plus 1.65 SD (experiment 1, 0.236; experiment 2, 0.427).
Effects of LigA7-13 oral immunization on renal infection.
As in previous studies examining immunoprotection with LigA (26, 30), oral immunization with LigA7-13 improved survival but did not prevent renal infection. In this study, kidney cultures of all hamsters were positive for leptospires, both from survivors and from those that met endpoint criteria early. Differences in renal leptospiral burden were observed depending on the animals' immunization and survival status. After i.p. challenge, the leptospiral burden in the kidneys of LigA7-13-immunized animals that survived (mean = 1.4 × 104 copies/mg of kidney tissue) was not different from that of control-immunized hamsters (mean = 8.4 × 103 copies/mg of kidney tissue) (P > 0.05) and was higher than in LigA7-13-immunized animals that met endpoint criteria (mean = 3.7 × 102 copies/mg of kidney tissue) (P = 0.0005). After i.d. challenge, there was no difference in the leptospiral burden in the kidneys of animals that met endpoint criteria, whether or not they were immunized with LigA7-13 (P > 0.05). The leptospiral burden in the kidneys of LigA7-13-immunized hamsters that survived (mean = 3.5 × 104 copies/mg of kidney tissue) was significantly higher than the number of bacteria in LigA7-13-immunized animals that met endpoint criteria (mean = 17 copies/mg of kidney tissue) (P < 0.02) and in control-immunized animals (mean = 20 copies/mg of kidney tissue) (P < 0.0001). These differences are most likely due to the different time of euthanasia; survivors were alive for >2 weeks longer than nonsurvivors, providing sufficient time for leptospires to multiply in the kidneys.
Histopathology.
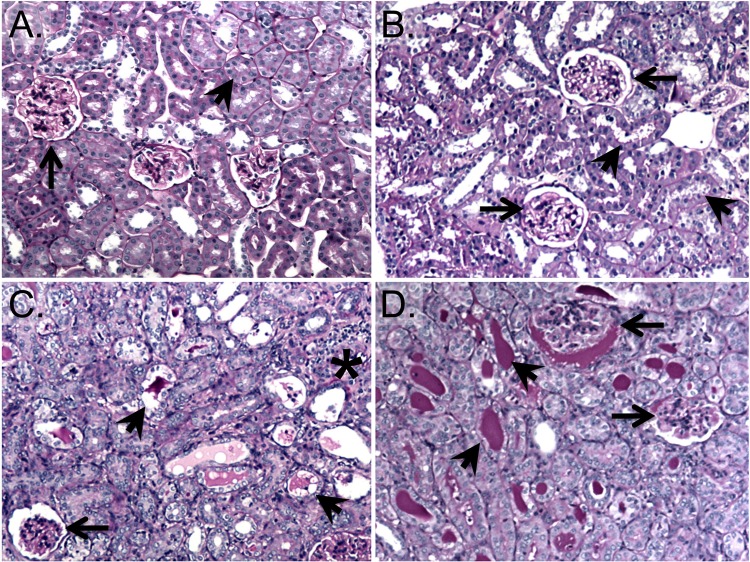
Kidneys were collected from all hamsters either when they met endpoint criteria or at 28 days after challenge. Kidney sections were stained with H&E and PAS and scored blindly using a scale of 0 to 15 (see Materials and Methods for scoring method details). LigA7-13-immunized animals that survived to 28 days postchallenge had less hyaline cast deposition, renal tubular damage, tubular and glomerular atrophy, and Bowman's space dilation (Fig. 5B) compared to control-immunized animals (Fig. 5C) or immunized hamsters that met endpoint criteria (Fig. 5D). LigA7-13-immunized animals that survived had significantly lower (i.e., better) total histopathology scores than either control-immunized animals (P < 0.0001) or LigA7-13-immunized animals that met endpoint criteria (P < 0.0001) (Fig. 6A). The lower histopathology scores in the survivors may be due in part to the fact that these animals had time to recover from the acute phase of leptospirosis infection. There was a correlation (R2 = 0.79, Pearson) between the total histopathology score and the percent weight gain or loss relative to the date of challenge (Fig. 6B).
FIG 5.
Renal histopathology. Representative PAS-stained kidney sections with glomeruli and tubules marked by arrows and arrowheads, respectively, showing normal renal tubular and glomerular morphology (10×) (A), mild tubular and glomerular damage in an LigA7-13-immunized hamster that survived to 28 days after challenge (10×) (B), interstitial inflammation and moderate glomerular and tubular damage and tubular casts in an LigA7-13-immunized hamster that did not survive challenge (10×) (C), and severe glomerular damage and extensive tubular cast formation in a control-immunized hamster that did not survive challenge (10×) (D).
FIG 6.
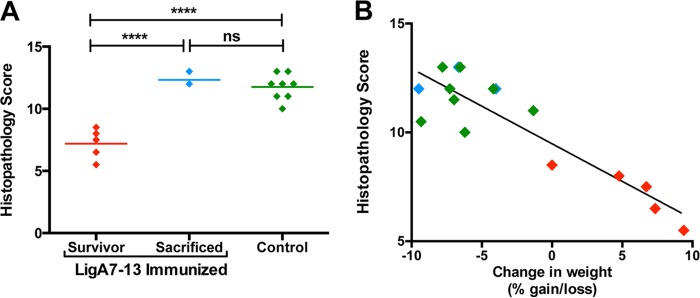
Renal histopathology scores. Kidneys were collected from all animals and examined by histopathology. Slides were stained with PAS, and lesions were read blindly and scored on a scale of 0 (normal) to 15 (severe) for the degree of hyaline cast deposition, interstitial inflammation, Bowman's space dilation, renal tubular damage, tubular and glomerular atrophy, and associated capsular depression (see the text for details). (A) Histopathology scores of all hamsters (experiment 2) of LigA7-13-immunized animals that survived were lower than those for either LigA7-13-immunized animals that were sacrificed or control-immunized hamsters. ****, P < 0.0001. The letters “ns” indicate nonsignificance (P > 0.05). (B) Histopathology scores correlated with the percent gain or loss of weight of each animal at the time of sacrifice relative to the weight on the challenge date.
DISCUSSION
This study examined whether an oral OMP-based leptospiral vaccine can provide protection against lethal infection. The vaccine consisted of E. coli expressing a fusion of the OspA lipoprotein signal peptide with LigA immunoglobulin-like domains 7 to 13. The OspA signal peptide resulted in lipidation of LigA, as demonstrated by intrinsic labeling with [14C]palmitate (Fig. 1A) and incorporation of LigA7-13 into the E. coli cell envelope fraction (Fig. 1B and C). Lipidation of OspA has been shown to be important for overcoming oral tolerance by inducing a Th1/Th2 immune response (19). Hamsters immunized by oral gavage with E. coli expressing the lipidated LigA7-13 antigen developed a protective immune response to lethal challenge by L. interrogans serovar Copenhageni. We performed two independent experiments using oral immunization involving two different vaccine doses and two different challenge doses and routes: 103 bacteria delivered by the intraperitoneal (i.p.) route and 102 bacteria delivered by the intradermal (i.d.) route of challenge. All control-immunized animals in both experiments were gavaged with E. coli containing the empty pET9c plasmid and failed to survive challenge, while 37.5% and 62.5% of hamsters immunized with 37 and 148 mg of LigA7-13, respectively, survived challenge (Fig. 3 and Table 2). A novel aspect of this study is that we are able to use an oral delivery system to raise a systemic antibody response to a leptospiral antigen, LigA7-13, demonstrating for the first time that it is feasible to protect animals from leptospirosis using an oral vaccine. Induction of a protective immune response can be attributed to two factors: (i) the delivery vehicle was E. coli, which itself may have natural adjuvant capabilities; and (ii) the lipidation of LigA7-13, which also was shown to have adjuvant capability in mice (16–18). Together, the delivery vehicle and the lipidated immunogen may contribute independently to production of a highly immunogenic oral vaccine.
The i.d. route is arguably a more biologically relevant challenge route than the i.p. route and is relatively easy to administer. Our results confirm those of previous studies (39, 40) showing no major differences in outcome for different challenge routes. In our study, we found that the ED50 for i.d. challenge of 11-week-old hamsters was <10 leptospires, whereas the ED50 for i.p. challenge was slightly higher: 20 leptospires (Table 1). These ED50 estimates are consistent with the LD50 previously reported using i.p. challenge of 9-week-old hamsters (26, 41) and that of i.p. challenge of 3-week-old guinea pigs (40). Our ED50 estimates are also consistent with the 0% survival results among control-immunized animals challenged with 1,000 leptospires i.p. in experiment 1 and 100 leptospires i.d. in experiment 2. Low challenge doses such as these are likely to be similar to those occurring during natural infection based on field studies showing that the concentrations of leptospires in natural bodies of water range from 50/ml to 103/ml, depending on whether samples were obtained from rural or urban areas (42). Despite the low challenge doses used in this study, culture and qPCR results of kidney tissue from all animals, LigA7-13 immunized or not, were positive for leptospires. This result is consistent with previous studies showing that LigA immunization afforded protection from lethality but not renal infection (26, 30). It is worth noting that our qPCR method involved TaqMan quantitation of the number of lipL32 gene copies per gram of kidney tissue, a method that has previously been applied to blood and urine (38, 43).
The strength of the hamster humoral response to immunization was completely predictive of survival. All animals that achieved an antibody response greater than the critical limit after the first 2 weeks of immunization survived, whereas all animals with an antibody level lower than the critical limit met endpoint criteria, even if they mounted a significant antibody level after the third or fourth weeks of immunization; one animal had an undetectable LigA7-13 antibody level after 2 weeks of immunization and a higher level than a survivor after the fourth week of immunization but still met endpoint criteria (Fig. 2 and 4). These findings suggest that generating a humoral immune response to the antigen at an early stage in the immunization process is critical to achieving protection. Additionally, these results represent opportunities to improve the oral immunization protocol and to use the LigA7-13 antibody response as a surrogate marker for vaccine efficacy.
In an effort to boost antibody responses, hamsters were given four times more LigA7-13 in the second experiment than in the first. The increase in LigA7-13 dose resulted in a higher percentage of animals achieving a critical LigA7-13 antibody level after the first boost (2 first weeks of immunization) and in a higher LigA7-13 antibody level after the third and fourth weeks of immunization (Table 2). While a total dose of 37 mg of LigA7-13 (experiment 1) did not result in an increase in LigA7-13 antibody levels after the third week of immunization, animals that received a total dose of 148 mg of LigA7-13 (experiment 2) more than doubled their LigA7-13 antibody levels after the third week of immunization. This increase in LigA7-13 dose resulted in 3.5- and 2.6-fold increases in the LigA7-13 antibody as measured by ELISA after the third and fourth weeks of immunization, respectively (see Table 2). Differences in LigA7-13 antibody levels between the first and second experiments were significant (t test, P < 0.05) and are reflected in the higher survival rate in the second experiment. These results suggest that methods of delivering larger amounts of LigA7-13 antigen by oral immunization could result in higher antibody levels, a higher percentage of animals responding to immunization, and higher survival rates.
As in an earlier study, we found that an endpoint criterion of weight loss of ≥10% of the animal's maximum body weight was extremely useful, because a decrease in body weight was the earliest observable sign of clinical leptospirosis (30). Further support for the importance of body weight in animal models of leptospirosis comes from our finding that the weight of animals that survived challenge was also useful as an indication of underlying renal health. There was a correlation (R2 = 0.79, Pearson) between renal histopathology and the change in weight of animals during the 28 days after challenge (Fig. 6B). Animals with fewer renal histopathology lesions and lower histopathology scores weighed more at the end of the study. This correlation reflects, in part, the accuracy of our renal histopathology scoring system, which was improved from our previously described scoring method (30), and the importance of kidney function in the overall health of the animals. The largest differences between the renal histopathology of LigA7-13-immunized survivors and nonsurvivors or control-immunized hamsters were the scores for the severity of tubular damage and the degree of hyaline cast formation (data not shown). The ability of a vaccine to protect against renal pathology is highly significant, given that renal failure is a major predictor of morbidity and mortality in leptospirosis (7). It is likely that the lower histopathology scores in the survivors reflect recovery from acute infection. In this context, it is interesting to note that the renal histopathology of survivors was better than that for animals that met endpoint criteria during acute infection, even though the burden of organisms in the kidneys of survivors was greater.
In summary, this study establishes oral immunization as a novel approach to inducing protective immunity against leptospirosis. In hamsters that received a total dose of 148 mg of LigA7-13, protection against lethal infection was observed in 62.5% hamsters after i.d. challenge. This challenge route is a more natural transmission route for pathogenic leptospires than the i.p. route commonly used in animal models of acute leptospirosis. Based on the strong predictive relationship between LigA7-13 antibody levels and protection, future efforts will be directed toward priming cellular immune responses in ways that stimulate strong antibody responses. If sterilizing immunity can be achieved by inclusion of additional antigens, an oral vaccine could be applied to the rat model of chronic leptospirosis with prevention of urinary shedding as a major milestone. Based on the previous success of studies on reservoir hosts for rabies and B. burgdorferi (15, 44), we believe that oral immunization of reservoir hosts may be an effective strategy to prevent human exposure to leptospirosis.
ACKNOWLEDGMENTS
We are thankful to Jane Babbitt for critical reading of the manuscript.
This work was supported by grants R01 AI034431 (to D.A.H.) and R43 AI096551 (to M.G.-S.) from the National Institutes of Health, grant UO1 CK000107 (to M.G.-S.) from the Centers for Disease Control and Prevention, and VA Merit Research Funds (to J.M. and D.A.H.).
D.A.H., J.M., and M.G.-S. have patents related to this work.
Footnotes
Published ahead of print 9 December 2013
REFERENCES
- 1.Levett PN. 2001. Leptospirosis. Clin. Microbiol. Rev. 14:296–326. 10.1128/CMR.14.2.296-326.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faine S, Adler B, Bolin C, Perolat P. 1999. Leptospira and leptospirosis, 2nd ed. MediSci, Melbourne, Australia [Google Scholar]
- 3.Weil A. 1886. Ueber eine eigenthümliche, mit milztumor, icterus und nephritis einhergehende, acute infectionskrankheit. Dtsch. Arch. Klin. Med. 39:209 [Google Scholar]
- 4.McBride AJ, Athanazio DA, Reis MG, Ko AI. 2005. Leptospirosis. Curr. Opin. Infect. Dis. 18:376–386. 10.1097/01.qco.0000178824.05715.2c [DOI] [PubMed] [Google Scholar]
- 5.Hartskeerl RA, Collares-Pereira M, Ellis WA. 2011. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clin. Microbiol. Infect. 17:494–501. 10.1111/j.1469-0691.2011.03474.x [DOI] [PubMed] [Google Scholar]
- 6.WHO 2003. Human leptospirosis: guidance for diagnosis, surveillance and control. WHO, Geneva, Switzerland [Google Scholar]
- 7.Ko AI, Galvao Reis M, Ribeiro Dourado CM, Johnson WD, Jr, Riley LW, Salvador Leptospirosis Study Group 1999. Urban epidemic of severe leptospirosis in Brazil. Lancet 354:820–825. 10.1016/S0140-6736(99)80012-9 [DOI] [PubMed] [Google Scholar]
- 8.Martínez Sanchez R, Obregon Fuentes AM, Perez Sierra A, Baly Gil A, Diaz Gonzalez M, Baro Suarez M, Menendez Capote R, Ruiz Perez A, Sierra Gonzalez G, Lopez Chavez AU. 1998. The reactogenicity and immunogenicity of the first Cuban vaccine against human leptospirosis. Rev. Cuba. Med. Trop. 50:159–166 [PubMed] [Google Scholar]
- 9.Baranton G. 1990. The human vaccine against Leptospirosis icterohaemorrhagiae. An effective vaccine which concerns only exposed subjects. Rev. Prat. Med. Gen. 93:17–19 [Google Scholar]
- 10.Koizumi N, Watanabe H. 2005. Leptospirosis vaccines: past, present, and future. J. Postgrad. Med. 51:210–214 [PubMed] [Google Scholar]
- 11.Lycke N. 2012. Recent progress in mucosal vaccine development: potential and limitations. Nat. Rev. Immunol. 12:592–605. 10.1038/nri3251 [DOI] [PubMed] [Google Scholar]
- 12.del Rio B, Dattwyler RJ, Aroso M, Neves V, Meirelles L, Seegers JF, Gomes-Solecki MJ. 2008. Oral immunization with recombinant lactobacillus plantarum induces a protective immune response in mice with Lyme disease. Clin. Vaccine Immunol. 15:1429–1435. 10.1128/CVI.00169-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmgren J, Svennerholm AM. 2012. Vaccines against mucosal infections. Curr. Opin. Immunol. 24:343–353. 10.1016/j.coi.2012.03.014 [DOI] [PubMed] [Google Scholar]
- 14.Scheckelhoff MR, Telford SR, Hu LT. 2006. Protective efficacy of an oral vaccine to reduce carriage of Borrelia burgdorferi (strain N40) in mouse and tick reservoirs. Vaccine 24:1949–1957. 10.1016/j.vaccine.2005.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomes-Solecki MJ, Brisson DR, Dattwyler RJ. 2006. Oral vaccine that breaks the transmission cycle of the Lyme disease spirochete can be delivered via bait. Vaccine 24:4440–4449. 10.1016/j.vaccine.2005.08.089 [DOI] [PubMed] [Google Scholar]
- 16.Erdile LF, Brandt MA, Warakomski DJ, Westrack GJ, Sadziene A, Barbour AG, Mays JP. 1993. Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect. Immun. 61:81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erdile LF, Guy B. 1997. OspA lipoprotein of Borrelia burgdorferi is a mucosal immunogen and adjuvant. Vaccine 15:988–996. 10.1016/S0264-410X(96)00295-2 [DOI] [PubMed] [Google Scholar]
- 18.Weis JJ, Ma Y, Erdile LF. 1994. Biological activities of native and recombinant Borrelia burgdorferi outer surface protein A: dependence on lipid modification. Infect. Immun. 62:4632–4636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.del Rio B, Seegers JF, Gomes-Solecki MJ. 2010. Immune response to Lactobacillus plantarum expressing Borrelia burgdorferi OspA is modulated by the lipid modification of the antigen. PLoS One 5:e11199. 10.1371/journal.pone.0011199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haake DA, Matsunaga J. 2010. Leptospira: a spirochaete with a hybrid outer membrane. Mol. Microbiol. 77:805–814. 10.1111/j.1365-2958.2010.07262.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nally JE, Whitelegge JP, Blanco SBDR, Lovett MA. 2007. Characterization of the outer membrane proteome of Leptospira interrogans expressed during acute lethal infection. Infect. Immun. 75:766–773. 10.1128/IAI.00741-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsunaga J, Barocchi MA, Croda J, Young TA, Sanchez Y, Siqueira I, Bolin CA, Reis MG, Riley LW, Haake DA, Ko AI. 2003. Pathogenic Leptospira species express surface-exposed proteins belonging to the bacterial immunoglobulin superfamily. Mol. Microbiol. 49:929–945. 10.1046/j.1365-2958.2003.03619.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsunaga J, Medeiros MA, Sanchez Y, Werneid KF, Ko AI. 2007. Osmotic regulation of expression of two extracellular matrix-binding proteins and a haemolysin of Leptospira interrogans: differential effects on LigA and Sph2 extracellular release. Microbiology 153:3390–3398. 10.1099/mic.0.2007/007948-0 [DOI] [PubMed] [Google Scholar]
- 24.Matsunaga J, Schlax PJ, Haake DA. 2013. Role for cis-acting RNA sequences in the temperature-dependent expression of the multiadhesive Lig proteins in Leptospira interrogans. J. Bacteriol. 195:5092–5101. 10.1128/JB.00663-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Croda J, Ramos JG, Matsunaga J, Queiroz A, Homma A, Riley LW, Haake DA, Reis MG, Ko AI. 2007. Leptospira immunoglobulin-like proteins as a serodiagnostic marker for acute leptospirosis. J. Clin. Microbiol. 45:1528–1534. 10.1128/JCM.02344-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva EF, Medeiros MA, McBride AJ, Matsunaga J, Esteves GS, Ramos JB, Santos CS, Croda J, Homma A, Dellagostin OA, Haake DA, Reis MG, Ko AI. 2007. The terminal portion of leptospiral immunoglobulin-like protein LigA confers protective immunity against lethal infection in the hamster model of leptospirosis. Vaccine 25:6277–6286. 10.1016/j.vaccine.2007.05.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koizumi N, Watanabe H. 2004. Leptospiral immunoglobulin-like proteins elicit protective immunity. Vaccine 22:1545–1552. 10.1016/j.vaccine.2003.10.007 [DOI] [PubMed] [Google Scholar]
- 28.Palaniappan RU, McDonough SP, Divers TJ, Chen CS, Pan MJ, Matsumoto M, Chang YF. 2006. Immunoprotection of recombinant leptospiral immunoglobulin-like protein A against Leptospira interrogans serovar Pomona infection. Infect. Immun. 74:1745–1750. 10.1128/IAI.74.3.1745-1750.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faisal SM, Yan W, Chen CS, Palaniappan RU, McDonough SP, Chang YF. 2008. Evaluation of protective immunity of Leptospira immunoglobulin like protein A (LigA) DNA vaccine against challenge in hamsters. Vaccine 26:277–287. 10.1016/j.vaccine.2007.10.029 [DOI] [PubMed] [Google Scholar]
- 30.Coutinho ML, Choy HA, Haake D. 2011. A LigA three-domain region protects hamsters from lethal infection by Leptospira interrogans. PLoS Neg. Trop. Dis. 5:e1422. 10.1371/journal.pntd.0001422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellinghausen HC, McCullough WG. 1965. Nutrition of “Leptospira pomona” and growth of 13 other serotypes: fractionation of oleic albumin complex and a medium of bovine albumin and polysorbate 80. Am. J. Vet. Res. 26:45–51 [PubMed] [Google Scholar]
- 32.Johnson RC, Harris VG. 1967. Differentiation of pathogenic and saprophytic letospires. I. Growth at low temperatures. J. Bacteriol. 94:27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller JN. 1971. Spirochetes in body fluids and tissues: manual of investigative methods. Charles C. Thomas Publisher, Springfield, IL [Google Scholar]
- 34.Nascimento AL, Ko AI, Martins EA, Monteiro-Vitorello CB, Ho PL, Haake DA, Verjovski-Almeida S, Hartskeerl RA, Marques MV, Oliveira MC, Menck CF, Leite LC, Carrer H, Coutinho LL, Degrave WM, Dellagostin OA, El-Dorry H, Ferro ES, Ferro MI, Furlan LR, Gamberini M, Giglioti EA, Goes-Neto A, Goldman GH, Goldman MH, Harakava R, Jeronimo SM, Junqueira-de-Azevedo IL, Kimura ET, Kuramae EE, Lemos EG, Lemos MV, Marino CL, Nunes LR, de Oliveira RC, Pereira GG, Reis MS, Schriefer A, Siqueira WJ, Sommer P, Tsai SM, Simpson AJ, Ferro JA, Camargo LE, Kitajima JP, Setubal JC, Van Sluys MA. 2004. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J. Bacteriol. 186:2164–2172. 10.1128/JB.186.7.2164-2172.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radolf JD, Chamberlain NR, Clausell A, Norgard MV. 1988. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp. pallidum by phase partitioning with the nonionic detergent triton X-114. Infect. Immun. 56:490–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9:671–675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 38.Stoddard RA, Gee JE, Wilkins PP, McCaustland K, Hoffmaster AR. 2009. Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagn. Microbiol. Infect. Dis. 64:247–255. 10.1016/j.diagmicrobio.2009.03.014 [DOI] [PubMed] [Google Scholar]
- 39.Stavitsky AB. 1945. Studies on the pathogenesis of leptospirosis. J. Infect. Dis. 76:179–192. 10.1093/infdis/76.3.179 [DOI] [Google Scholar]
- 40.Lourdault K, Aviat F, Picardeau M. 2009. Use of quantitative real-time PCR for studying the dissemination of Leptospira interrogans in the guinea pig infection model of leptospirosis. J. Med. Microbiol. 58:648–655. 10.1099/jmm.0.008169-0 [DOI] [PubMed] [Google Scholar]
- 41.Silva EF, Santos CS, Athanazio DA, Seyffert N, Seixas FK, Cerqueira GM, Fagundes MQ, Brod CS, Reis MG, Dellagostin OA, Ko AI. 2008. Characterization of virulence of Leptospira isolates in a hamster model. Vaccine 26:3892–3896. 10.1016/j.vaccine.2008.04.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganoza CA, Matthias MA, Collins-Richards D, Brouwer KC, Cunningham CB, Segura ER, Gilman RH, Gotuzzo E, Vinetz JM. 2006. Determining risk for severe leptospirosis by molecular analysis of environmental surface waters for pathogenic Leptospira. PLoS Med. 3:e308. 10.1371/journal.pmed.0030308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoddard RA. 2013. Detection of pathogenic Leptospira spp. through real-time PCR (qPCR) targeting the LipL32 gene. Methods Mol. Biol. 943:257–266. 10.1007/978-1-60327-353-4_17 [DOI] [PubMed] [Google Scholar]
- 44.Moss B. 1996. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc. Natl. Acad. Sci. U. S. A. 93:11341–11348. 10.1073/pnas.93.21.11341 [DOI] [PMC free article] [PubMed] [Google Scholar]