Abstract
High concentrations of lung tissue-associated interleukin-10 (IL-10), an anti-inflammatory and immunosuppressive cytokine, correlate with susceptibility of mice to Coccidioides spp. infection. In this study, we found that macrophages, dendritic cells, neutrophils, and both CD8+ and CD4+ T cells recruited to Coccidioides posadasii-infected lungs of nonvaccinated and vaccinated mice contributed to the production of IL-10. The major IL-10-producing leukocytes were CD8+ T cells, neutrophils, and macrophages in lungs of nonvaccinated mice, while both Foxp3+ and Foxp3− subsets of IL-10+ CD4+ T cells were significantly elevated in vaccinated mice. Profiles of the recruited leukocytes in lungs revealed that only CD4+ T cells were significantly increased in IL-10−/− knockout mice compared to their wild-type counterparts. Furthermore, ex vivo recall assays showed that CD4+ T cells isolated from vaccinated IL-10−/− mice compared to vaccinated wild-type mice produced significantly higher amounts of IL-2, gamma interferon (IFN-γ), IL-4, IL-6, and IL-17A in the presence of a coccidioidal antigen, indicating that IL-10 suppresses Th1, Th2, and Th17 immunity to Coccidioides infection. Analysis of absolute numbers of CD44+ CD62L− CD4+ T effector memory T cells (TEM) and IFN-γ- and IL-17A-producing CD4+ T cells in the lungs of Coccidioides-infected mice correlated with better fungal clearance in nonvaccinated IL-10−/− mice than in nonvaccinated wild-type mice. Our results suggest that IL-10 suppresses CD4+ T-cell immunity in nonvaccinated mice during Coccidioides infection but does not impede the development of a memory response nor exacerbate immunopathology of vaccinated mice over at least a 4-month period after the last immunization.
INTRODUCTION
Coccidioides is a fungal pathogen and the causative agent of a human respiratory disease known as coccidioidomycosis, or San Joaquin Valley fever (1). Human infections typically occur by inhalation of spores (arthroconidia) released into the air from the saprobic phase of the soilborne fungus. In the approximately 40% of human exposures that result in symptomatic infection, the initial clinical manifestation is characterized by onset of an acute respiratory response that can occur within 1 to 3 weeks after inhalation of the pathogen. In a few patients (<5%), Coccidioides species infections may progress to life-threatening, chronic pneumonia, extrapulmonary nonmeningeal disease, or meningitis. The latter is the most feared complication of coccidioidomycosis (1). The number of reported cases of primary coccidioidal pneumonia in Arizona and California has significantly increased during the last decade (2), which has raised the level of awareness among people who live in regions where this mycosis is endemic. Development of a vaccine and effective therapeutic strategies against coccidioidomycosis would promote the well-being of at-risk populations in the areas of endemicity.
Interleukin-10 (IL-10) is a pleiotropic cytokine with anti-inflammatory and immunosuppressive functions and the ability to impact both innate and adaptive immunity to microbial infections (3–5). Studies using IL-10 knockout mice have suggested that this cytokine is an essential immune regulator in a variety of fungal infections, including infections caused by Candida spp. (6), Cryptococcus neoformans (7), Histoplasma capsulatum (8), Pneumocystis carinii (9), and Aspergillus fumigatus (10). A correlation has been revealed between susceptibility to Coccidioides infection and the amount of IL-10 produced (11–14). Loss of IL-10 production significantly improves the outcome of coccidioidomycosis in nonvaccinated mice (12, 13). IL-10 can exert direct inhibition on CD4+ T cell proliferation and cytokine synthesis (15). In the latter case, IL-10 has been shown to suppress the production of IL-2, gamma interferon (IFN-γ), IL-4, and IL-5 (16) and, thereby, hamper protective responses of both Th1 and Th2 cells during early stages of microbial and viral infections (15, 17). Recently, IL-10 has also been shown to inhibit murine macrophages and T cells in the secretion of Th17-related cytokines (18). The latter are required for development of Th17-type immunity, which is essential for vaccine-induced protection against Coccidioides infection and other dimorphic fungal diseases (19, 20). Thus, treatment with anti-IL-10 antibody and vaccination strategies aimed at neutralizing excess IL-10 following microbial infection should provide therapeutic advantages (21, 22).
On the other hand, IL-10 is required to control fungal infections caused by Candida albicans and P. carinii (9, 23) as well as numerous viral, bacterial, and parasitic pathogens (24–26). Although IL-10-deficient mice infected with Candida revealed significantly reduced fungal burden, the mice presented with severe inflammatory pathology and susceptibility to reinfection (23). An attempt to treat Leishmania major infection in mice by immunization with an IL-10 peptide-based vaccine revealed increased parasitic burden and exacerbated disease (27). These contradictory effects of IL-10 raise concerns about application of supplemental IL-10 therapy to treat inflammatory diseases or neutralization of IL-10 to improve the efficacy of vaccines against microbial infections (21, 22).
Despite the considerable information available regarding the regulatory functions of IL-10 for the immune response and in immunopathology, there is less known about the major sources of this cytokine during specific microbial infections. IL-10 can be produced by CD4+ T regulatory (Treg) cells, CD8+ T cells, and numerous members of the innate immune repertoire, including dendritic cells (DCs), macrophages, mast cells, natural killer cells, neutrophils (polymorphonuclear leukocytes [PMNs]), and B cells (3). In a murine model of acute brucellosis, CD4+ CD25+ T cells were identified as the major source of IL-10 (28). These cells were shown to play an important role in modulating the early immune response to Brucella abortus infection. Similarly, T-cell-derived, but not B-cell-derived, IL-10 was reported to contribute to the suppression of the antigen-specific CD4+ T-cell response to a helminth parasite infection in mice (29). In the case of Yersinia enterocolitica infection, the main sources of IL-10 were neutrophils (30). In this study, we explored the following questions related to the IL-10 response to Coccidioides posadasii infection. (i) What are the cellular sources of IL-10 in vaccinated versus nonvaccinated C57BL/6 mice following pulmonary infection? (ii) Are the composition and numbers of immune cells in the lungs of Coccidioides-infected mice altered in the absence of IL-10? (iii) Can IL-10-deficient mice develop long-term T-cell immunity and polarization of the CD4+ T-cell response to infection? (iv) Does ablation of IL-10 cause immunopathology during early stages of coccidioidomycosis?
MATERIALS AND METHODS
Fungal strain, growth conditions, and spore preparation.
The virulent fungal strain used to challenge mice in this study was clinical isolate C735 of Coccidioides posadasii. A previously described genetically engineered, live, attenuated mutant strain (Δcts2/ard1/cts3) derived from the parental C735 isolate was employed as a vaccine and designated ΔT (19, 31). The saprobic phases of both the wild-type (WT) and mutant strains were grown on GYE agar (1% glucose, 0.5% yeast extract, 1.5% agar) for 3 to 4 weeks at 30°C to generate a confluent layer of spores on the surface of the solid culture medium. Spore suspensions used for vaccination or intranasal challenge of mice as described below were prepared as previously reported (32). All culturing and preparatory procedures that involved live cells of C. posadasii were conducted in a biosafety level 3 (BSL3) laboratory.
Mouse strain.
Breeder pairs of C57BL/6 and B6.129P2-IL10tm1Cgn/J (IL-10−/−) mice were purchased from Jackson Laboratory. Mice were housed in a specific-pathogen-free (SPF) animal facility at the University of Texas at San Antonio (UTSA) and handled according to guidelines approved by the university's Institutional Animal Care and Use Committee. Both strains of mice were gender matched. Animals 8 to 10 weeks old with an average weight of 18 to 22 g were used in this study. Mice were relocated to an animal biosafety level 3 laboratory prior to vaccination and infection.
Vaccination protocol, animal challenge, and evaluation of protection.
Primary immunization of C57BL/6 and IL-10−/− mice with 5.0 × 104 viable spores of the live ΔT vaccine in 100 μl phosphate-buffered saline (PBS) was performed by the subcutaneous route in the abdominal region. This initial vaccination step was followed 14 days later with a boost of 2.5 × 104 spores by the same route of immunization. Control mice were injected with PBS following the same vaccination protocol as above. The animals were challenged 16 weeks after completion of the vaccination protocol by intranasal instillation with a suspension of approximately 80 viable spores of the virulent, parental isolate of C. posadasii (C735) in 35 μl PBS as previously reported (31). The fungal burden in the lungs and spleen was determined at the time when the animal approached the moribund state or at 14 days postchallenge (DPC) by plating serial dilutions of separate lung and spleen homogenates on GYE agar containing 50 μg/ml chloramphenicol, as reported elsewhere (31). The number of CFU of Coccidioides was expressed on a log scale and reported for each group of 10 animals as previously described (31). Survival studies of vaccinated versus nonvaccinated mice were conducted over 60 days postchallenge as reported previously (31).
Cytokine assays.
Concentrations of IL-10 in supernatants of lung homogenates were compared between nonvaccinated and ΔT-vaccinated mice sacrificed at 5, 7, 9, 11, and 14 DPC (4 animals per group). Lung homogenates of individual mice were prepared as previously described (33), supernatants were obtained by centrifugation (8,000 × g at 4°C for 10 min), and samples were stored at −80°C until ready for analysis. Assays of cytokine and chemokine concentrations were conducted using a Bio-Plex suspension array system (Bio-Rad Laboratories, Hercules, CA) as previously reported (33).
Quantitative RT-PCR assays.
Total RNA was extracted from isolated pulmonary cells of Coccidioides-infected lungs of nonvaccinated and vaccinated C57BL/6 mice (three animals per group) using an RNeasy kit (Qiagen, Valencia, CA). The samples were treated with DNase (Promega, San Luis Obispo, CA) to remove traces of contaminating DNA. Reverse transcription (RT) was performed using 5 μg total RNA in a 50-μl reaction mixture containing oligo(dT) and SuperScript III (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The relative amounts of specific gene transcripts encoding IL-10 and the forkhead box P3 transcription factor (Foxp3) (34) in the lungs of nonvaccinated and vaccinated mice were examined by quantitative RT-PCR (QRT-PCR) as previously reported (32). The data are presented as the mean fold change ± the standard error of the mean (SEM).
Quantification of Treg cells.
Regulatory T cells are characterized by expression of CD3, CD4, and CD25 surface markers as well as expression of the Foxp3 transcription factor gene. Total pulmonary leukocytes were isolated from nonvaccinated mice or mice immunized with the ΔT vaccine and sacrificed at 5 to 14 days postchallenge (3 mice per group) as previously reported (19). A standard methodology was employed for direct monoclonal antibody (MAb) labeling and enumeration of selected pulmonary leukocyte phenotypes by fluorescence-activated cell sorting (FACS) with a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ) as previously described (19). Isolated pulmonary leukocytes were blocked and labeled with fluorochrome-conjugated monoclonal antibodies against CD3 (clone 17A2), CD4 (clone RM4-5), and CD25 (clone PC61). The cells were then chemically fixed and permeabilized using a mouse Foxp3 buffer set (BD Bioscience) and incubated with 0.25 μg of fluorochrome-conjugated anti-Foxp3 MAb (clone MF23) in 40 μl of FACS buffer for 30 min at 4°C. The absolute number of Treg cells was determined by multiplying the percentage of the gated population by the total number of CD45+ cells.
Characterization of IL-10-producing leukocyte subpopulations by flow cytometry.
Pulmonary leukocytes were isolated from nonvaccinated mice or mice vaccinated with the ΔT vaccine as well as naive (untreated) IL-10−/− mice. The latter were used as negative controls for intracellular staining of IL-10. Pulmonary cells were stimulated with 50 ng/ml phorbol myristate acetate (PMA; Sigma-Aldrich, St. Louis, MO) and 500 ng/ml ionomycin (Sigma-Aldrich) in the presence of GolgiStop and GolgiPlug (BD Biosciences) in 10% fetal bovine serum (FBS)-supplemented RPMI 1640 for 5 h at 37°C. After stimulation, aliquots of cells were first stained for surface markers of T cells (CD4, CD8α, and CD45) and B cells (CD19 and CD45) in separate wells and then stained for intracellular IL-10 as previously described (19). A separate aliquot was first labeled with monoclonal antibodies against surface markers CD3 and CD4, followed by intracellular labeling for Foxp3 and IL-10 to determine numbers of IL-10-producing Treg cells. Pulmonary cells were gated on CD45+ leukocytes for T- and B-cell panels and then analyzed for IL-10 labeling. The amount of baseline label of IL-10 in leukocytes (CD45+) isolated from IL-10−/− mice was used as the control. Percentages of CD4+ CD8−, CD4− CD8+, and CD19+ cells in the CD45+ IL-10+ population were determined and used to calculate the absolute number of IL-10-producing CD4+ T cells, CD8+ T cells, and B cells, respectively. The double-positive CD4+ CD8+ cells, which could also be DCs, were not included within the gates. Pulmonary leukocytes identified as IL-10+/CD8− CD4+ Foxp3+ were defined as pulmonary Treg cells. Aliquots of IL-10+-labeled, PMA/ionomycin-activated pulmonary cells were stained for surface markers to identify different types of granulocytes (CD11b, Ly6G, and CD11c). FACS data were first gated on cells which were IL-10+ and then further analyzed for their expression of Ly6G, CD11b, and CD11c. Percentages of Ly6G+ CD11b+, CD11b+ CD11c+, and CD11b+ CD11c− Ly6G− cells in the IL-10+ population were determined and used to calculate the absolute number of IL-10-producing neutrophils, dendritic cells, and macrophages, respectively.
Quantification of leukocyte cell types in Coccidioides-infected lungs.
Flow cytometry was used to determine the numbers of CD4+ T cells, CD8+ T cells, B cells, natural killer cells (NK), neutrophils (PMNs), alveolar macrophages (AM), inflammatory macrophages (IM), and DCs in the lung homogenates as previously described (19, 33). The gating strategies were the following: CD3+ CD4+ CD8−, CD3+ CD4− CD8+, CD19+, CD161c (NK1.1)+ CD3−, Ly6G+ CD11b+ CD11c−, intermediate levels of CD11b (CD11bM) CD11c+, Mac3+ CD11c− Ly6G−, and high levels of CD11b (CD11bH) CD11c+, respectively (19, 32, 33).
Recall assays of CD4+ T cells.
Splenocytes of wild-type C57BL/6 and IL-10−/− mice immunized with the ΔT vaccine were separately harvested, pooled, and macerated for isolation of CD4+ T cells by using a CD4+ T-cell isolation kit (Miltenyi Biotec, Inc., Gladbach, Germany) as previously reported (35). The purity of CD4+ T cells was verified by staining the cells with fluorescein-conjugated anti-CD3 and anti-CD4 MAbs, followed by flow cytometry. The purity of CD4+ T cells was routinely above 90%. The isolated CD4+ T cells were cultured in 24-well plates (2.5 × 106 cells/well) with 2.5 × 106 splenocytes isolated from naive IL-10−/− mice in 2 ml RPMI 1640 containing 10% heat-inactivated FBS, 1% streptomycin (Strep), and 1% l-glutamine, to which 40 μg/ml of a coccidioidal antigen extract (T27K) was added. Examination of in vitro T-cell stimulation was conducted by incubation of immune splenocytes in growth medium alone or with the addition of T27K antigen (19) for 72 h at 37°C in the presence of 5% CO2. T cells incubated in growth medium alone served as negative controls. After incubation, a cocktail of protease inhibitors (Complete, EDTA-free; Roche Diagnostics, Pleasanton, CA) was added to each well, and culture supernatants were collected from the centrifuged samples (11,950 × g for 10 min at 4°C). The concentrations of selected cytokines were determined by using a Bio-Plex suspension array system.
Intracellular cytokine staining.
Aliquots of pulmonary leukocytes were stimulated with anti-CD3 and anti-CD28 in the presence of GolgiStop in 10% FBS-supplemented RPMI 1640 for 4 h at 37°C. Permeabilized cells were stained with selected fluorochrome-conjugated monoclonal antibodies (anti-IFN-γ, anti-IL-5, or anti-IL-17A) to determine absolute numbers of the specific cytokine-producing CD4+ T cells as previously described (19, 36). The leukocytes were gated for CD4+ CD8− T cells, and numbers of cytokine-producing cells were determined. The absolute numbers of the specific cytokine-producing CD4+ T cells relative to the total lung-infiltrating leukocytes per lung homogenate at 8 and 12 days postchallenge were calculated by multiplying the percentage of each gated population by the total number of viable pulmonary leukocytes determined by hemocytometer counts as described above.
Histopathology.
Comparative histopathology was conducted with excised whole lung organs from Coccidioides-infected, nonvaccinated, and vaccinated wild-type C57BL/6 and IL-10−/− mice. The animals were sacrificed at 14 days postchallenge (4 mice per group) as reported elsewhere (31). Tissue fixation and embedding procedures were performed as described previously (31). Paraffin sections (5 μm thick) were stained with hematoxylin and eosin (H&E) or Gomori methenamine silver stain (GMS) by standard procedures. Sections were examined using a Leica DMI6000 microscope equipped with an automated turboscan stage (Objective Imaging, Ltd., Cambridge, United Kingdom), and microscope images of infected lung tissue were acquired and analyzed using Surveyor software (Objective Imaging) as reported elsewhere (32).
Statistical analyses.
The Student Newman-Keuls test, a type of analysis of variance statistical method for multisample comparisons, was used to analyze the enzyme-linked immunosorbent assay results, cytokine concentrations, absolute numbers of lung-infiltrating immune cells, and percentages of specific cytokine-producing T cells as previously reported (36). All pairwise comparisons of the fungal burden, measured as the CFU between nonvaccinated versus vaccinated mice and between wild-type versus IL-10−/− mice, were analyzed using the Mann-Whitney U test as reported previously (31). Survival data were examined by the Kaplan-Meier test using log rank analysis to compare survival plots, as reported previously (31). A P value of <0.05 was considered statistically significant.
RESULTS
Vaccinated C57BL/6 mice revealed early but limited production of IL-10 after Coccidioides infection.
C57BL/6 mice were vaccinated with the live, attenuated vaccine strain (ΔT) and challenged with a potentially lethal dose of Coccidioides spores as previously described (19, 31, 32), except that the interval between the last vaccination and challenge was extended from 4 to 16 weeks. At 8 weeks postvaccination, there was no detectable live, attenuated Coccidioides ΔT cells in any of the tissue or organ homogenates examined, including cutaneous tissue at the injection site, draining lymph nodes, lungs, spleen, liver, and kidneys (the detection limit was 5 CFU per organ). We extended the interval prior to challenge to 16 weeks in order to evaluate the memory T-cell response. Cytokine assays of infected lung homogenates of mice revealed distinct differences in the kinetics of IL-10 production between the vaccinated (ΔT) and nonvaccinated mice during the first 14 DPC (Fig. 1A). The concentration of IL-10 was significantly elevated in vaccinated mice starting at 7 DPC, peaked at 9 DPC, and then declined between 11 and 14 DPC (Fig. 1A). In contrast, lung homogenates of nonvaccinated mice showed a steady increase in IL-10 production between 7 and 14 DPC, which correlated with the increase in fungal burden in the lungs and dissemination of the pathogen to the spleen, as previously reported (19). Since recruitment of leukocyte subpopulations in lungs of WT mice has been shown to be comparable at 12 and 14 DPC (19), we decided to conduct our immunological assays at 8 and 12 DPC, which represented the time points before and after dissemination occurred. In addition, because IL-10 gene expression is expected to precede an increase in IL-10 cytokine production, QRT-PCR analysis of the amounts of IL-10 transcript was conducted at these same time points before the peaks of IL-10 concentration were detected in the lungs of vaccinated mice (9 DPC) and nonvaccinated mice (14 DPC). The results were coincident with upregulation of IL-10 production in the lungs of nonvaccinated but not vaccinated mice (Fig. 1B). The amount of IL-10 transcript in the lungs of vaccinated mice showed no significant change between 8 and 12 DPC, while upregulation of IL-10 expression in lung homogenates of nonvaccinated mice revealed an approximate 35-fold increase during this same period postchallenge. These observations support previous findings indicating that there is a direct correlation between amounts of IL-10 production and susceptibility to Coccidioides infection (12, 13).
FIG 1.
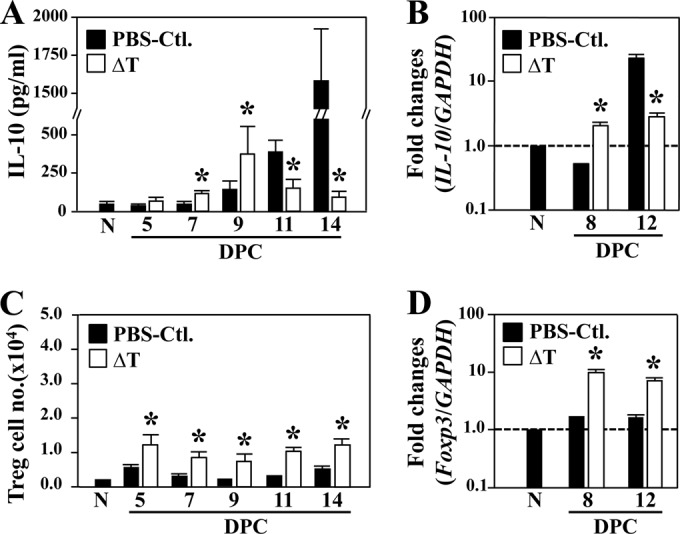
IL-10 production in lungs of vaccinated (ΔT) and nonvaccinated (PBS-Ctl.) C57BL/6 mice did not directly correlate with recruitment of CD4+ Treg cells. (A and B) IL-10 cytokine levels (A) and fold changes of transcripts (B) in lung homogenates at the indicated DPC following intranasal challenge with approximately 60 to 80 Coccidioides spores were determined for C57BL/6 mice that were challenged at 16 weeks after the final vaccination. Nonvaccinated and noninfected mice (N) of the same age and gender were included for determination of the baseline amounts of IL-10 transcript and protein. (C and D) The absolute number of Foxp3+ CD4+ CD25+ Treg cells (C) in lung homogenates of the mice was determined at the indicated time points. Total RNA was also isolated from duplicated sets of mice and used for determination of the fold changes of Foxp3 expression at 8 and 12 DPC (D). The assays were conducted in two independent experiments. Data are means ± SEM of 4 mice per group. Asterisks indicate P < 0. 05 compared to nonvaccinated mice.
Treg cells contributed to IL-10 production in the vaccinated mice.
We next attempted to determine which lung-infiltrated immune cells contributed to IL-10 production in the Coccidioides-infected lungs. Since it is well established that CD3+ CD4+ Foxp3+ T cells (Treg phenotype) can produce high amounts of IL-10 during microbial infection (4, 5), we employed cytometric techniques to determine the absolute numbers of Tregs that were recruited to the Coccidioides-infected lungs of vaccinated and nonvaccinated mice during the initial 14 days postchallenge. Our results revealed that Treg cells were sustained at a significantly elevated number in vaccinated compared to nonvaccinated mice during this period (Fig. 1C). We also compared expression of Foxp3 in lung homogenates of the two groups of infected mice (Fig. 1D). The amount of Foxp3 transcript was significantly higher in homogenates of vaccinated than in nonvaccinated mice at 8 and 12 DPC, and both showed little change during this interval. Unexpectedly, the kinetics of Treg recruitment did not correlate with the peak of IL-10 production in nonvaccinated mice, suggesting that other immune cells in lung homogenates of this group of mice contribute to the sharp increase in IL-10 concentration.
Comparison of the relative contributions of IL-10 production by immune cell subpopulations recruited to lungs of vaccinated versus nonvaccinated mice.
We systematically investigated leukocyte subpopulations that were capable of producing IL-10 in Coccidioides-infected lungs. The percentages of leukocytes positive for IL-10 production in lungs of vaccinated mice were comparable to nonvaccinated mice at 8 and 12 DPC (Fig. 2A). Further analyses showed that a significantly higher percentage and number of the gated IL-10-producing lymphocytes were CD4+ T cells of vaccinated than in nonvaccinated mice at 8 and 12 DPC (Fig. 2B, D and E). The total numbers of CD4+ IL-10+ T cells were further examined for their expression of Foxp3. The numbers of IL-10-producing Foxp3+ CD4+ cells were significantly elevated in the lungs of vaccinated mice at 8 and 12 DPC (Fig. 2D and E). On the other hand, the lungs of nonvaccinated mice had a higher percentage and number of CD8+ T cells producing IL-10 at 12 DPC (Fig. 2B and E). Interestingly, a significantly elevated percentage and total number of PMNs in Coccidioides-infected lungs of nonvaccinated mice were capable of producing IL-10 at both 8 and 12 DPC compared to vaccinated mice (Fig. 2C, D, and E). IL-10-producing CD19+ B cells were not detected in Coccidioides-infected lungs during the first 2 weeks postchallenge (Fig. 2D and E). IL-10-producing granulocytes, including PMNs, DCs, and macrophages, were the major innate cell types recruited to the Coccidioides-infected lungs of both nonvaccinated and vaccinated mice. In summary, our results showed that CD4+ T cells, both Foxp3+ and Foxp3− cells, and DCs and macrophages were the major activated immune cells that produced IL-10 in the lungs of vaccinated mice, while CD8+ T cells, PMNs, and macrophages were the major cell types that contributed to the sharp increase of IL-10 in the lungs of nonvaccinated mice after 11 days postchallenge (Fig. 1A and 2D and E).
FIG 2.
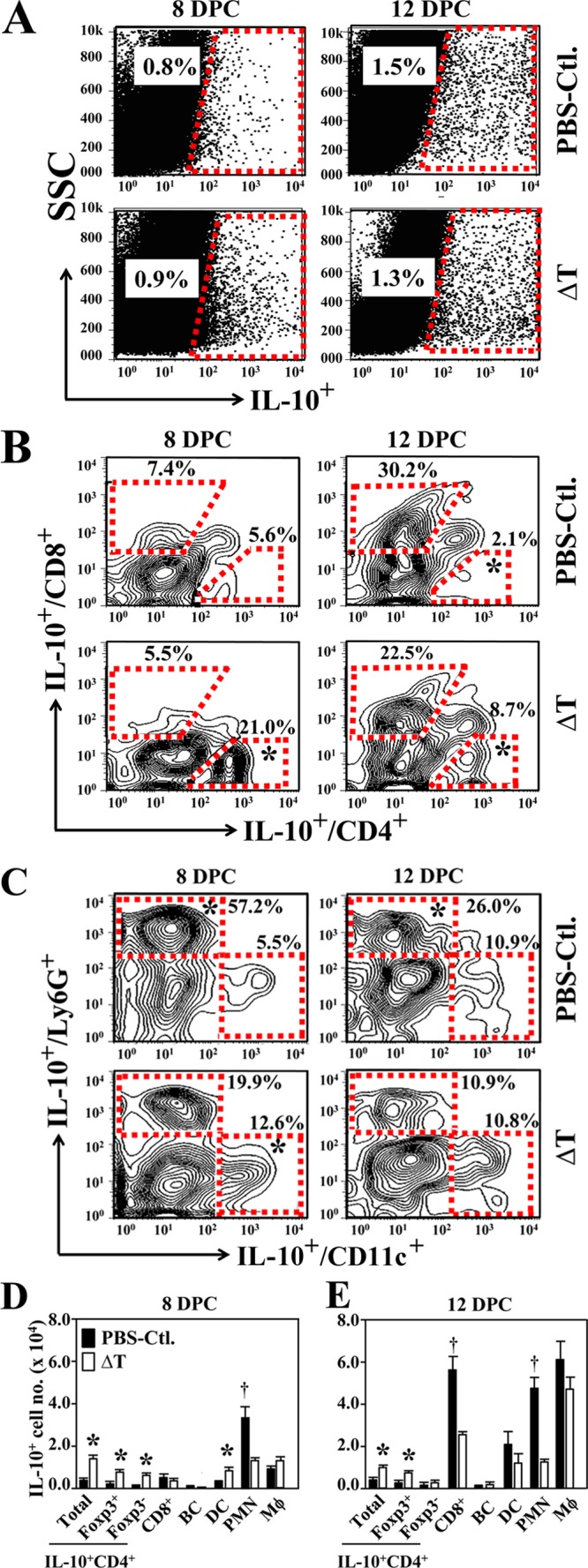
Diverse subsets of lymphocytes and granulocytes produced IL-10 in the lungs during Coccidioides infection. Pulmonary cells were isolated from the nonvaccinated (PBS-Ctl.) or vaccinated (ΔT) C57BL/6 mice at 8 or 12 DPC. (A) Dot plots of gated IL-10-producing cells in the pulmonary leukocyte population (CD45+). (B) Percentages of CD4+ versus CD8+ cells in the gated IL-10+ populations. Numbers juxtaposed to the gates give the mean percentage of the subset populations within the gated IL-10+ cells. (C) Percentages of CD11c+ versus Ly6G+ subsets of granulocytes, which represented DCs and PMNs in the gated IL-10+ cell population. (D and E) Total numbers of IL-10-producing CD4+ T cells in the lungs were further divided into Foxp3+ and Foxp3− subpopulations. The numbers of IL-10-producing CD8+ T cells, B cells (BC), dendritic cells, neutrophils (PMN), and macrophages (Mϕ) per lung organ were also measured as described in Materials and Methods at 8 and 12 DPC. Asterisks indicate significantly higher numbers of IL-10-producing cell numbers in vaccinated compared to nonvaccinated mice, while daggers indicate higher numbers in the nonvaccinated mice compared to vaccinated mice (P < 0.05). Data are representative of 2 independent experiments with similar results.
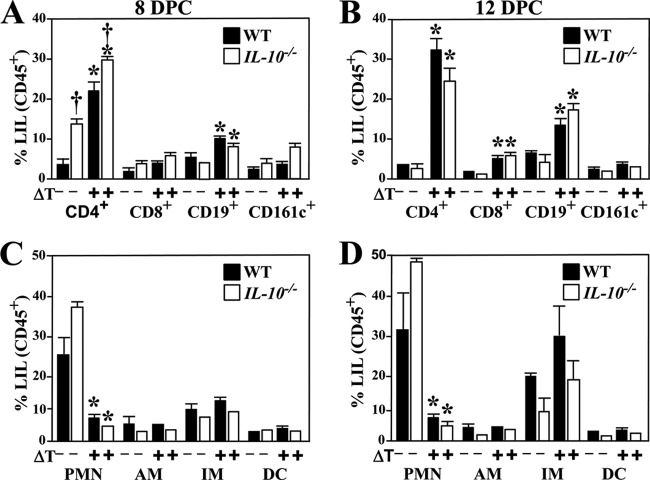
Enhanced recruitment of CD4+ T cells to lungs of IL-10−/− mice during the first 8 days postchallenge.
To investigate whether acquisition of leukocyte subpopulations in lungs was altered in the absence of IL-10, we compared the inventory of CD4+ and CD8+ T cells, B cells, NK cells, PMNs, alveolar and inflammatory macrophages, and dendritic cells that had infiltrated Coccidioides-infected lungs of WT and IL-10−/− mice at 8 and 12 DPC. The numbers of these examined immune cells of both naive IL-10−/− and WT mice were comparable to vaccinated WT mice before challenge (0 DPC), as we previously reported (19). No significant difference in the numbers of these immune cells was detectable between vaccinated WT and IL-10−/− mice before challenge. Total numbers of CD45+ leukocytes were comparable in lungs of IL-10−/− and wild-type mice during the first 12 DPC. Significantly elevated levels of CD4+ T cells and B cells accompanied by reduced percentages of pulmonary-infiltrating PMNs were observed in lung homogenates of vaccinated compared to nonvaccinated IL-10-deficient mice at 8 and 12 DPC (Fig. 3A to D). In addition, the percentage of CD8+ T cells of vaccinated IL-10−/− mice was significantly higher at 12 DPC than in the nonvaccinated mice (Fig. 3B). These results were comparable to those of the nonvaccinated and vaccinated wild-type mice (Fig. 3A to D) (19), except that a significantly higher percentage of CD4+ T cells was revealed in the lungs of nonvaccinated and vaccinated IL-10−/− mice at 8 DPC (Fig. 3A to D). These results suggested that IL-10 mainly impacts the acquisition of CD4+ T cells following pulmonary infection with Coccidioides, particularly during the first 8 days postchallenge.
FIG 3.
Enhanced acquisition of CD4+ T cells in lung homogenates of Coccidioides-infected IL-10−/− mice. Pulmonary cells were isolated from the nonvaccinated or vaccinated Coccidioides-infected WT and IL-10−/− mice that were sacrificed at 8 DPC (A and C) or 12 DPC (B and D). Percentages of each immune cell subset within the gated CD45+ population were determined as described in Materials and Methods. Data are presented as means ± SEM of 4 mice per group. Asterisks indicate significant differences between the vaccinated and nonvaccinated mice of the same strain, while the dagger represents a significant difference between the IL-10−/− and WT mice (P < 0.05).
CD4+ T cells isolated from vaccinated IL-10−/− mice respond ex vivo to Coccidioides antigen by increased production of Th1-, Th2-, and Th17-type cytokines.
We compared cytokine secretion by CD4+ T cells isolated from ΔT-vaccinated wild-type and IL-10−/− mice at 16 weeks after the final step of the immunization protocol. Splenocytes isolated from naive IL-10−/− mice were used as antigen-presenting cells (APCs) to ensure that the source of IL-10 was from CD4+ T cells. The concentrations of selected cytokines, which are indicators of Th1 (Fig. 4A and B), Th2 (Fig. 4C and D), and Th17 (Fig. 4E and F) polarized responses, were highest in T cells isolated from ΔT-vaccinated IL-10−/− mice upon stimulation ex vivo with Coccidioides-specific T27K antigen (P < 0.01). CD4+ T cells isolated from nonvaccinated wild-type or IL-10 knockout mice showed minimal ex vivo production of the selected cytokines when cocultured with the T27K antigen. Likewise, isolated immune CD4+ T cells cultured in the absence of T27K consistently showed low levels of cytokine production. Taken together, these data suggest that vaccine-induced CD4+ memory T cells differentiated in both wild-type and IL-10−/− mice at least by 16 weeks after the final vaccination step. On the other hand, the presence of IL-10 suppressed the secretion of cytokines by CD4+ T cells in a self-regulatory manner.
FIG 4.
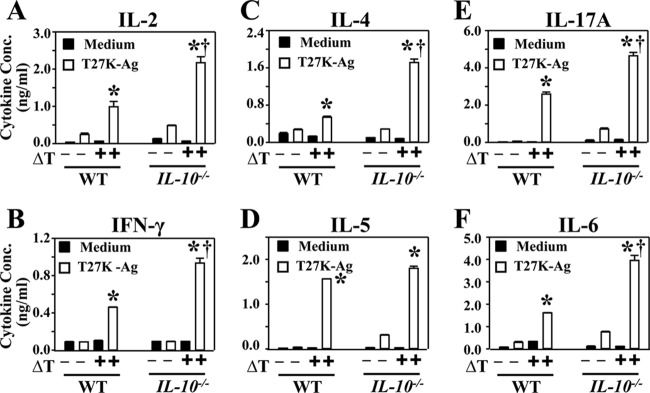
CD4+ T cells isolated from vaccinated IL-10−/− mice secreted higher amounts of Th1-, Th2-, and Th17-type cytokines upon stimulation with Coccidioides antigen than did nonvaccinated mice. CD4+ T cells were isolated from splenocytes of WT and IL-10−/− mice immunized with the ΔT vaccine or PBS at 16 weeks postvaccination. Isolated CD4+ T cells were cultured in the presence or absence of Coccidioides T27K antigen. Concentrations of representative Th1 cytokines (IL-2 and IFN-γ) (A and B), Th2 cytokines (IL-4 and IL-5) (C and D), and Th17 cytokines (IL-6 and IL-17A) (E and F) in supernatants of antigen-stimulated immune cells were compared. Statistically significant differences in cytokine production between the ΔT-vaccinated and nonvaccinated mice of the same strain are indicated by asterisks (P < 0.05). Daggers indicate a significant difference between WT and IL-10−/− mice (P < 0.01). The results are representative of 2 separate experiments with 4 mice per group.
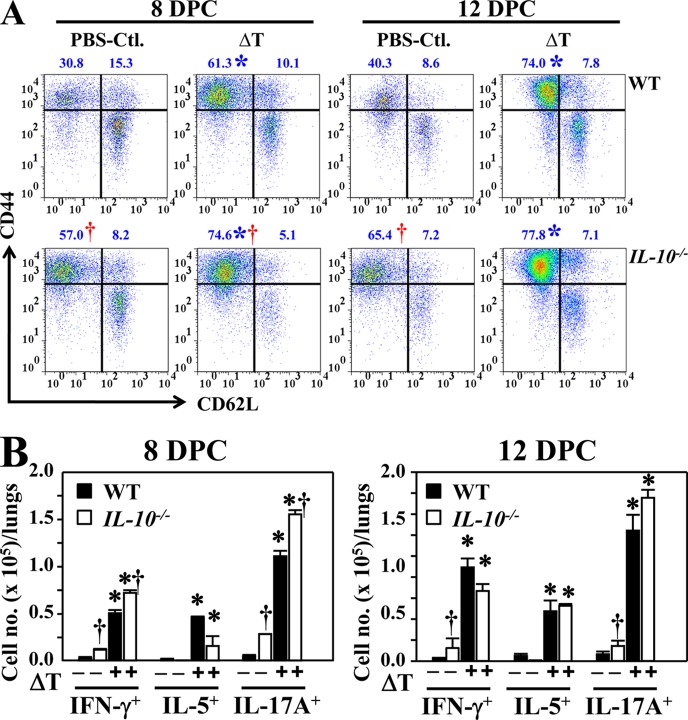
Vaccinated IL-10−/− mice undergo expansion of effector memory T cells during the first 8 DPC.
To determine whether the elevated, ex vivo response of CD4+ T cells isolated from vaccinated IL-10−/− mice described above translated into changes in T effector cell memory (TEM) function in the infected sites, we isolated pulmonary CD4+ T cells from each mouse group and compared their expression of CD44 and CD62L at 8 and 12 DPC. Both vaccinated and nonvaccinated IL-10−/− mice consistently showed higher percentages and numbers of CD44+ CD62L− CD4+ T cells (TEM) during the initial 12 days postchallenge compared to wild-type mice (Fig. 5A). Phenotypic analysis of activated, pulmonary CD4+ T cells revealed that significantly higher numbers of Th1 (IFN-γ+) and Th17 (IL-17A+) cells were present in lungs of vaccinated and nonvaccinated IL-10−/− mice than WT mice at 8 DPC (Fig. 5B). At 12 DPC, significant differences were observed between absolute numbers of Th1 and Th17 cells isolated from nonvaccinated IL-10−/− versus WT mice, while vaccinated mice of both strains had comparable numbers of Th1 and Th17 cells. No significant difference was detected in the absolute numbers of Th2 (IL-5) cells between these two groups of mice during the first 12 days postchallenge (Fig. 5B).
FIG 5.
Both ΔT-vaccinated WT and IL-10−/− mice revealed elevated percentages and numbers of TEM cells as well as IFN-γ-, IL-5-, and IL-17A-expressing CD4+ T cells compared to nonvaccinated mice. (A) Percentages of TEM (CD4+ CD44+ CD62−) and TCM (CD4+ CD44+ CD62L+) cells within the gated CD4+ T-cell population in the lungs of nonvaccinated (PBS-Ctl.) versus vaccinated (ΔT) mice are shown at 8 or 12 DPC. Numbers above the gates indicate the mean percentages of the gated TEM and TCM subsets. (B) The cell numbers of gated, specific cytokine-producing CD4+ T cells per lung were determined by intracellular cytokine staining. Asterisks indicate significantly higher cell numbers of responsive T-cell subpopulations in lungs of vaccinated compared to nonvaccinated mice, while the daggers indicate higher numbers of selected cytokine-producing cells in IL-10−/− mice compared to WT counterparts. Error bars indicate standard errors. The results reported are representative of two independent experiments.
Nonvaccinated IL-10−/− mice showed significantly better clearance of Coccidioides and prolonged survival compared to nonvaccinated WT mice.
Relative degrees of protection against coccidioidomycosis were determined by measuring numbers of CFU in the lungs and spleen of wild-type and IL-10−/− mice at 14 DPC. The mean CFU in the lungs of nonvaccinated IL-10−/− mice (5.2 ± 1.4 log10) was significantly lower than that of wild-type mice (6.9 ± 1.3 log10; P = 0.0002) (Fig. 6A). Similarly, nonvaccinated IL-10−/− mice showed significantly reduced fungal burdens in their spleens (2.6 ± 1.7 log10) than did nonvaccinated WT mice (4.1 ± 1.8 log10; P = 0.0037) (Fig. 6B). As expected, both vaccinated IL-10−/− and wild-type mice showed comparable reductions of fungal burden in both their lungs (2.2 ± 1.1 log10 and 2.3 ± 0.9 log10, respectively) and spleens (1.1 ± 0.4 log10 and1.6 ± 0.8 log10, respectively). Additional comparative studies revealed that the nonvaccinated IL-10−/− mice showed a significant increase in the percent survival compared to nonvaccinated wild-type mice after a lethal intranasal challenge with approximately 80 spores (P < 0.01) (Fig. 6C). Both vaccinated IL-10−/− and wild-type mice showed 100% survival after 60 days postchallenge (P < 0.001) (Fig. 6C).
FIG 6.
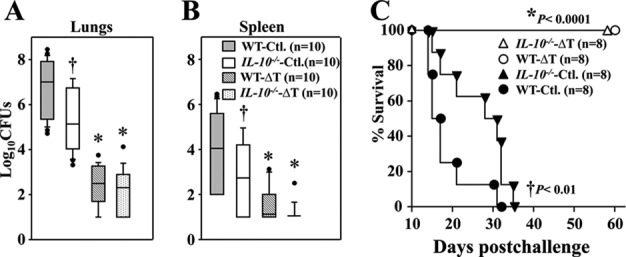
Both vaccinated WT and IL-10−/− mice significantly reduced the fungal burden in their lungs and spleen and showed prolonged survival compared to nonvaccinated mice. The numbers of CFU of C. posadasii detected in dilution plate cultures of lung (A) and spleen (B) homogenates of WT and IL-10−/− mice that had been vaccinated with the ΔT vaccine (ΔT) or immunized with PBS (Ctl.) as controls are reported at 14 DPC. All mice were challenged by the intranasal route with 80 viable spores isolated from the virulent strain. The horizontal lines indicate the mean CFU determinations. (C) Survival plots are presented for WT and IL-10−/− mice vaccinated with the ΔT vaccine or treated with PBS as controls. Asterisks indicate a statistically significant difference (P < 0.05) between CFU in the lungs and spleen of the vaccinated versus nonvaccinated mice of the same strain, while the daggers indicate significantly reduced CFU in the IL-10−/− mice versus the wild-type counterpart. The results are representative of 2 separate experiments.
Comparative histopathology of nonvaccinated IL-10−/− and wild-type mice showed differences in concentrations of parasitic cells in infected lung tissue.
At 14 DPC, lungs of both nonvaccinated WT and IL-10−/− mice had coalescing abscesses that constituted over 50% of the organs. While 50% of the vaccinated WT mice had 1 to 2 small granulomas (∼1 to 2 mm in diameter), the others revealed no gross evidence of abscess in their lungs. No vaccinated IL-10−/− mice had visible lesions. Cross-sections of visible abscesses in lungs of both nonvaccinated WT and IL-10−/− mice were examined (4 mice per strain). Since most of the vaccinated mice did not show any visible lesions in their lungs, we randomly examined tissue sections obtained from anterior, middle, and posterior regions (6 sections per mouse). Our results revealed that both nonvaccinated WT and IL-10−/− mice had intense suppurative inflammation associated with Coccidioides infection (Fig. 7A to D), although there were significant differences between the CFU in the lungs of these two strains of mice (see Fig. 6A). Sectioned lungs of wild-type mice revealed large numbers of spherules in various stages of development. When viewed at higher magnification (Fig. 7B), densely stained regions corresponding to concentrated inflammatory cells were visible, juxtaposed to mature spherules. Most of the spherules had ruptured and released their endospores. Inflammatory cells appeared to have converged to the sites of spherule rupture and in some cases were visible within the spherule matrix (Fig. 7B, arrow). The histopathology of sectioned lungs of nonvaccinated IL-10−/− mice revealed significantly fewer spherules. Unlike the wild-type mice, mature spherules that had released their endospores were rarely observed, although dense clusters of infiltrated inflammatory cells were visible, often juxtaposed to young spherules in stages of development prior to endosporulation (Fig. 7D, labeled with an S). The histopathology of lungs obtained from both vaccinated wild-type and IL-10−/− mice clearly showed more condensed inflammation and consolidation of the infection than did infected lungs of either nonvaccinated WT or IL-10−/− mice (Fig. 7E to H). Rarely were parasitic cells observed within an inflammation region in the lungs of the two groups of vaccinated mice (Fig. 7F and H), and the host tissue adjacent to the abscesses revealed structural features of normal lung tissue.
FIG 7.
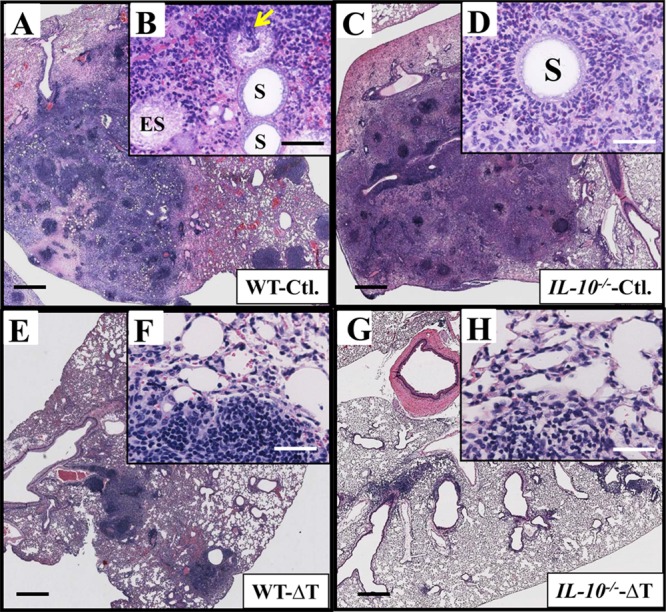
Comparative histopathology of Coccidioides-infected lungs revealed greater consolidation of inflammatory tissue in both vaccinated WT and IL-10−/− mice compared to nonvaccinated mice. Tissue sections were prepared from lungs of the nonvaccinated (A to D) and vaccinated (E to H) mice at 14 DPC. The large abscesses of the nonvaccinated WT and IL-10−/− mice contained concentrated inflammatory cells without apparent organization (A and C). Representative images revealed higher numbers of Coccidioides spherules surrounded by areas of necrosis in the nonvaccinated WT mice (B) compared to the IL-10−/− mice (D). Inflammatory cells converged to the sites of spherule rupture and in some cases were visible within the spherule matrix of the nonvaccinated mice (arrows in panel B). High numbers of inflammatory cells also surrounded spherules (S) prior to endosporulation in IL-10−/− mice. In contrast, both vaccinated wild-type and IL-10−/− mice showed localized recruitment of inflammatory cells surrounded by normal tissue (E to H) representive of a protective response, compared to nonvaccinated mice. Bars, 500 μm (A, C, E, and G) or 100 μm (B, D, E, F, and H).
DISCUSSION
IL-10 has a major impact on the regulation of inflammation during the course of a wide range of infectious diseases (reviewed in references 3 and 5). Extensive evidence supports the notion that persistently elevated IL-10 production during onset of coccidioidomycosis contributes to pathogen proliferation, dissemination, and disease severity (11–14). Fierer and coworkers (13, 14) reported that nonvaccinated, Coccidioides-challenged C57BL/6 mice produce higher amounts of IL-10 than resistant DBA2 mice during the early stage of pulmonary infection (10 to 16 days postchallenge) when the pathogen is tissue invasive and disseminates from the lungs to other body organs. To explore the effects of IL-10 on development of vaccine immunity to Coccidioides infection, we analyzed the kinetics of IL-10 secretion in the lungs of C57BL/6 mice that had been vaccinated with a protective live, attenuated ΔT vaccine (31). Our results show that in nonvaccinated and vaccinated mice during the onset of pulmonary infection with Coccidioides, distinct patterns of IL-10 secretion exist, perhaps involving different sources of the cytokine, and result in unique regulatory pathways. Due to temporal and spatial differences in cell-specific IL-10 expression, it is conceivable that the cytokine has different effects, depending on its source (37). Published studies on the role of IL-10 in coccidioidomycosis have focused on its role in disease progression and impact on innate immunity of nonvaccinated mice. This study is the first to analyze the cellular sources of IL-10 in Coccidioides-infected lungs of nonvaccinated and vaccinated C57BL/6 mice and the effects of loss of IL-10 production on vaccine immunity to infection.
We employed ex vivo assays and intracellular staining of IL-10 using pulmonary immune cells isolated from the Coccidioides-challenged C57BL/6 mice to measure sizes of each subpopulation of immune, cytokine-secreting lymphocytes and granulocytes. Although this approach has limitations of detection of IL-10 production in situ, we observed that recruitment of IL-10-producing CD8+ T cells, dendritic cells, neutrophils, and macrophages in lungs of nonvaccinated mice were elevated from 8 to 12 DPC, which coincided with the sharp increase of IL-10 concentration during this period. Since recruitment of these leukocyte subpopulations in lungs of nonvaccinated mice was sustained at constant numbers during 12 to 14 DPC, as previously reported (19), it is possible that the same cell types contributed to the sharp increase of IL-10 at 14 DPC (Fig. 1A). In vaccinated and infected mice, CD4+ T cells, DCs, and macrophages are the main producers of IL-10. We observed that Treg cells were not elevated in the nonvaccinated mice compared to vaccinated mice. This finding is in accordance with a previous confocal microscopy study that found that an elevated IL-10 concentration was colocalized with T cells and B cells in granulomata of Coccidioides-infected human lung tissue, but not with CD4+ CD25+ Treg cells (38). IL-10-producing CD4+, CD8+ T cells, and B cells have been reported to regulate immune responses during microbial infection (39–42). However, no IL-10-producing B cells were detected in the lungs of either nonvaccinated or vaccinated mice during the course of Coccidioides infection. It has been reported that T-cell-derived, but not B-cell-derived, IL-10 contributes to the suppression of the antigen-specific CD4+ T-cell response to helminth parasite infection (29). Similarly, IL-10 produced by T cells but not macrophages or PMNs influences the outcome of Leishmania infection (43). To further explore the role of IL-10-producing subsets of immune cells during Coccidioides infection, we plan to utilize IL-10 reporter mice that are conditionally deficient in IL-10 production in T cells or CD11b granulocytes (30, 42, 44, 45). These investigations are in progress, and our results will be presented in a separate report.
We observed that lung-infiltrated neutrophils, but not dendritic cells, represented a major elevated myeloid cell type among IL-10-producing pulmonary cells of nonvaccinated mice compared to vaccinated mice. Although Coccidioides-infected murine macrophages are capable of producing IL-10 in vitro (46), their roles in vivo remain to be explored. Neutrophils are one of the first lines of defense against microbial pathogens and are rapidly recruited to Coccidioides-infected lungs. Similar results have been reported for systemic infection with Yersinia enterocolitica, in which neutrophils are the major source of IL-10 (30). Coactivation of MyD88- and C-type lectin receptor-mediated signal pathways is essential for triggering neutrophils to produce IL-10 during bacterial infections (47). Host recognition of Coccidioides is dependent on Toll-like receptor 2 and Dectin-1, a C-type lectin receptor (48). Our preliminary data have shown that both MyD88 and caspase adaptor recruitment domain family member 9 (Card9), an intracellular signal adaptor for C-type lectin receptors, are essential for vaccine immunity to Coccidioides infection (unpublished data). Both MyD88- and C-type lectin-mediated pathways appear to be activated during Coccidioides infection. Taken together, our data suggest that IL-10-producing CD4+ T cells play a regulatory role in the development of protective immunity to Coccidioides infection in vaccinated mice, while IL-10-producing CD8+ T cells and neutrophils are associated with impairment of resistance to pulmonary coccidioidomycosis in nonvaccinated mice.
Jimenez and coworkers (13) reported that the IL-10 increased susceptibility to pulmonary coccidioidomycosis in mice is partly due to downregulation of inducible nitric oxide synthase 2 (iNOS2) expression. Although iNOS2−/− mice have significantly elevated lung-infiltrating PMNs, macrophages, and dendritic cells at 11 DPC compared to wild-type mice, iNOS2−/− and wild-type mice have comparable fungal burdens (33). It appears that iNOS2 activity has limited influence on the control of Coccidioides infection in susceptible mice (33). In our study, we observed that IL-10 mainly impacted acquisition of CD4+ T cells to sites of Coccidioides infection. Th17 cells have been shown to be the major responding immune cell type during the course of coccidioidomycosis and are essential for protection against Coccidioides infection and other dimorphic fungal pathogens (19, 20, 32, 36). Both CD4+ Treg and Th17 cells are derived from a common progenitor cell, but they have reciprocal maturation pathways and exert mostly opposing functions (49). We observed that both Treg IL-10-producing CD4+ T cells and Th17 cells were elevated in vaccinated mice upon Coccidioides infection, possibly as a consequence of maintenance of balanced immune responses through a self-regulatory mechanism of CD4+ T cells (4). Both CD4+ and CD8+ T cells can mediate vaccine immunity and resistance to Coccidioides infection (50). Furthermore, CD8+ T cells, especially IL-17-producing Th17 cells, can be primed and activated in the absence of CD4+ T cells and confer resistance to Blastomyces infection (51). The possibility that IL-10 production impedes acquisition of CD8+ T cells in lungs of Coccidioides-infected mice cannot be excluded.
Although preventing IL-10 production and/or blocking the binding of IL-10 to IL-10 receptors as vaccination strategies may favor the establishment of immune protection, we need to consider the possibility that IL-10 is required for development of long-term memory T-cell immunity (23). IL-10 has been shown to be essential for the normal recruitment of Aspergillus-specific CD4+ T cells to the airways of infected mice, although the mechanism is not yet known (52). In addition, loss of IL-10 can lead to a detrimental immune response accompanied by both excessive production of inflammatory cytokines and recruitment of immune cells (26). Memory T cells, which express the activation marker CD44, can be subdivided into two subpopulations: TEM and T central memory (TCM) cells, which are distinguished by their surface expression of the lymph node-homing receptor L-selectin (CD62L). TCM cells have high surface expression of CD62L and reside in the lymphoid tissue, where they replicate and expand, comprising TEM cells. The latter can subsequently be recruited to the infection sites to combat microbial infection (53). TEM cells are the major memory cell type, whereas the size of the CD4+ TCM population is transient in Coccidioides-infected lungs. As expected, the numbers of TEM, Th1, and Th17 cells were higher in lungs of the nonvaccinated IL-10−/− mice than in nonvaccinated wild-type mice during the first 12 DPC, which is consistent with reduced fungal burden and prolonged survival after Coccidioides infection. Both vaccinated IL-10−/− and wild-type mice after infection activate comparable numbers of these three protective cell types and reveal similarly reduced fungal burdens and 100% survival. Durable elevated numbers of antifungal memory CD4+ T cells were revealed in both vaccinated and Coccidioides-infected IL-10−/− and wild-type mice for at least 16 weeks after vaccination. It is possible that activated TEM cells, including Th1 and Th17 cells, can enhance recruitment of phagocytes to alveoli and promote early reduction of fungal burden while dampening inflammatory pathology at infection sites. Hence, our data show that loss of IL-10 production does not impact the acquisition of T effector memory cells nor exacerbate immunopathology in the lungs of vaccinated mice after pulmonary Coccidioides infection. IL-10 depletion has a beneficial influence on protection during the course of coccidioidomycosis in nonvaccinated mice and no alarming effect on vaccine immunity against Coccidioides infection.
ACKNOWLEDGMENT
This work was supported by a research grant from the National Institutes of Health, NIAID (R01 AI-071118), awarded to G.T.C.
Footnotes
Published ahead of print 9 December 2013
REFERENCES
- 1.Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Johnson RH, Stevens DA, Williams PL. 2005. Coccidioidomycosis. Clin. Infect. Dis. 41:1217–1223. 10.1086/496991 [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 2013. Increase in reported coccidioidomycosis—United States, 1998–2011. MMWR Morb. Mortal. Wkly. Rep. 62:217–221 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6212a1.htm [PMC free article] [PubMed] [Google Scholar]
- 3.Saraiva M, O'Garra A. 2010. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 10:170–181. 10.1038/nri2711 [DOI] [PubMed] [Google Scholar]
- 4.Jankovic D, Kugler DG, Sher A. 2010. IL-10 production by CD4+ effector T cells: a mechanism for self-regulation. Mucosal Immunol. 3:239–246. 10.1038/mi.2010.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couper KN, Blount DG, Riley EM. 2008. IL-10: the master regulator of immunity to infection. J. Immunol. 180:5771–5777 http://www.jimmunol.org/content/180/9/5771.long [DOI] [PubMed] [Google Scholar]
- 6.Tavares D, Ferreira P, Arala-Chaves M. 2000. Increased resistance to systemic candidiasis in athymic or interleukin-10-depleted mice. J. Infect. Dis. 182:266–273. 10.1086/315674 [DOI] [PubMed] [Google Scholar]
- 7.Hernandez Y, Arora S, Erb-Downward JR, McDonald RA, Toews GB, Huffnagle GB. 2005. Distinct roles for IL-4 and IL-10 in regulating T2 immunity during allergic bronchopulmonary mycosis. J. Immunol. 174:1027–1036 http://www.jimmunol.org/content/174/2/1027.long [DOI] [PubMed] [Google Scholar]
- 8.Deepe GS, Jr, Gibbons RS. 2003. Protective and memory immunity to Histoplasma capsulatum in the absence of IL-10. J. Immunol. 171:5353–5362 http://www.jimmunol.org/content/171/10/5353.long [DOI] [PubMed] [Google Scholar]
- 9.Qureshi MH, Harmsen AG, Garvy BA. 2003. IL-10 modulates host responses and lung damage induced by Pneumocystis carinii infection. J. Immunol. 170:1002–1009 http://www.jimmunol.org/content/170/2/1002.long [DOI] [PubMed] [Google Scholar]
- 10.Clemons KV, Grunig G, Sobel RA, Mirels LF, Rennick DM, Stevens DA. 2000. Role of IL-10 in invasive aspergillosis: increased resistance of IL-10 gene knockout mice to lethal systemic aspergillosis. Clin. Exp. Immunol. 122:186–191. 10.1046/j.1365-2249.2000.01382.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fierer J. 2006. IL-10 and susceptibility to Coccidioides immitis infection. Trends Microbiol. 14:426–427. 10.1016/j.tim.2006.07.009 [DOI] [PubMed] [Google Scholar]
- 12.Fierer J. 2007. The role of IL-10 in genetic susceptibility to coccidioidomycosis on mice. Ann. N. Y. Acad. Sci. 1111:236–244. 10.1196/annals.1406.048 [DOI] [PubMed] [Google Scholar]
- 13.Jimenez MdP, Walls L, Fierer J. 2006. High levels of interleukin-10 impair resistance to pulmonary coccidioidomycosis in mice in part through control of nitric oxide synthase 2 expression. Infect. Immun. 74:3387–3395. 10.1128/IAI.01985-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fierer J, Walls L, Eckmann L, Yamamoto T, Kirkland TN. 1998. Importance of interleukin-10 in genetic susceptibility of mice to Coccidioides immitis. Infect. Immun. 66:4397–4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks DG, Walsh KB, Elsaesser H, Oldstone MB. 2010. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proc. Natl. Acad. Sci. U. S. A. 107:3018–3023. 10.1073/pnas.0914500107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groux H, Bigler M, de Vries JE, Roncarolo MG. 1996. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J. Exp. Med. 184:19–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Sero G, Mencacci A, Cenci E, d'Ostiani CF, Montagnoli C, Bacci A, Mosci P, Kopf M, Romani L. 1999. Anti-fungal type 1 responses are upregulated in IL-10-deficient mice. Microbes Infect. 1:1169–1180. 10.1016/S1286-4579(99)00245-2 [DOI] [PubMed] [Google Scholar]
- 18.Gu Y, Yang J, Ouyang X, Liu W, Li H, Bromberg J, Chen SH, Mayer L, Unkeless JC, Xiong H. 2008. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur. J. Immunol. 38:1807–1813. 10.1002/eji.2008838331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung CY, Gonzalez A, Wuthrich M, Klein BS, Cole GT. 2011. Vaccine immunity to coccidioidomycosis occurs by early activation of three signal pathways of T helper cell response (Th1, Th2, and Th17). Infect. Immun. 79:4511–4522. 10.1128/IAI.05726-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wüthrich M, Gern B, Hung C-Y, Ersland K, Rocco N, Pick-Jacobs J, Galles K, Filutowicz H, Warner T, Evans M, Cole GT, Klein B. 2011. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J. Clin. Invest. 121:554–568. 10.1172/JCI43984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Döcke W-D, Asadullah K, Belbe G, Ebeling M, Höflich C, Friedrich M, Sterry W, Volk H-D. 2009. Comprehensive biomarker monitoring in cytokine therapy: heterogeneous, time-dependent, and persisting immune effects of interleukin-10 application in psoriasis. J. Leukoc. Biol. 85:582–593. 10.1189/jlb.0408249 [DOI] [PubMed] [Google Scholar]
- 22.Kalli F, Machiorlatti R, Battaglia F, Parodi A, Conteduca G, Ferrera F, Proietti M, Tardito S, Sanguineti M, Millo E, Fenoglio D, De Palma R, Inghirami G, Filaci G. 2013. Comparative analysis of cancer vaccine settings for the selection of an effective protocol in mice. J. Transl. Med. 11:120. 10.1186/1479-5876-11-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montagnoli C, Bacci A, Bozza S, Gaziano R, Mosci P, Sharpe AH, Romani L. 2002. B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J. Immunol. 169:6298–6308 http://www.jimmunol.org/content/169/11/6298.long [DOI] [PubMed] [Google Scholar]
- 24.Foulds KE, Rotte MJ, Seder RA. 2006. IL-10 is required for optimal CD8 T cell memory following Listeria monocytogenes infection. J. Immunol. 177:2565–2574 http://www.jimmunol.org/content/177/4/2565.long [DOI] [PubMed] [Google Scholar]
- 25.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. 2011. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity 35:792–805. 10.1016/j.immuni.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ and TNF-α. J. Immunol. 157:798–805 [PubMed] [Google Scholar]
- 27.Zhou G, Ma Y, Jia P, Guan Q, Uzonna JE, Peng Z. 2010. Enhancement of IL-10 bioactivity using an IL-10 peptide-based vaccine exacerbates Leishmania major infection and improves airway inflammation in mice. Vaccine 28:1838–1846. 10.1016/j.vaccine.2009.11.081 [DOI] [PubMed] [Google Scholar]
- 28.Xavier MN, Winter MG, Spees AM, Nguyen K, Atluri VL, Silva TMA, Bäumler AJ, Müller W, Santos RL, Tsolis RM. 2013. CD4+ T cell-derived IL-10 promotes Brucella abortus persistence via modulation of macrophage function. PLoS Pathog. 9(6):e1003454. 10.1371/journal.ppat.1003454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haben I, Hartmann W, Specht S, Hoerauf A, Roers A, Muller W, Breloer M. 2013. T-cell-derived, but not B-cell-derived, IL-10 suppresses antigen-specific T-cell responses in Litomosoides sigmodontis-infected mice. Eur. J. Immunol. 43:1799–1805. 10.1002/eji.201242929 [DOI] [PubMed] [Google Scholar]
- 30.Bouabe H, Liu Y, Moser M, Bösl MR, Heesemann J. 2011. Novel highly sensitive IL-10–β-lactamase reporter mouse reveals cells of the innate Immune system as a substantial source of IL-10 in vivo. J. Immunol. 187:3165–3176. 10.4049/jimmunol.1101477 [DOI] [PubMed] [Google Scholar]
- 31.Xue J, Chen X, Selby D, Hung CY, Yu JJ, Cole GT. 2009. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect. Immun. 77:3196–3208. 10.1128/IAI.00459-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hung CY, Hurtgen BJ, Bellecourt M, Sanderson SD, Morgan EL, Cole GT. 2012. An agonist of human complement fragment C5a enhances vaccine immunity against Coccidioides infection. Vaccine 30:4681–4690. 10.1016/j.vaccine.2012.04.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez A, Hung C-Y, Cole GT. 2011. Nitric oxide synthase activity has limited influence on the control of Coccidioides infection in mice. Microb. Pathog. 51:161–168. 10.1016/j.micpath.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feuerer M, Hill JA, Mathis D, Benoist C. 2009. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat. Immunol. 10:689–695. 10.1038/ni.1760 [DOI] [PubMed] [Google Scholar]
- 35.Herr RA, Hung CY, Cole GT. 2007. Evaluation of two homologous proline-rich proteins of Coccidioides posadasii as candidate vaccines against coccidioidomycosis. Infect. Immun. 75:5777–5787. 10.1128/IAI.00807-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurtgen BJ, Hung CY, Ostroff GR, Levitz SM, Cole GT. 2012. Construction and evaluation of a novel recombinant T cell epitope-based vaccine against coccidioidomycosis. Infect. Immun. 80:3960–3974. 10.1128/IAI.00566-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedrich C, Bream J. 2010. Cell type-specific regulation of IL-10 expression in inflammation and disease. Immunol. Res. 47:185–206. 10.1007/s12026-009-8150-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Dial SM, Schmelz M, Rennels MA, Ampel NM. 2005. Cellular immune suppressor activity resides in lymphocyte cell clusters adjacent to granulomata in human coccidioidomycosis. Infect. Immun. 73:3923–3928. 10.1128/IAI.73.7.3923-3928.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molloy MJ, Zhang W, Usherwood EJ. 2011. Suppressive CD8+ T cells arise in the absence of CD4 help and compromise control of persistent virus. J. Immunol. 186:6218–6226. 10.4049/jimmunol.1003812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noble A, Giorgini A, Leggat JA. 2006. Cytokine-induced IL-10-secreting CD8 T cells represent a phenotypically distinct suppressor T-cell lineage. Blood 107:4475–4483. 10.1182/blood-2005-10-3994 [DOI] [PubMed] [Google Scholar]
- 41.Trandem K, Zhao J, Fleming E, Perlman S. 2011. Highly activated cytotoxic CD8 T cells express protective IL-10 at the peak of coronavirus-induced encephalitis. J. Immunol. 186:3642–3652. 10.4049/jimmunol.1003292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, Yogev N, Gu Y, Khodoun M, Hildeman D, Boespflug N, Fogolin MB, Grobe L, Greweling M, Finkelman FD, Cardin R, Mohrs M, Muller W, Waisman A, Roers A, Karp CL. 2009. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J. Immunol. 183:2312–2320. 10.4049/jimmunol.0900185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarz T, Remer KA, Nahrendorf W, Masic A, Siewe L, Muller W, Roers A, Moll H. 2013. T cell-derived IL-10 determines leishmaniasis disease outcome and is suppressed by a dendritic cell-based vaccine. PLoS Pathog. 9(6):e1003476. 10.1371/journal.ppat.1003476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roers A, Siewe L, Strittmatter E, Deckert M, Schlüter D, Stenzel W, Gruber AD, Krieg T, Rajewsky K, Müller W. 2004. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J. Exp. Med. 200:1289–1297. 10.1048/jem.20041789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siewe L, Bollati-Fogolin M, Wickenhauser C, Krieg T, Müller W, Roers A. 2006. Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. Eur. J. Immunol. 36:3248–3255. 10.1002/eji.200636012 [DOI] [PubMed] [Google Scholar]
- 46.Viriyakosol S, Jimenez Mdel P, Gurney MA, Ashbaugh ME, Fierer J. 2013. Dectin-1 is required for resistance to coccidioidomycosis in mice. mBio 4(1):e00597–00512. 10.1128/mBio.00597-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Majlessi L, Deriaud E, Leclerc C, Lo-Man R. 2009. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity 31:761–771. 10.1016/j.immuni.2009.09.016 [DOI] [PubMed] [Google Scholar]
- 48.Viriyakosol S, Fierer J, Brown GD, Kirkland TN. 2005. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1. Infect. Immun. 73:1553–1560. 10.1128/IAI.73.3.1553-1560.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. 2006. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature 441:235–238. 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- 50.Fierer J, Waters C, Walls L. 2006. Both CD4+ and CD8+ T cells can mediate vaccine-induced protection against Coccidioides immitis infection in mice. J. Infect. Dis. 193:1323–1331. 10.1086/502972 [DOI] [PubMed] [Google Scholar]
- 51.Nanjappa SG, Heninger E, Wuthrich M, Gasper DJ, Klein BS. 2012. Tc17 cells mediate vaccine immunity against lethal fungal pneumonia in immune deficient hosts lacking CD4+ T cells. PLoS Pathog. 8(7):e1002771. 10.1371/journal.ppat.1002771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rivera A, Collins N, Stephan MT, Lipuma L, Leiner I, Pamer EG. 2009. Aberrant tissue localization of fungus-specific CD4+ T cells in IL-10-deficient mice. J. Immunol. 183:631–641. 10.4049/jimmunol.0900396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sallusto F, Geginat J, Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745–763. 10.1146/annurev.immunol.22.012703.104702 [DOI] [PubMed] [Google Scholar]