Abstract
Clostridium difficile is a colonizer of the human gut, and toxin-producing strains may cause diarrhea if the infectious burden is heavy. Infants are more frequently colonized than adults, but they rarely develop C. difficile disease. It is not known whether strains of C. difficile differ in the capacity to colonize and persist in the human gut microbiota. Here, we strain typed isolates of C. difficile that had colonized 42 healthy infants followed from birth to ≥12 months of age by using PCR ribotyping of the 16S-23S rRNA intergenic spacer region. The isolates were also characterized regarding carriage of the toxin genes tcdA, tcdB, and cdtA/B and the capacity to produce toxin B in vitro. Most strains (71%) were toxin producers, and 51% belonged to the 001 or 014 ribotypes, which often cause disease in adults. These ribotypes were significantly more likely than others to persist for ≥6 months in the infant micobiota, and they were isolated from 13/15 children carrying such long-term-colonizing strains. Ribotype 001 strains were often acquired in the first week of life and attained higher population counts than other C. difficile ribotypes in newborn infants' feces. Several toxin-negative ribotypes were identified, two of which (GI and GIII) were long-term colonizers, each found in one infant. Our results suggest that the toxin-producing C. difficile ribotypes 001 and 014 have special fitness in the infantile gut microbiota. Toxin-producing strains colonizing young children for long time periods may represent a reservoir for strains causing disease in adults.
INTRODUCTION
Clostridium difficile is a spore-forming gut anaerobe, and toxin-producing strains may cause diarrhea and colitis (1). The responsible toxins, termed toxin A and toxin B, induce cell death, fluid secretion, and inflammation. In severe cases, the gut wall may be perforated, a condition with high mortality (1, 2). However, most individuals carrying C. difficile have no symptoms, but if competing bacteria are suppressed by treatment with broad-spectrum antibiotics, C. difficile may expand and trigger diarrhea and mucosal inflammation. Some strains of C. difficile also produce a binary toxin whose clinical significance is yet unclear (1).
C. difficile may be separated into ribotypes by PCR amplification of the 16S-23S rRNA intergenic spacer region (3). Certain ribotypes, e.g., 001 and 014, are frequently associated with disease (4, 5). C. difficile infections are a growing clinical problem. Since the year 2000, a highly virulent C. difficile clone of ribotype 027 has spread in North America and Europe and has caused outbreaks with high mortality rates (6).
Colonization by C. difficile in adults varies with geographical location and between populations; carriage rates between 2 and 15% have been reported in different studies (7–9). Colonization in infants is much more common, but also variable, and 25 to 80% of infants harbor C. difficile (10–13). The high carriage rate in infancy has been ascribed to the low capacity of the infantile gut microbiota to suppress the growth of C. difficile. Colonization rates are increased in bottle-fed and caesarean section-delivered infants (14–16); in the latter case, carriage is probably dependent on delayed acquisition of suppressive anaerobes (17). A significant portion of C. difficile strains colonizing infants are toxin producers (13), but the vast majority of colonized infants have no overt symptoms. The reason for this is unknown, but it could relate to an absence of toxin receptors or poorly developed cellular signaling pathways in the infantile gut mucosa, or to protective factors present in the infantile gut.
Studies from the 1980s showed that colonization by C. difficile increased during the first half year of life, peaked around 6 months of age, and thereafter declined (11, 13, 16), in parallel with an increased complexity of the microbiota (18). We have reported that in Swedish infants born around the year 2000, colonization by C. difficile increased up to 12 months of age (17). This increased carriage rate in older infants suggests that a complex gut microbiota able to suppress the growth of C. difficile is acquired at a later age today than some decades ago.
C. difficile-colonized infants may provide a reservoir for strains causing disease in vulnerable individuals, and prolonged carriage of C. difficile in infants may, thus, increase the risk of C. difficile disease in a population. The aim of the present study was to determine whether individual C. difficile strains persisted in the infantile gut microbiota and to characterize the strains with regard to ribotype, toxin gene carriage, and toxin production. C. difficile isolates derived from 42 infants followed longitudinally during the first year of life and, in some cases, up to 3 years of age, were studied. Individual C. difficile strains were identified using PCR ribotyping and mapping of toxin genes, and their fecal population counts and time of persistence in the gut microbiota were determined. Our results suggest that certain toxin-producing ribotypes have a pronounced capacity for long-term colonization of the infant gut.
MATERIALS AND METHODS
Study cohort.
Forty-two children who yielded at least one stool culture positive for C. difficile during their first year of life were included in the study. The children were selected (see below) from the AllergyFlora birth cohort recruited during 1998 to 2003. This birth cohort includes 184 children who were followed with the primary aim to examine the relation between the infantile gut colonization pattern, development of the immune system, and allergy development (17, 19, 20). Informed consent was obtained from the parents, and the study was approved by the Human Research Ethics Committee of the Medical Faculty, Göteborg University, Sweden.
One aim of the AllergyFlora study was to describe the gut colonization pattern in Swedish infants. Stool samples were collected at 1, 2, and 4 weeks and 2, 6, and 12 months of age. Children born after July 1999 were also sampled at 18 months, and children born after July 2000 were also sampled at 36 months of age. The rationale for the close spacing in time between samples in the first months of life was to cover the time of establishment of common culturable gut bacteria (17). Stool samples were collected by the parents, placed in an airtight plastic bag in which an anaerobic atmosphere was created (AnaeroGen Compact, Oxoid Ltd., Basingstoke, United Kingdom), transported to the laboratory, and cultured quantitatively within 24 h for major facultative and anaerobic bacteria (17). Clostridium difficile was isolated after anaerobic incubation of serial dilutions of feces plated on cycloserine-cefoxitin-fructose egg yolk agar (CCFA) (21). Clostridia, i.e., anaerobic spore formers, were also isolated by ethanol treatment of fecal samples, followed by anaerobic culture on brucella blood agar (BBA) (17, 19). After enumeration and subculture for purity, all isolates were subjected to biotyping with the Rapid ID32A system (bioMérieux, Marcy L'Etoile, France), and isolates identified as C. difficile were frozen at −80°C. C. difficile population counts were calculated from the counts on the original CCFA plate cultures of the colony types identified as C. difficile and were expressed as CFU/g of feces. The limit of detection was 102.52 CFU/g of feces (17).
Selection procedure.
In the entire AllergyFlora cohort of 184 children, 137 (74%) yielded at least one stool sample that was positive for C. difficile during their first year of life. We aimed to include C. difficile-colonized infants born over the entire inclusion period (1998 to 2003) and all infants delivered by caesarean section, since we wanted to compare strain characteristics between strains obtained from vaginally and caesarean section-delivered infants. We included the first 20 C. difficile-positive children in the cohort (born in 1998 to 1999, including 3 of whom were delivered by caesarean section), all caesarean section-delivered C. difficile-positive infants born during 2000 to 2003 (n = 12), and for each of these caesarean section-delivered infants, the next born C. difficile-positive vaginally delivered infant (n = 12). Isolates from 2 children failed to grow, and 42 children (13 delivered by caesarean section) remained in our sample. This group of colonized infants differed from the full AllergyFlora cohort by having a higher frequency of caesarean delivery (31% versus 15%; P = 0.012), whereas no differences were observed regarding gender, siblings, duration of breastfeeding, gastrointestinal symptoms, or antibiotic consumption in the first year of life (data not shown).
All, or all but 1, of the 42 children provided fecal samples at the sampling points at 1 week to 12 months of age, 23 yielded an 18-month sample, and 17 yielded a 36-month sample.
PCR ribotyping, toxin gene carriage, and production of toxin B.
C. difficile isolates were ribotyped on the basis of profiles obtained after PCR amplification of the 16S-23S rRNA intergenic spacer region, as described by Stubbs and coworkers (3). Ribotypes recognized by the PHLS Anaerobic Reference Unit (Cardiff, United Kingdom) were numbered according to this nomenclature. Ribotypes not recognized by the PHLS but previously identified by the Swedish Institute for Communicable Disease Control (SMI) were given the designation SE followed by a number. Ribotypes not recognized by the PHLS or SMI were assigned a roman number and the prefix G (Gothenburg).
All C. difficile isolates were investigated for carriage of toxin genes by using PCR for the detection of genes for toxin A (tcdA) and multiplex real-time PCR for the detection of genes for toxin B (tcdB) and binary toxin (cdtA/B) (22, 23). In addition, culture supernatants from all isolates were used to detect toxin B production by a direct cell cytotoxicity neutralization assay (TechLab, Blacksburg, VA) (22).
Definition of long-term resident C. difficile strains.
A child's isolates belonging to a specific ribotype and showing identical patterns regarding toxin genes and toxin production were defined as belonging to the same strain. Strains isolated repeatedly from a child over a period of at least 6 months were defined as long-term resident strains, as opposed to strains present over a period shorter than 6 months (24). For strains present in a child solely on the last sampling occasion (at 12, 18, or 36 months of age), the colonization time could not be determined.
Statistical analyses.
Frequencies were compared by using Fisher's exact test, and population counts were compared by using the Mann-Whitney U test (SPSS version 16.0).
RESULTS
PCR ribotypes, toxin gene carriage, and toxin production in C. difficile.
In total, 106 of the 288 samples obtained from the 42 children were positive for C. difficile, yielding a total of 183 C. difficile isolates. All isolates were characterized by PCR ribotyping, toxin gene carriage (tcdA, tcdB, and cdtA/B), and toxin B production in vitro. In total, 21 different PCR ribotypes were identified. All isolates belonging to one ribotype shared the same toxin profile (Table 1). The most common ribotypes were 001 and 014 (PHLS Anaerobic Reference Unit nomenclature), which colonized 45% and 26% of the infants, respectively. Ribotypes 001 and 014 were positive for tcdA and tcdB and for production of toxin B, but negative for cdtA/B (Table 1). Other ribotypes recognized by the PHLS Anaerobic Reference Unit included 020, which colonized two infants, and 002, 012, 015, 046, 117, and 131, each isolated from one infant (Table 1). All of these ribotypes shared a toxin profile with 001 and 014.
TABLE 1.
C. difficile ribotypes detected in 42 Swedish children followed from birth to 1 to 3 years of age
| Nomenclature source and ribotype | Toxin gene carriagea |
Toxin B productionb | No. (%) of infants colonized | % of isolates | ||
|---|---|---|---|---|---|---|
| tcdA | tcdB | cdtA/B | ||||
| PHLSc | ||||||
| 001 | + | + | − | + | 19 (45) | 48 |
| 014 | + | + | − | + | 11 (26) | 19 |
| 020 | + | + | − | + | 2 (5) | 2 |
| 002 | + | + | − | + | 1 (2) | 2 |
| 012 | + | + | − | + | 1 (2) | 2 |
| 015 | + | + | − | + | 1 (2) | 0.5 |
| 046 | + | + | − | + | 1 (2) | 0.5 |
| 117 | + | + | − | + | 1 (2) | 1 |
| 131 | + | + | − | + | 1 (2) | 0.5 |
| SMId | ||||||
| SE2 | + | + | − | + | 1 (2) | 0.5 |
| SE5 | − | − | − | − | 1 (2) | 0.5 |
| SE6 | − | − | − | − | 1 (2) | 0.5 |
| SE14a | + | + | − | + | 1 (2) | 0.5 |
| SE21 | + | + | − | + | 1 (2) | 0.5 |
| SE36 | + | + | − | + | 1 (2) | 1 |
| This study | ||||||
| GI | − | − | − | − | 5 (12) | 5 |
| GII | − | − | − | − | 3 (7) | 3 |
| GIII | − | − | − | − | 3 (7) | 5 |
| GIV | − | − | − | − | 2 (5) | 5 |
| GV | − | − | − | − | 1 (2) | 2 |
| GVI | − | − | − | − | 1 (2) | 0.5 |
Isolates of C. difficile colonizing 42 Swedish infants were ribotyped and characterized regarding toxin gene carriage (presence [+] or absence [−] of tcdA, tcdB, and cdtA/B) in vitro. Toxin genes were identified by PCR (tcdA) or real-time PCR (tcdB and cdtA/B).
Toxin B production was determined in direct cell cytotoxicity neutralization assays.
PHLS Anaerobic Reference Unit (Cardiff, United Kingdom).
The Swedish Institute for Communicable Disease Control (http://www.smittskyddsinstitutet.se).
Six ribotypes not recognized by the PHLS Anaerobic Reference Unit but previously identified by the Swedish Institute for Communicable Disease Control (SMI) were identified in one infant each. They had various toxin profiles, although 4 out of 6 were toxin producers (Table 1).
Six PCR ribotypes not recognized by the PHLS or SMI were identified. Three of these, GI, GII, and GIII, were, second to 001 and 014, the most common ribotypes and colonized 5, 3, and 3 children, respectively (Table 1). None of these ribotypes carried the investigated toxin genes, nor did they produce toxin B in vitro.
Time of acquisition of C. difficile strains.
From each infant, isolates that belonged to the same ribotype were defined as one strain. In total, 59 strains were identified. Two children acquired 3 different strains, 13 children acquired 2 strains, and 27 children acquired only 1 strain during the study period. Table 2 shows the age at which the strains were first isolated. Most new strains were found in the 12-month samples or the 1-week samples. However, the chance of acquiring a C. difficile strain was highest in the first week of life (Table 2), since the period between sampling occasions was much shorter during the first months than after 6 months of age, when 6 months had passed between two sampling occasions. After 12 months of age, acquisition of new strains dropped sharply; only one new strain was found in the samples from 18 months of age (n = 23), and none appeared in the 36-month samples (n = 17).
TABLE 2.
Frequency of new C. difficile strains isolated on different culture occassionsa
| Age of child | No. of children sampled | No. (%) of newly acquired strains | No. of new strains/wkb |
|---|---|---|---|
| 1 wk | 41 | 12 (20) | 12 |
| 2 wks | 41 | 1 (1.7) | 1.0 |
| 4 wks | 42 | 2 (3.4) | 1.0 |
| 2 mos | 41 | 7 (12) | 1.8 |
| 6 mos | 42 | 11 (19) | 0.7 |
| 12 mos | 41 | 25 (42) | 1.0 |
| 18 mos | 23 | 1 (1.7) | 0.04 |
| 36 mos | 17 | 0 (0) | 0 |
| Total | 42 | 59 |
C. difficile was isolated by culture of fecal samples from 42 Swedish infants positive for C. difficile at least once in the first year of life.
Number of new strains acquired per week since the previous sampling occasion. The calculation was based on the assumption that no strains that were acquired between two sampling occasions were lost before the second of the samplings.
Figure 1 shows the PCR ribotype distribution and toxin production among strains in relation to the time when the isolates first appeared in the feces. Among the strains isolated at 1 week of age, 66% belonged to ribotype 001. Thereafter, the proportion of 001 strains among newly acquired strains declined steadily to 8% in 12- to 18-month-old children (P = 0.0004 for week 1 strains versus strains detected at 12 to 18 months) (Fig. 1). PCR ribotype 014 instead increased in prevalence with time and was more common among strains first isolated at 6 to 18 months of age than at 0 to 2 months of age (P = 0.041) (Fig. 1). No more than one-third of the strains were toxin negative (lacking tcdA, tcdB, and cdtA/B), regardless of when they were first isolated (Fig. 1).
FIG 1.
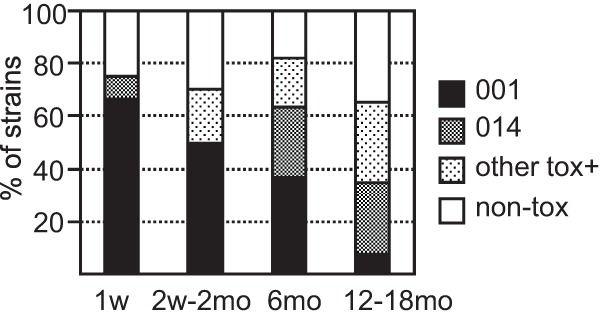
The relative frequencies (as percentages) of newly acquired C. difficile strains belonging to ribotype 001, ribotype 014, other toxin-producing ribotypes (other tox+), and toxin-negative ribotypes (non-tox; negative for tcdA, tcdB, and cdtA/B) on different sampling dates.
PCR ribotype, toxin profile, and persistence in the gut microbiota.
C. difficile strains were defined as long-term residents if they persisted in the gut microbiota over a period of at least 6 months. Fifteen children (36%) harbored such a long-term colonizer (Fig. 2). In six of these children (subject numbers 13, 19, 107, 128, 140, and 144), one or two additional C. difficile strains were isolated, each only on a single sampling occasion. Thus, no child had more than one strain that was a long-term colonizer (Fig. 2).
FIG 2.
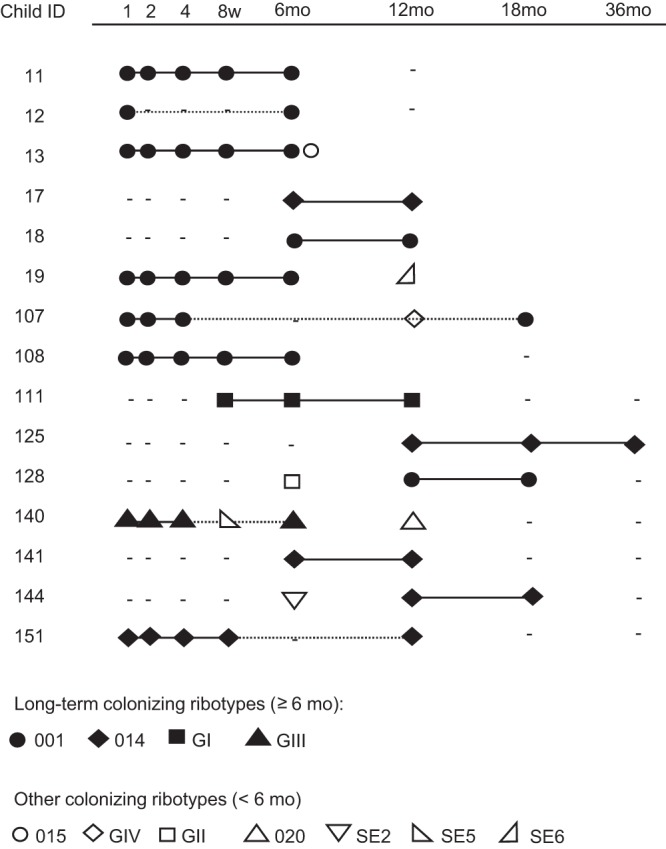
Persistence of C. difficile strains in the infantile gut microbiota. Fifteen infants were colonized by a single C. difficile strain over at least 6 months. The child ID number is indicated in the left column, and sampling occasions are indicated at the top (1, 2, 4, and 8 weeks and 6, 12, 18, and 36 months). Solid lines indicate the presence of a strain on consecutive sampling occasions; the dotted line denotes that the strain was not found on an intervening occasion. The latter situation could result from the strain being present at a level below the detection limit or result from the strain being lost and reacquired at a later time point. Dashes indicate time points at which the culture was negative for C. difficile.
Among the long-term resident strains, 53% (8/15) were already established during the first week of life; among all strains, 22% were established that early (P = 0.020). Ribotype 001 was most common among the long-term colonizers (53%), followed by 014 (33%). The two toxin-negative ribotypes, GI and GIII, were long-term colonizers in one child each (Fig. 2).
Whether a strain persisted in the gut microbiota of an infant for ≥6 months or not could be determined for 47/59 C. difficile strains. The remaining strains were isolated at the last sampling occasion only, in which case it could not be established if it was lost or kept thereafter. Among ribotype 001 and 014 strains, the proportions of strains that established as long-term colonizers were significantly higher than for strains of other ribotypes (P = 0.03 and P = 0.0095, respectively) (Fig. 3A). Furthermore, the proportion of ribotype 014 strains that became long-term colonizers was significantly higher than the corresponding proportion among strains of toxin-producing ribotypes other than 001 or 014 (P = 0.026), and a similar tendency was observed for ribotype 001 strains (P = 0.061) (Fig. 3A). Overall, 37% (13/35) of strains belonging to toxin-producing ribotypes became established as long-term-colonizers, compared to 17% (2/12) of toxin-negative strains (negative for tcdA, tcdB, and cdtA/B) (P = 0.29).
FIG 3.
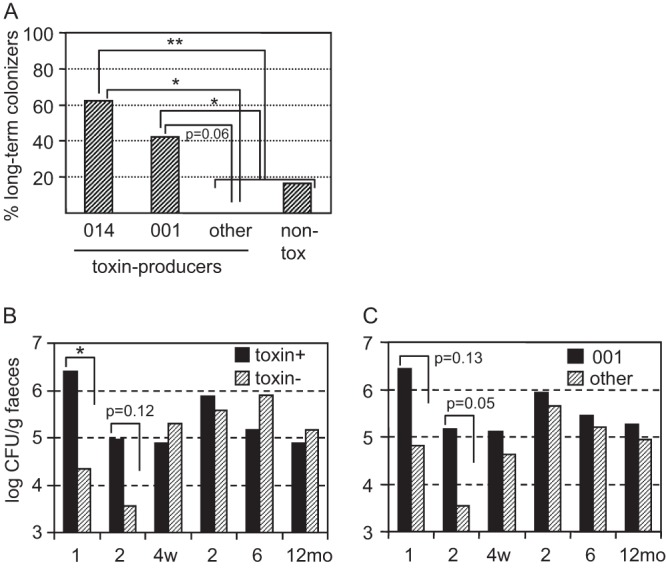
Colonization data for C. difficile strains. (A) The percentage of strains that established as long-term colonizers in the gut microbiota of infants, shown separately for strains of ribotype 014, ribotype 001, other toxin-producing ribotypes, and toxin-negative ribotypes (non-tox; negative for tcdA, tcdB, and cdtA/B). *, P < 0.05; **, P < 0.01. (B and C) C. difficile population counts in fecal samples, shown separately for toxin-producing (toxin+) and toxin-negative (toxin-) strains (B) and for strains of ribotype 001 and all other strains (C) at 1, 2, and 4 weeks and 2, 6, and 12 months. *, P < 0.05.
PCR ribotype, toxin profile, and population counts in the gut microbiota.
All stool samples were subjected to quantitative anaerobic culture. Usually, only one C. difficile strain was isolated at each time point. However, as colonies differing in appearance were enumerated separately, the population counts of each strain in a sample could be determined separately, in case of there was simultaneous occurrence of more than one strain (e.g., child 13) (Fig. 2).
Toxin-producing strains reached significantly higher population counts than toxin-negative ones in 1-week-old infants (106.4 versus 104.3 CFU/g; P = 0.041) (Fig. 3B). A similar tendency was observed at 2 weeks of age (105.0 versus 103.6 CFU/g; P = 0.12), but not at later time points. Ribotype 001 strains tended to reach higher fecal population counts than other strains in the infants at 1 week of age (106.4 versus 104.8 CFU/g; P = 0.13) and at 2 weeks of age (105.2 versus 103.5 CFU/g; P = 0.052) (Fig. 3C). No such tendency was observed for strains of ribotype 014 (data not shown).
C. difficile ribotypes and strain characteristics in relation to delivery mode.
Since delivery by caesarean section is a risk factor for acquisition of C. difficile, we compared the C. difficile ribotype distributions between children delivered by caesarean section (n = 13) and children delivered vaginally (n = 29). Caesarean-delivered children tended to carry strains of ribotype 014 more frequently than vaginally delivered children (46% versus 17%; P = 0.066), whereas no clear difference was observed regarding ribotype 001 strains (38% versus 48%) or regarding toxin-producing strains in general (85% versus 79%).
Toxin-producing C. difficile ribotypes in infants with loose stools.
Although colonization by C. difficile in infants is generally assumed to be asymptomatic (13), a Swedish study performed in the 1980s pointed to an increased risk of diarrhea in colonized infants (16). In the present study, we thought to examine if carriage of toxin-producing, rather than toxin-negative, C. difficile strains could be linked to gastrointestinal symptoms. The parents recorded medical events in a diary that was checked by telephone interview when the infants were 6 and 12 months old. No strict definition for diarrhea was used, but parents reported episodes of loose stools lasting ≥3 weeks. At least one such episode was reported during the first year of life for 6 of the 42 infants studied. All six harbored at least one toxin-producing C. difficile strain in the first 12 months, but this was also true of the majority (28/36; 78%) of infants not reported to have loose stools (P = 0.58).
The colonization pattern of children with loose stools is depicted in Fig. 4. In three cases, there was a possible relation between colonization by a toxin-producing C. difficile strain and a period of loose stools. Infant 151 was colonized by a strain of ribotype 014 from 1 week of age and had loose stools between 2 weeks and 4 months of age (Fig. 4). Infant 13 had no reported loose stools during carriage of the toxin-producing 001 ribotype but experienced a period of loose stools in conjunction with acquisition of the toxin-producing ribotype 015 at 6 months of age. Infant 140 experienced loose stools between 9 and 11 months age, and a toxin-producing ribotype 020 strain was isolated in the 12-month sample.
FIG 4.
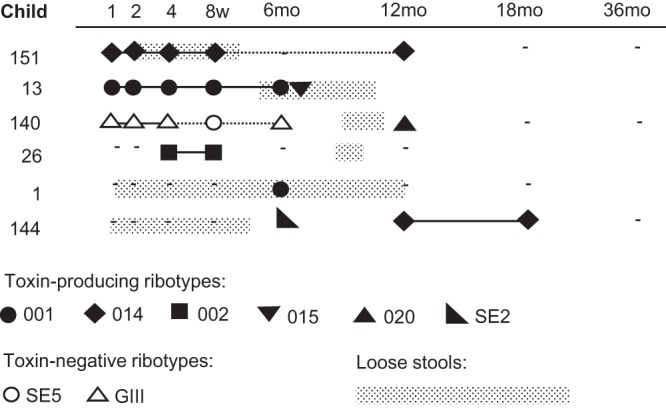
Longitudinal C. difficile colonization patterns in 6 infants reported to have had loose stools over a period of at least 3 weeks in the first year of life. The children's ID numbers are indicated in the left column, and sampling dates (1, 2, 4, and 8 weeks and 6, 12, 18, and 36 months) are indicated at the top. A solid line indicates the presence of the strain on consecutive sampling occasions; a dotted line denotes that the strain was not found on an intervening occasion. Periods of loose stools are indicated in the figure. Dashes indicate time points at which the culture was negative for C. difficile.
In two cases, episodes of loose stools were clearly unrelated to C. difficile colonization (infants 1 and 144), as these children were not colonized by C. difficile when symptoms first occurred. In a third case (infant 26), there was a period of loose stools between 6 and 12 months of age when there was no sampling, and no C. difficile was isolated prior to or after the episode. Thus, symptoms occurred in relation to colonization by toxin-producing C. difficile in only 3 of the 6 infants with loose stools.
DISCUSSION
In the present study, we investigated the C. difficile colonization pattern at the strain level based on quantitative cultures of fecal samples for 42 children who were followed from birth to 12, 18, or 36 months of age. Individual C. difficile strains were identified by PCR ribotyping, and their carriage of toxin genes and capacity to produce toxin B in vitro were determined. Among the 59 strains identified, 71% were toxin producers. This proportion is higher than the 20% reported in a Swedish study performed in the 1980s (16) and also higher than the 40% calculated from data pooled from nine studies in a recent review (13). More than half of the strains isolated from the infants in our study belonged to the 001 and 014 ribotypes (32% and 19%, respectively). Both of these ribotypes are toxin producers, and both have commonly been isolated from adult and elderly people with C. difficile toxin-mediated disease in Sweden (25) and in other countries (4). Ribotype 001 is especially likely to cause relapsing infection (25), possibly related to efficient toxin production and a high sporulation rate (5). Strains of ribotype 014 were isolated from 25% of the infants. The 014 strains were almost exclusively acquired after 2 months of age, indicating acquisition occurred outside the hospital. Ribotype 014 strains may be acquired from several sources, as this ribotype is found in both animals and humans, and it is also one of the most prevalent ribotypes in environmental samples (26, 27). Interestingly, ribotype 014 strains tended to be more common (P = 0.066) among infants delivered by caesarean section than among vaginally delivered infants. Possibly, some characteristics of the gut microbiota of caesarean section-delivered infants, which clearly differs from the microbiota acquired after a vaginal delivery (19), promote the establishment of ribotype 014 strains.
We also identified several toxin-negative ribotypes, and three of them (GI, GII, and GIII) were second only to 001 and 014 for being most prevalent in the infantile gut microbiota. Toxin-producing ribotypes other than 001 and 014 were isolated from only one or two infants each. Some of these ribotypes, i.e., 020, 002, 012, 046, and SE21, are today common causes of C. difficile disease in Sweden (http://www.smittskyddsinstitutet.se). Unfortunately, we do not have data regarding the ribotype distributions for clinical C. difficile isolates in Sweden during the years when the children in the present study were sampled. This is a limitation of our study, since the epidemiology of C. difficile has changed dramatically since the early 2000s (28).
The highest risk of becoming colonized by C. difficile was in the first week of life. Clostridial spores occur in all environments, and acquisition of C. difficile in maternity wards is commonplace (29). Two-thirds of the strains that were established in the first week belonged to the toxin-producing ribotype 001, compared to only 8% of the strains acquired after 6 months of age. The high prevalence of ribotype 001 strains and the overall high colonization rate in the first week of life likely reflects pronounced exposure to C. difficile in the hospital milieu. Currently, ribotype 001 strains are common in Swedish hospitals, where their spores may be spread (25).
After the first week and up to 1 year of age, new strains were isolated from the 42 infants at an estimated rate of 1 to 2 per week, but after 1 year of age, acquisition of new strains was negligible. As clostridial spores are common in the environment, this phenomenon demonstrates an increased colonization resistance toward C. difficile with increasing age, in parallel with the acquisition of a successively more complex gut microbiota.
Toxin-producing ribotypes attained significantly higher fecal population counts in 1-week-old infants than did strains negative for the examined toxins (toxin A, toxin B, and binary toxin). We speculate that toxin production confers an ecologic advantage during commensal colonization of the neonatal gut, possibly by promoting leakage into the gut lumen of nutrients acting as growth substrates for the microbes. We previously showed that Escherichia coli strains carrying the genes encoding the toxin hemolysin have a superior capacity to colonize the infant gut (30, 31). Along the same lines, Staphylococcus aureus strains producing certain toxins are superior to toxin-negative strains as gut colonizers of young children (32). Thus, production of certain toxins might, in fact, have evolved to promote commensal colonization, and virulence may be a side effect to this adaptation.
One-third of the C. difficile-colonized children carried a single ribotype over at least 6 months in the gut microbiota. This is a similar proportion to that reported in a recent study that followed 10 infants over 12 months (33). In our study, 53% of long-term-colonizing strains belonged to ribotype 001 and 33% to 014, and these ribotypes were significantly more likely to establish long-term colonization than other ribotypes. Toxin production per se was not significantly more common among long-term versus short-term colonizers, and two toxin-negative strains (of ribotypes GI and GIII) persisted for more than 6 months in the gut microbiota. These findings indicated that bacterial traits other than toxin production may be essential for the capacity of C. difficile to persist in the neonatal gut microbiota but do not exclude that toxins also contribute to the capacity for long-term colonization by ribotypes 001 and 014. Clearly, a larger study is needed to explore the possible role of toxin production for long-term colonization by C. difficile.
C. difficile has been increasingly recognized as a cause of diarrhea in infants and children (34, 35). We found little evidence for symptoms related to colonization by toxin-producing C. difficile in the infants we studied. For only three of six infants suffering from loose stools at some time in the first year of life was a possible link to colonization by toxin-producing C. difficile observed, and carriage of toxin-producing C. difficile was not significantly more common among infants suffering from loose stools than among other infants in the subgroup studied. However, only children colonized by C. difficile were included in the present study. A larger study that included infants colonized by toxin-producing or toxin-negative C. difficile, as well as infants not harboring these bacteria, is needed to confirm or rule out toxin-producing C. difficile as a cause of mild gastrointestinal symptoms in otherwise-healthy infants.
Recent studies have indicated that toxin-producing C. difficile strains that have colonized in infants may spread and cause disease in adults. For example, a case report implicated spread of the highly virulent ribotype 027 clone from an infant to his mother, in whom clinical disease appeared (36). Furthermore, community-acquired C. difficile disease is more prevalent in adults who have infant contacts than in those lacking such contacts (37). As pathogenic ribotypes seem to be highly prevalent among strains persisting for prolonged periods in the gut of infants and young children, there is ample opportunity for spread to individuals at risk for C. difficile disease.
ACKNOWLEDGMENTS
This work was supported by the European Commission (grant number QLK-2000-00538), the medical faculty of the University of Gothenburg (ALFGBG138401), the Swedish Medical Research Council (grant number K98-06X-12612-01A), and the Torsten and Ragnar Söderberg Foundation.
We thank study nurse Birgitta Åberg for excellent help in the recruitment process, collection of stool samples, and interviews. We also thank technicians Eva Ågren, Ingela Kinell, and Jolanta Bonislavska for their skillful technical assistance.
We declare that there are no conflicts of interest.
Footnotes
Published ahead of print 30 October 2013
REFERENCES
- 1.Carroll KC, Bartlett JG. 2011. Biology of Clostridium difficile: implications for epidemiology and diagnosis. Annu. Rev. Microbiol. 65:501–521. 10.1146/annurev-micro-090110-102824 [DOI] [PubMed] [Google Scholar]
- 2.Rupnik M, Wilcox MH, Gerding DN. 2009. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 7:526–536. 10.1038/nrmicro2164 [DOI] [PubMed] [Google Scholar]
- 3.Stubbs SL, Brazier JS, O'Neill GL, Duerden BI. 1999. PCR targeted to the 16S-23S rRNA gene intergenic spacer region of Clostridium difficile and construction of a library consisting of 116 different PCR ribotypes. J. Clin. Microbiol. 37:461–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer MP, Notermans DW, van Benthem BH, Brazier JS, Wilcox MH, Rupnik M, Monnet DL, van Dissel JT, Kuijper EJ, ECDIS Study Group 2011. Clostridium difficile infection in Europe: a hospital-based survey. Lancet 377:63–73. 10.1016/S0140-6736(10)61266-4 [DOI] [PubMed] [Google Scholar]
- 5.Vohra P, Poxton IR. 2011. Comparison of toxin and spore production in clinically relevant strains of Clostridium difficile. Microbiology 157:1343–1353. 10.1099/mic.0.046243-0 [DOI] [PubMed] [Google Scholar]
- 6.Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, Frost E, McDonald LC. 2005. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366:1079–1084. 10.1016/S0140-6736(05)67420-X [DOI] [PubMed] [Google Scholar]
- 7.Aronsson B, Möllby R, Nord CE. 1985. Antimicrobial agents and Clostridium difficile in acute enteric disease: epidemiological data from Sweden, 1980–1982. J. Infect. Dis. 151:476–481 [DOI] [PubMed] [Google Scholar]
- 8.Kato H, Kita H, Karasawa T, Maegawa T, Koino Y, Takakuwa H, Saikai T, Kobayashi K, Yamagishi T, Nakamura S. 2001. Colonisation and transmission of Clostridium difficile in healthy individuals examined by PCR ribotyping and pulsed-field gel electrophoresis. J. Med. Microbiol. 50:720–727 [DOI] [PubMed] [Google Scholar]
- 9.Nakamura S, Mikawa M, Nakashio S, Takabatake M, Okado I, Yamakawa K, Serikawa T, Okumura S, Nishida S. 1981. Isolation of Clostridium difficile from the feces and the antibody in sera of young and elderly adults. Microbiol. Immunol. 25:345–351 [DOI] [PubMed] [Google Scholar]
- 10.Collignon A, Ticchi L, Depitre C, Gaudelus J, Delmee M, Corthier G. 1993. Heterogeneity of Clostridium difficile isolates from infants. Eur. J. Pediatr. 152:319–322 [DOI] [PubMed] [Google Scholar]
- 11.Holst E, Helin I, Mårdh PA. 1981. Recovery of Clostridium difficile from children. Scand. J. Infect. Dis. 13:41–45 [DOI] [PubMed] [Google Scholar]
- 12.Matsuki S, Ozaki E, Shozu M, Inoue M, Shimizu S, Yamaguchi N, Karasawa T, Yamagishi T, Nakamura S. 2005. Colonization by Clostridium difficile of neonates in a hospital, and infants and children in three day-care facilities of Kanazawa, Japan. Int. Microbiol. 8:43–48 [PubMed] [Google Scholar]
- 13.Jangi S, Lamont JT. 2010. Asymptomatic colonization by Clostridium difficile in infants: implications for disease in later life. J. Pediatr. Gastroenterol. Nutr. 51:2–7. 10.1097/MPG.0b0013e181d29767 [DOI] [PubMed] [Google Scholar]
- 14.Bacon AE, Fekety R, Schaberg DR, Faix RG. 1988. Epidemiology of Clostridium difficile colonization in newborns: results using a bacteriophage and bacteriocin typing system. J. Infect. Dis. 158:349–354 [DOI] [PubMed] [Google Scholar]
- 15.Cooperstock MS, Steffen E, Yolken R, Onderdonk A. 1982. Clostridium difficile in normal infants and sudden infant death syndrome: an association with infant formula feeding. Pediatrics 70:91–95 [PubMed] [Google Scholar]
- 16.Tullus K, Aronsson B, Marcus S, Möllby R. 1989. Intestinal colonization with Clostridium difficile in infants up to 18 months of age. Eur. J. Clin. Microbiol. Infect. Dis. 8:390–393 [DOI] [PubMed] [Google Scholar]
- 17.Adlerberth I, Lindberg E, Åberg N, Hesselmar B, Saalman R, Strannegård IL, Wold AE. 2006. Reduced enterobacterial and increased staphylococcal colonization of the infantile bowel: an effect of hygienic lifestyle? Pediatr. Res. 59:96–101. 10.1023/01.pdr.0000191137.12774.b2 [DOI] [PubMed] [Google Scholar]
- 18.Adlerberth I, Wold AE. 2009. Establishment of the gut microbiota in Western infants. Acta Paediatr. 98:229–238. 10.1111/j.1651-2227.2008.01060.x [DOI] [PubMed] [Google Scholar]
- 19.Adlerberth I, Strachan DP, Matricardi PM, Ahrne S, Orfei L, Aberg N, Perkin MR, Tripodi S, Hesselmar B, Saalman R, Coates AR, Bonanno CL, Panetta V, Wold AE. 2007. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J. Allergy Clin. Immunol. 120:343–350. 10.1016/j.jaci.2007.05.018 [DOI] [PubMed] [Google Scholar]
- 20.Lundell AC, Hesselmar B, Nordström I, Saalman R, Karlsson H, Lindberg E, Aberg N, Adlerberth I, Wold AE, Rudin A. 2009. High circulating immunoglobulin A levels in infants are associated with intestinal toxigenic Staphylococcus aureus and a lower frequency of eczema. Clin. Exp. Allergy 39:662–670. 10.1111/j.1365-2222.2008.03176.x [DOI] [PubMed] [Google Scholar]
- 21.George WL, Sutter VL, Citron D, Finegold SM. 1979. Selective and differential medium for isolation of Clostridium difficile. J. Clin. Microbiol. 9:214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang H, Weintraub A, Fang H, Nord CE. 2009. Comparison of a commercial multiplex real-time PCR to the cell cytotoxicity neutralization assay for diagnosis of Clostridium difficile infections. J. Clin. Microbiol. 47:3729–3731. 10.1128/JCM.01280-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato H, Kato N, Watanabe K, Iwai N, Nakamura H, Yamamoto T, Suzuki K, Kim SM, Chong Y, Wasito EB. 1998. Identification of toxin A-negative, toxin B-positive Clostridium difficile by PCR. J. Clin. Microbiol. 36:2178–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Östblom A, Adlerberth I, Wold AE, Nowrouzian FL. 2011. Pathogenicity island markers, virulence determinants malX and usp, and the capacity of Escherichia coli to persist in infants' commensal microbiotas. Appl. Environ. Microbiol. 77:2303–2308. 10.1128/AEM.02405-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnusson C, Wullt M, Löfgren S, Iveroth P, Akerlund T, Matussek A. 2013. Ribotyping of Clostridium difficile strains associated with nosocomial transmission and relapses in a Swedish county. APMIS 121:153–157. 10.1111/j.1600-0463.2012.02950.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koene MG, Mevius D, Wagenaar JA, Harmanus C, Hensgens MP, Meetsma AM, Putirulan FF, van Bergen MA, Kuijper EJ. 2011. Clostridium difficile in Dutch animals: their presence, characteristics and similarities with human isolates. Clin. Microbiol. Infect. 18:778–784. 10.1111/j.1469-0691.2011.03651.x [DOI] [PubMed] [Google Scholar]
- 27.Janezic S, Ocepek M, Zidaric V, Rupnik M. 2012. Clostridium difficile genotypes other than ribotype 078 that are prevalent among human, animal and environmental isolates. BMC Microbiol. 12:48. 10.1186/1471-2180-12-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burnham CA, Carroll KC. 2013. Diagnosis of Clostridium difficile infection: an ongoing conundrum for clinicians and for clinical laboratories. Clin. Microbiol. Rev. 26:604–630. 10.1128/CMR.00016-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martirosian G, Kuipers S, Verbrugh H, van Belkum A, Meisel-Mikolajczyk F. 1995. PCR ribotyping and arbitrarily primed PCR for typing strains of Clostridium difficile from a Polish maternity hospital. J. Clin. Microbiol. 33:2016–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nowrouzian F, Hesselmar B, Saalman R, Strannegard IL, Aberg N, Wold AE, Adlerberth I. 2003. Escherichia coli in infants' intestinal microflora: colonization rate, strain turnover, and virulence gene carriage. Pediatr. Res. 54:8–14. 10.1203/01.PDR.0000069843.206550.EE [DOI] [PubMed] [Google Scholar]
- 31.Nowrouzian F, Wold AE, Adlerberth I. 2001. P fimbriae and aerobactin as intestinal colonization factors for Escherichia coli in Pakistani infants. Epidemiol. Infect. 126:19–23. 10.1017/S095026880100512X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nowrouzian FL, Dauwalder O, Meugnier H, Bes M, Etienne J, Vandenesch F, Lindberg E, Hesselmar B, Saalman R, Strannegård IL, Aberg N, Adlerberth I, Wold AE, Lina G. 2011. Adhesin and superantigen genes and the capacity of Staphylococcus aureus to colonize the infantile gut. J. Infect. Dis. 204:714–721. 10.1093/infdis/jir388 [DOI] [PubMed] [Google Scholar]
- 33.Rousseau C, Poilane I, De Pontual L, Maherault AC, Le Monnier A, Collignon A. 2012. Clostridium difficile carriage in healthy infants in the community: a potential reservoir for pathogenic strains. Clin. Infect. Dis. 55:1209–1215. 10.1093/cid/cis637 [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Shaklee JF, Smathers S, Prasad P, Asti L, Zoltanski J, Dul M, Nerandzic M, Coffin SE, Toltzis P, Zaoutis T. 2012. Risk factors and outcomes associated with severe Clostridium difficile infection in children. Pediatr. Infect. Dis. J. 31:134–138. 10.1097/INF.0b013e3182352e2c [DOI] [PubMed] [Google Scholar]
- 35.Zilberberg MD, Shorr AF, Kollef MH. 2008. Increase in Clostridium difficile-related hospitalizations among infants in the United States, 2000–2005. Pediatr. Infect. Dis. J. 27:1111–1113. 10.1097/INF.obo13e31817eef13 [DOI] [PubMed] [Google Scholar]
- 36.Hecker MT, Riggs MM, Hoyen CK, Lancioni C, Donskey CJ. 2008. Recurrent infection with epidemic Clostridium difficile in a peripartum woman whose infant was asymptomatically colonized with the same strain. Clin. Infect. Dis. 46:956–957. 10.1086/527568 [DOI] [PubMed] [Google Scholar]
- 37.Wilcox MH, Mooney L, Bendall R, Settle CD, Fawley WN. 2008. A case-control study of community-associated Clostridium difficile infection. J. Antimicrob. Chemother. 62:388–396. 10.1083/jac/dkn163 [DOI] [PubMed] [Google Scholar]


