Abstract
We sequenced pncA and rpsA genes plus flanking regions of 161 Mycobacterium tuberculosis isolates and found 10 new pncA and 3 novel rpsA mutations in pyrazinamide-resistant strains determined by the Bactec MGIT 960 system. The 3′ end of rpsA might be added as the target of molecular detection of pyrazinamide susceptibility.
TEXT
Pyrazinamide (PZA) is an indispensable first-line antituberculosis drug which exhibits unique sterilizing activity for both drug-sensitive and multidrug-resistant (MDR) tuberculosis. It can kill semidormant bacilli which persist in acidic-pH environments inside macrophages (1), where other drugs may not act so well. It is a prodrug that needs to be converted into its active form, pyrazinoic acid (POA), by the Mycobacterium tuberculosis pyrazinamidase (PZase), which is encoded by the 561-nucleotide (nt) pncA gene (2, 3). In 1996, the pncA gene was confirmed to be strongly associated with PZA resistance in M. tuberculosis (3), and in the following year, investigators found that pncA mutations constituted the primary mechanism of PZA resistance (4). In 2011, through systematic review with meta-analyses, Chang et al. drew the conclusion that molecular assays based on the pncA mutations were probably the way forward for detecting pyrazinamide resistance in M. tuberculosis (5). Molecular detection of PZA resistance-related mutations in the pncA gene is indeed more rapid than traditional mycobacterial susceptibility testing methods that depend on the growth of M. tuberculosis and are hampered by the complication of testing PZA activity (3).
Shi et al. recently confirmed that the ribosomal protein S1 (RpsA), encoded by the rpsA gene, was a target of POA which might be associated with PZA resistance in clinical M. tuberculosis isolates (6), so it is worth investigating the molecular characterization of rpsA gene mutations in both PZA-resistant and PZA-susceptible M. tuberculosis clinical isolates. However, some reports suggested that rpsA gene sequencing might not play a role in detecting PZA susceptibility by molecular methods (7).
Rapid and accurate detection of drug resistance contributes to early optimization of M. tuberculosis treatment, which is an effective way to further avoid the emergence of other drug resistance and potential PZA toxicity. However, the mutations in pncA are highly diverse in different regions (8–10), which hampers the development and application of molecular assays that are always based on the molecular characterization of gene mutations. In southern China, there are no data so far published describing pncA mutations in PZA-resistant clinical strains of M. tuberculosis, so it is necessary to analyze the profile of pncA and rpsA mutations of clinical M. tuberculosis strains in this region for the convenience of rapid and accurate detection of PZA resistance.
Drug susceptibility profile of clinical isolates.
It is well known that testing for PZA susceptibility (3) in vitro is very hard, and the CLSI-recommended Bactec 460TB radiometric system (460TB; Becton, Dickinson, Sparks, MD) was replaced recently in many laboratories by the nonradiometric Bactec MGIT 960 system (BT960; Becton, Dickinson, Sparks, MD) (11). In the present study, 161 clinical M. tuberculosis strains isolated from January 2011 to October 2012 from southern China, mostly (115 of 161) from Guangdong province, were selected to determine their susceptibilities to PZA by BT960 as recommended by the manufacturer with modified Middlebrook 7H9 broth (pH 5.9) containing 100 μg/ml PZA. Mycobacterium bovis BCG ATCC 34540 and M. tuberculosis H37Rv ATCC 27294 were used as PZA-resistant and PZA-susceptible controls, respectively. The susceptibilities of these isolates to rifampin (RIF), isoniazid (INH), ethambutol (EMB), and streptomycin (STR) were previously determined by BT960 when the patients were hospitalized in Guangzhou Chest Hospital, the biggest tuberculosis treatment center located in southern China (12). Of the 161 M. tuberculosis clinical isolates, 109 isolates were PZA susceptible and 52 were PZA resistant. Their susceptibilities to RIF, INH, EMB, and STR are summarized in Table 1 and Table 2.
TABLE 1.
Drug susceptibility and genotypic characteristics of PZA-resistant clinical isolatesa
| Resistance | pncA + FR mutation(s) | rpsA + FR mutation | No. of isolates |
|---|---|---|---|
| INH, RIF | D8Ab (GAC→GCC) | — | 1 |
| INH, RIF | Q10P (CAG→CCG) | — | 1 |
| INH, RIF | F13L (TTC→CTC) | — | 1 |
| INH, RIF | S18Pb (TCG→CCG)/P54L (CCG→CTG) | — | 1 |
| INH, RIF | G24D (GGC→GAC) | — | 2 |
| INH, RIF | L27b deletion | — | 1 |
| INH, RIF | T47A (ACC→GCC) | — | 1 |
| INH, RIF | H57Y (CAC→TAC) | — | 2 |
| INH, RIF | F58Sb (TTC→TCC) | — | 1 |
| INH, RIF | S66P (TCG→CCG) | — | 1 |
| INH, RIF | T76P (ACT→CCT) | — | 2 |
| INH, RIF | F81V (TTC→GTC) | — | 1 |
| INH, RIF | H82R (CAT→CGG) | — | 1 |
| INH, RIF | E91 deletionb | — | 1 |
| INH, RIF | F94L (TTC→CTC) | — | 2 |
| INH, RIF | F94S (TTC→TCC) | — | 1 |
| INH, RIF | K96E (AAG→GAG) | — | 2 |
| INH, RIF | E111 deletionb | — | 1 |
| INH, RIF | Q122 deletionb | — | 2 |
| INH, RIF | VVG130-132 deletionb | — | 1 |
| INH, RIF | V131 deletionb | — | 2 |
| INH, RIF | G132A (GGT→GCT) | — | 1 |
| INH, RIF | T135P (ACC→CCC) | — | 2 |
| INH, RIF | D136G (GAT→GGT) | — | 2 |
| INH, RIF | V139G (GTG→GGG) | — | 1 |
| INH, RIF | V139A (GTG→GCG) | — | 1 |
| INH, RIF | V139L (GTG→CTG) | — | 1 |
| STR, RIF | Q141P (CAG→CCG) | — | 1 |
| INH, RIF | L159R (CTG→CGG) | — | 1 |
| INH, STR, EMB | G162D (GGT→GAT) | — | 1 |
| INH | T168P (ACC→CCC) | — | 3 |
| INH, RIF | E174Gb (GAG→GGG)/M175Rb (ATG→AGA) | — | 2 |
| INH, RIF | — | R474W (CGG→TGG) | 1 |
| INH | — | R474L (CGG→CTG) | 1 |
| STR | −11 (A→G) | — | 1 |
| INH, STR, EMB | — | E433D (GAG→GAC) | 1 |
| STR | — | — | 4 |
| Total | 45 | 3 | 48 + 4 |
Abbreviations and symbol: INH, isoniazid; RIF, rifampin; STR, streptomycin; EMB, ethambutol; FR, flanking region, which means the upstream and downstream regions of the gene; —, no mutation.
First nonsynonymous mutations of pncA found.
TABLE 2.
Drug susceptibility and genotypic characteristics of PZA-sensitive clinical isolatesa
| Resistance | pncA + FR mutation(s) | rpsA + FR mutation | No. of isolates |
|---|---|---|---|
| — | — | — | 57 |
| INH, RIF | — | — | 17 |
| INH, RIF | — | Q162R (CAG→CGG) | 1 |
| INH, STR | — | — | 8 |
| INH | — | — | 6 |
| RIF | — | — | 4 |
| STR | — | — | 14 |
| EMB | — | — | 1 |
| INH, STR, EMB | — | — | 1 |
| Total | 108 | 1 | 109 |
Abbreviations and symbol: INH, isoniazid; RIF, rifampin; STR, streptomycin; EMB, ethambutol; FR, flanking region, which means the upstream and downstream regions of the gene; —, no mutation.
Sequencing pncA and rpsA genes of the clinical isolates.
As pncA and rpsA genes are the only known genes related to PZA susceptibility and their corresponding flanking regions (FR) may contain regulatory elements which may affect gene expression, it is reasonable that mutation in FR may alter the mutants' susceptibility to PZA. We analyzed the pncA and rpsA genes and their corresponding FR. No typical promoter was found upstream of the pncA gene from nt −1 to nt −358 including its upstream gene Rv2044c. Though there are only 162 nt between rpsA and its upstream gene (Rv1629), a typical putative prokaryotic promoter was found as CCGAGTTTGTCCAGCGTGTACCCGTCGAGTAGCCTCGTCAGGTACCAATC (nt −90 to nt −41 of rpsA), which was predicted with an online program (http://www.fruitfly.org/seq_tools/promoter.html). The score was 0.86, with the total score being 1.0. The bold italic G was supposed to be the transcription starting site. There were only 25 nt between rpsA and its downstream gene, and no regulatory element was found. We designed primers to amplify and sequence the whole pncA and rpsA genes plus FR of the clinical strains according to sequences of M. tuberculosis H37Rv (GenBank accession no. NC000962) using the software Primer Premier 5.0 (Premier, Canada). The primers included the following: for the pncA gene plus FR, primers F1, 5′-TGCCACTCGCCGGTAACCGG (nt 321 to 340 downstream of pncA), and R1, 5′-GGTGGCCGCCGCTCAGCTGG (nt −119 to −100 of pncA); for the rpsA gene plus FR, primers F2, 5′-GGCCGCAGCTGGGACGCGGC (nt −192 to −173 of rpsA), and R2, 5′-CGGTCCAGCGCTCCGTCTGC (nt 203 to 222 downstream of rpsA). The additional rpsA sequencing primers (HC-0815-3-R1, 5′-GTCCTCATTGGCTTGC; HC-0815-3-R2, 5′-CGTTGTTGCGGTTCTTGTC) and all other primers in this study were synthesized at BGI, China, where DNA sequencing was performed. Susceptibility testing was repeated for all the PZA-resistant isolates that had no pncA-plus-FR mutation, and their rpsA genes and pncA genes were resequenced in a double-blind form. The results were exactly the same as the first time.
Transcription of M. tuberculosis pncA gene in a polycistron.
Only 40 nt exist between pncA and Rv2044c, and 1 nt overlapped between pncA and its downstream gene (Rv2042c), so we inferred that pncA might be cotranscribed with its surrounding genes in a polycistron (Fig. 1A). We thus carried out a reverse transcription-PCR (RT-PCR) experiment to prove this suggestion. Four pairs of primers were designed: Af (5′-ATCCGCATCAGCACCG)-Ar (5′-CCCTCGCAGAAGTCGTTC), Cf (5′-GGCACGCCACTGCTGAA)-Cr (5′-CGAACCCACCGGGTCTT), and Df (5′-ATGACTTTAGGCGAGGATGA)-Dr (5′-TCGAACGGCTTATTGACC) were used for amplifying the fragments spanning Rv2045c-Rv2044c-pncA, pncA-Rv2042c, and Rv2042c-Rv2041c, respectively; primer pair Bf (5′-CAATCGAGGCGGTGTTC)-Br (5′-CAATGATCGGTGGCAATAC) was used to amplify a fragment in pncA as a positive control. Total RNA was extracted from M. tuberculosis H37Ra or the clinical nt −11 mutant at its log phase by the RNAiso Plus kit. Then, the samples were treated with recombinant DNase I to remove the possible contaminated genomic DNA. RT-PCR was performed using the DRR036S kit. PCR products amplified with the above 4 pairs of primers using the RT-PCR products from M. tuberculosis H37Ra as the templates were electrophoresed. All the reagents for these experiments were from TaKaRa, China, and the corresponding procedures were carried out according to the manufacturer's instructions. The sizes of the PCR products with these 4 pairs of primers were all exactly as expected: Af-Ar, 543 bp; Bf-Br, 148 bp; Cf-Cr, 356 bp; and Df-Dr, 255 bp (Fig. 1B). The samples with templates from RT-PCR produced without adding reverse transcriptase all produced no bands, so there was no genomic DNA contamination after the treatment with recombinant DNase I (Fig. 1B). We verified that the pncA gene was transcribed in a polycistron in M. tuberculosis. So, an accurate description of the mutations at −11 and the nearby region should be that the mutation is at a putative upstream regulatory region of the pncA gene, but not at its promoter.
FIG 1.
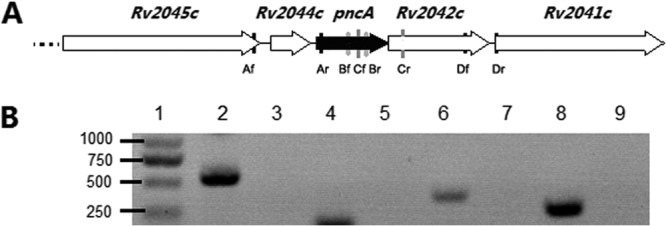
(A) Diagram of pncA gene and its surrounding genes on the M. tuberculosis chromosome. Af-Ar, Bf-Br, Cf-Cr, and Df-Dr were primer pairs for the amplification of the corresponding regions. (B) Electrophoresis results of the reverse transcription-PCR experiments. Lane 1, DNA markers (bp); lanes 2, 4, 6, and 8, reverse transcription-PCR products with primer pairs A, B, C, and D, respectively, using the cDNA as the templates; lanes 3, 5, 7, and 9, PCR products with primer pairs A, B, C, and D, respectively, using the reverse transcription products as the control templates without adding the reverse transcriptase.
pncA mutations.
There was no pncA mutation in all 109 PZA-susceptible isolates (0%), but 44 out of 52 (84.6%) PZA-resistant isolates showed pncA mutations (Table 1), which showed the strong correlation between mutations in pncA and phenotypic resistance to PZA and supported the finding that a pncA mutation could cause PZA resistance in M. tuberculosis (2). The pncA gene mutation frequency in the PZA phenotypically resistant strains here is much higher than those reported previously in other provinces of China (27 to 48.1%) (13–17) but similar to those from other countries (69% to 98.8%) in which the PZA phenotypic resistance was determined by the BT960 or 460TB system (18–21). It is well known that testing for the susceptibility of PZA in vitro is very hard (3) because PZA only has very weak activity even in an acid-pH environment, in which even the control without PZA could not grow well. Many factors, such as the inoculum size and the metabolic state of the culture, can have a great influence on the susceptibility testing results. In the previously published papers from China, an absolute concentration method was used to determine the PZA phenotypic susceptibility. This method may have both higher false-positive and higher false-negative resistance results than the BT960/460TB systems do, which may contribute to the disparities in mutation frequencies in pncA, especially in the PZA phenotypically sensitive ones from different regions. This is partially supported by the finding that obvious discrepancies existed between BT960 and 460TB, even when they were manipulated by the same persons working on the same samples (7, 11). Furthermore, a 90.5% pncA mutation rate in the PZA-resistant strains in eastern China determined using the BT960 system was reported very recently, which was very similar to our finding here (22).
Among the 44 mutant isolates, 8 harbored deletions and 3 exhibited double point mutations, while the remaining 33 isolates had single point mutations. The mutations were randomly distributed almost along the entire 561-bp-long pncA gene, from nt 23 to nt 525 in the clinical isolates. This was similar to other reports (23–25) and similar to the “all pncA mutations” collected and analyzed in the TB Drug Resistance Database (http://www.tbdreamdb.com/PZA_Rv2043c_AllMutations.html), in which mutations were randomly distributed from nt 1 to nt 554. Only 13 of 44 (29.5%) mutants in this study located in the clustering of mutations of G132-T142 and P69-L85 found by previous investigators (25), which showed that the location of mutations in the pncA gene was more variable than expected before (25) and that the mutations were not clustered as in other genes associated with drug resistance, such as the rpoB gene (12). None of the isolates with identical pncA mutations were epidemiologically linked, so the PZA-resistant strains might not have been obtained from transmission. For the mutations in pncA, 23 types of point mutations had been reported previously (8, 9, 21, 26–29), and to our best knowledge, 10 novel types of mutations (13 strains) were first found in this study. The latter included all six types of the deletion mutations (L27, 91E, 111E, 122Q, 130-132VVG, and 131V) and four types of amino acid substitutions (D8A, S18P, F58S, and E174G+M175R double mutation) in PZases as summarized in Table 1. Such novel mutants accounted for 25% (13 of 52) of all the PZA-resistant isolates. The PCR–single-strand conformational polymorphism (PCR-SSCP) and line probe assay (LiPA) developed previously (30, 31) could detect DNA mutations quickly, but they could not tell if the mutation was synonymous or nonsynonymous. No silent mutations were found in the pncA gene in our study, which indicated that PCR-SSCP and LiPA might be used to detect PZA resistance quickly with a high success rate by detecting the pncA mutation in southern China.
There were still 8 PZA-resistant M. tuberculosis isolates that had no mutation in the pncA gene. Four of them did not harbor any mutation, including FR; three of them showed an rpsA single point mutation (see below); and one other strain had a point mutation in the putative regulatory region (A-11G) of the pncA gene, which was a very common mutation found in isolates from other countries (10, 23–25). To explore the possible reason why this can affect PZA resistance, we checked the transcription levels of pncA genes in this clinical isolate and the laboratory control M. tuberculosis H37Ra strain. RT-PCR products were prepared as described in the section demonstrating transcription of pncA gene in a polycistron. Real-time PCR with SYBR green dye was used for detecting the initial concentration of pncA mRNA with sigA as inner reference. The primers used were Bf-Br for pncA and sigAF (5′-CTCGACGCTGAACCAGACCT)-sigAR(5′-AGGTCTTCGTGGTCTTCGTC) for sigA (32). Levene's test for equality of variances and the t test for equality of means were calculated using the software SPSS13.0 (SPSS Inc., Chicago, IL). This A-11G mutation may not affect pncA expression at the transcription level, because it can still be transcribed in a polycistron with flanking genes in the clinical −11 mutant (data not shown) and its expression level showed no difference from that in M. tuberculosis H37Ra. This mutation, however, may disturb the translation of the pncA gene by affecting the ribosome movement, because the nt −11 mutation is close to the ribosome binding site.
These new findings displayed the regional disparities in pncA mutations and the highly diverse patterns in pncA mutations (4, 5, 22), which partially make molecular detection of PZA susceptibility difficult to be adopted worldwide. Therefore, systematic establishment of the relationship between the mutation characterization of PZA resistance-related genes and the PZA resistance phenotype is highly needed for development and application of rapid molecular assays.
Of multidrug-resistant (MDR) strains, 68.4% (39/57) were PZA resistant, but only 12.5% (13/104) of non-MDR strains harbored such resistance. The former was significantly higher than the latter (P ≪ 0.01), which implied that the MDR strains were more likely to be PZA resistant than the drug-sensitive ones. This result is similar to those in South Africa and Thailand (4, 27). The importance of PZA in treating MDR-TB has been proved recently in a murine model in that all PZA-containing regimens tested showed better activities than those corresponding regimens without PZA when combined with second-line drugs (33). All these findings emphasized the importance of rapid detection of PZA resistance in controlling MDR-TB.
rpsA mutations.
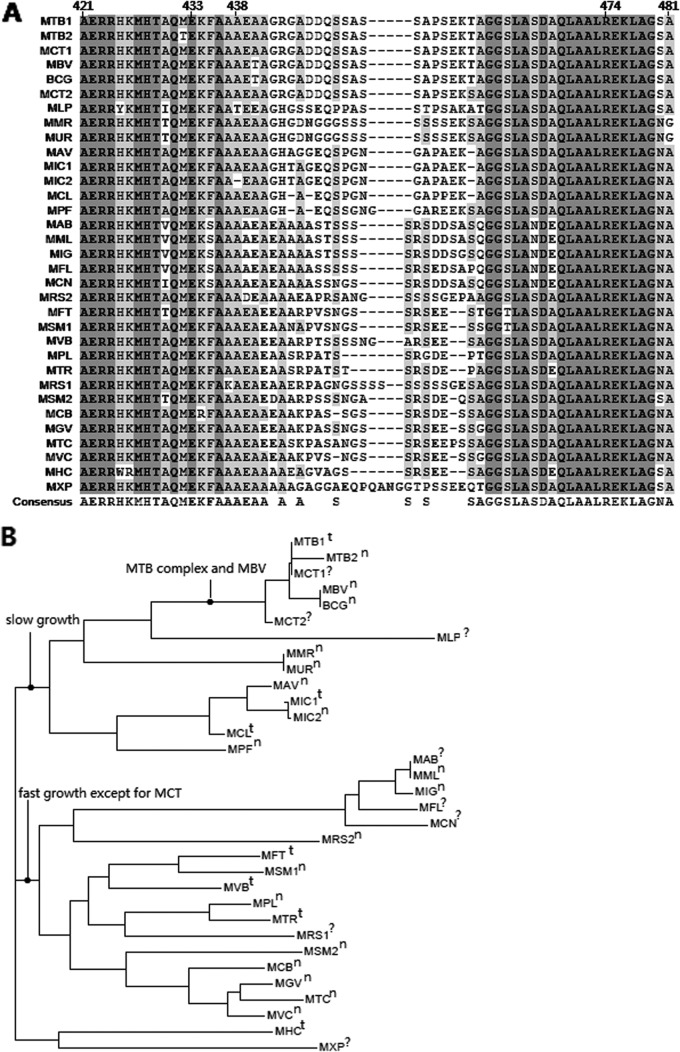
Three PZA-resistant clinical isolates with wild-type pncA (pncAWT) harbored single mutations R474L, R474W, and E433D, respectively. One PZA-susceptible clinical isolate also contained one Q162R single mutation. The frequency of rpsA mutations showed no significant statistical difference (P = 0.099) between PZA-resistant and PZA-susceptible isolates, which may be because of the small sample size for PZA-resistant isolates. The low frequency of rpsA mutations proved again that the rpsA mutation was not the main mechanism of PZA resistance in M. tuberculosis, though it can affect the susceptibility to PZA (6). Alexander et al. (7) could not find any rpsA mutations in PZA-resistant isolates, but one RpsA protein mutation (A364G) in 13 PZA-sensitive strains was discovered. In addition, the rpsA gene plus FR is long and not very convenient for amplification and sequencing, so the authors concluded that analysis of the rpsA gene had no role in the detection of pyrazinamide resistance (7). This suggestion was further supported by Simons and colleagues, who reported that only 1 of 5 PZA-resistant isolates with pncAWT had an RpsA (V260I) mutation (34) and that this so-called mutation was even questionable based on the disparity of the reported mutated codon and the amino acid that it encoded. However, in the current study, 3 of 7 phenotypically PZAr but genotypically pncAWT + FRWT isolates had rpsA mutations which were all clustered in the C terminus of RpsA protein (3′ of rpsA gene). This was concordant with the findings by Shi et al. (6), who reported that deletion of amino acid 438 in the C terminus of RpsA (Fig. 2A) could cause PZA resistance in an M. tuberculosis clinical isolate with pncAWT. We noticed that rpsA mutations in the PZA-sensitive strains found until now were mostly away from the C-terminal end of RpsA. We then made an alignment of C-terminal amino acid sequences of ribosomal protein S1 (RpsA) from 33 strains of 28 mycobacterial species (Fig. 2A) and found the same phenomenon as described by Shi et al. (6) that, unlike other parts, C-terminal ends of RpsA proteins were highly variable. At the same time, we drew a phylogenetic tree according to the C-terminal amino acid sequences of RpsAs from these mycobacterial species (Fig. 2B) using Vector NTI Suite 7.0 (Invitrogen, USA). It is similar to the phylogenetic trees based on either the nearly complete 16S rRNA gene sequence or the concatenated hypervariable sequences of 16S rRNA and rpoB and hsp65 genes (35). Two different species, for example, Mycobacterium marinum and Mycobacterium ulcerans, have RpsAs that are identical not only in the C terminus, while different strains of the same species, for example, Mycobacterium smegmatis MC2 155 and M. smegmatis JS263, or Mycobacterium rhodesiae NBB3 and another strain, have very different RpsA C-terminal ends but are even closer to other species in the phylogenetic tree. This agrees with the fact that the genus Mycobacterium has very limited interspecies genetic variability (35). In the current study, we found that all 3 mutated sites (E433, A438, and R474) were highly conserved among all the Mycobacterium species. It is interesting that the A438 deletion that took place at AAA (436 to 438) had the same effect as did A436, which seemed more conserved than A438 (Fig. 2A). If E433 and R474 mutations did cause PZA resistance as the A438 deletion did, sequencing the C-terminal end of the rpsA gene product in isolates with pncAWT may be needful for more rapid and accurate detection of PZA susceptibility, especially in southern China, though the role of this region is modest. Of phenotypically PZA-resistant isolates, 86.5% (45 of 52) could be predicted by sequencing only pncA plus FR and 92.3% (48 of 52) could be predicted if sequencing the 3′ end of rpsA was added in our study. In the meantime, it is needful to point out that not all mutations at the C-terminal ends of RpsA may cause PZA resistance. For example, M. bovis BCG has a nonfunctional pncA and an rpsA gene encoding mutated RpsA (A440T) and is naturally resistant to PZA. M. smegmatis pncA and pzaA both encode proteins with PZase activity and can transform PZA into POA. Introduction of M. smegmatis pncA or pzaA into M. bovis BCG can restore its sensitivity to PZA (36). Therefore, the resistance of M. bovis BCG to PZA is possibly because of the pncA mutation but not the rpsA mutation, and so RpsA (A440T) may not play a role in PZA resistance. Another strain, M. tuberculosis CTRI-2, contained a mutated RpsA (M432T) at its C-terminal end and was PZA sensitive (37). In this regard, therefore, the actual role of E433 and R474 mutations of RpsA found here in PZA resistance needs to be verified further.
FIG 2.
(A) Alignment of C-terminal amino acid sequences of ribosomal protein S1 (RpsA) from different mycobacterial species. Species are abbreviated as follows with GenBank accession numbers indicated in parentheses: MTB1, M. tuberculosis H37Rv (NP_216146.1); MTB2, M. tuberculosis CTRI-2 (YP_005916702.1); MCT1, M. canettii CIPT 140070008 (YP_007287527.1); MBV, M. bovis AF2122/97 (NP_855309); BCG, M. bovis BCG Pasteur 1173P2 (NP_855309.1); MCT2, M. canettii CIPT 140070010 (YP_007264352.1); MLP, M. leprae TN (NP_301983.1); MMR, M. marinum M (YP_905584.1); MUR, M. ulcerans Agy99 (YP_905584.1); MAV, M. avium subsp. paratuberculosis K-10 (NP_960259); MIC1, M. intracellulare 13950 (YP_005338525.1); MIC2, M. intracellulare (WP_009954955.1); MCL, M. colombiense CECT 3035 (WP_007776137.1); MPF, M. parascrofulaceum (WP_007169711.1); MAB, M. abscessus (YP_001703031.1); MML, M. massiliense GO 06 (YP_006520985.1); MIG, M. immunogenum MTCC 9506 (YP_006730099.1); MFL, M. franklinii (AEI54876.1); MCN, M. chelonae (AEI54878.1); MRS2, M. rhodesiae NBB3 (YP_005002313.1); MFT, M. fortuitum (WP_003884756); MSM1, M. smegmatis MC2 155 (YP_888124.1); MVB, M. vanbaalenii PYR-1 (YP_954159.1); MPL, M. phlei (WP_003884756.1); MTR, M. thermoresistibile (WP_003923841.1); MRS1, M. rhodesiae (WP_005149060); MSM2, M. smegmatis JS623 (YP_007292760.1); MCB, M. chubuense NBB4 (YP_006453027.1); MGV, M. gilvum PYR-GC (YP_001134823.1); MTC, M. tusciae (WP_006246193.1); MVC, M. vaccae (WP_003931928.1); MHC, M. hassiacum (WP_005627902.1); and MXP, M. xenopi (WP_003920384.1). Amino acid numbers are indicated on the top according to the order of RpsA from M. tuberculosis. The identical amino acids from all species are highlighted in dark gray, and the similar resides are highlighted in light gray. (B) The phylogenetic tree drawn according to the C-terminal amino acid sequences of RpsA from the above mycobacterial species. t, type strain; n, nontype strain; ?, not clearly indicated in web sources as type strain or nontype strain (http://www.bacterio.net/ [updated and expanded on 9 July 2013] and http://www.atcc.org).
In conclusion, the pncA mutations were randomly dispersed along with the entire gene and observed at a high ratio in PZA-resistant clinical isolates in southern China. Of clinical isolates with pncA-plus-FR mutations, 28.9% (13 of 45) were new, which displayed the high diversity of mutations in the pncA gene in different regions. It was first shown here that the pncA gene was transcribed in a polycistron and that the A-11G mutation of pncA did not affect its expression at transcription level. The 3′ end of the rpsA gene, if not the whole rpsA gene plus FR, should be added as the target for a two-step approach, where only pncAWT isolates from southern China are subjected to sequencing of this fragment. However, the influence of these rpsA mutations on PZA susceptibility needs to be proved further in the future, as well as that of many pncA mutations. For more accurate results, phenotypic PZA susceptibility testing, especially using the BT960 system, is needed for M. tuberculosis, and in particular MDR M. tuberculosis clinical isolates with no known gene mutations.
ACKNOWLEDGMENTS
We are grateful to BGI for assistance in DNA sequencing. We thank Wing Wai Yew at The Chinese University of Hong Kong for helpful discussion.
This work was supported by the National Great Research Program of China (2008zx10003-009). T.Z. was supported by the Chinese Academy of Sciences One Hundred Talents Program (Category A) and the key layout program of the Chinese Academy of Sciences (KSZD-EW-Z-006).
Footnotes
Published ahead of print 16 October 2013
REFERENCES
- 1.Heifets L, Lindholm-Levy P. 1992. Pyrazinamide sterilizing activity in vitro against semi-dormant Mycobacterium tuberculosis bacterial populations. Am. Rev. Respir. Dis. 145:1223–1225. 10.1164/ajrccm/145.5.1223 [DOI] [PubMed] [Google Scholar]
- 2.Scorpio A, Zhang Y. 1996. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat. Med. 2:662–667. 10.1038/nm0696-662 [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Mitchison D. 2003. The curious characteristics of pyrazinamide: a review. Int. J. Tuberc. Lung Dis. 7:6–21 [PubMed] [Google Scholar]
- 4.Hirano K, Takahashi M, Kazumi Y, Fukasawa Y, Abe C. 1997. Mutation in pncA is a major mechanism of pyrazinamide resistance in Mycobacterium tuberculosis. Tuber. Lung Dis. 78:117–122 [DOI] [PubMed] [Google Scholar]
- 5.Chang KC, Yew WW, Zhang Y. 2011. Pyrazinamide susceptibility testing in Mycobacterium tuberculosis: a systematic review with meta-analyses. Antimicrob. Agents Chemother. 55:4499–4505. 10.1128/AAC.00630-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi W, Zhang X, Jiang X, Yuan H, Lee JS, Barry CE, III, Wang H, Zhang W, Zhang Y. 2011. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science 333:1630–1632. 10.1126/science.1208813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander DC, Ma JH, Guthrie JL, Blair J, Chedore P, Jamieson FB. 2012. Gene sequencing for routine verification of pyrazinamide resistance in Mycobacterium tuberculosis: a role for pncA but not rpsA. J. Clin. Microbiol. 50:3726–3728. 10.1128/JCM.00620-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juréen P, Werngren J, Toro JC, Hoffner S. 2008. Pyrazinamide resistance and pncA gene mutations in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 52:1852–1854. 10.1128/AAC.00110-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues VDFS, Telles MA, Ribeiro MO, Cafrune PI, Rossetti ML, Zaha A. 2005. Characterization of pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis in Brazil. Antimicrob. Agents Chemother. 49:444–446. 10.1128/AAC.49.1.444-446.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Portugal I, Barreiro L, Moniz-Pereira J, Brum L. 2004. pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis isolates in Portugal. Antimicrob. Agents Chemother. 48:2736–2738. 10.1128/AAC.48.7.2736-2738.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chedore P, Bertucci L, Wolfe J, Sharma M, Jamieson F. 2010. Potential for erroneous results indicating resistance when using the Bactec MGIT 960 system for testing susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Clin. Microbiol. 48:300–301. 10.1128/JCM.01775-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan Y, Hu Z, Zhao Y, Cai X, Luo C, Zou C, Liu X. 2012. The beginning of the rpoB gene in addition to the rifampin resistance determination region might be needed for identifying rifampin/rifabutin cross-resistance in multidrug-resistant Mycobacterium tuberculosis isolates from southern China. J. Clin. Microbiol. 50:81–85. 10.1128/JCM.05092-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang TS, Lee SSJ, Tu HZ, Tu HZ, Huang WK, Chen YS, Huang CK, Wann SR, Lin HH, Liu YC. 2003. Correlation between pyrazinamide activity and pncA mutations in Mycobacterium tuberculosis isolates in Taiwan. Antimicrob. Agents Chemother. 47:3672–3673. 10.1128/AAC.47.11.3672-3673.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Y, Peng J, Yang F, Yu L, Wang Y, Jin Q. 2007. Molecular basis of Mycobacterium tuberculosis to pyrazinamide resistance. Prog. Microbiol. Immunol. 35:5–9 (In Chinese.) [Google Scholar]
- 15.Sun B, Yu H, Zhang K. 2007. Detection of pyrazinamide-resistant gene pncA of Mycobacterium tuberculosis isolates in Qingdao area. Acta Acad. Med. Qingdao Univ. 43:125–127 (In Chinese.) [Google Scholar]
- 16.Wu X, Zhang J, Zhong M, Yu W, Ding B, Zhang J, Jia S. 2000. Studies on pncA gene mutations in Mycobacterium tuberculosis isolates. Chin. J. Tuberc. Respir. Dis. 23:40–42 (In Chinese.) [PubMed] [Google Scholar]
- 17.Xu L, Yu H, Sun B, Zhou B, Zhang W. 2009. Detection of pncA gene mutation in Mycobacterium tuberculosis. Acta Acad. Med. Qingdao Univ. 45:424–426 (In Chinese.) [Google Scholar]
- 18.McCammon MT, Gillette JS, Thomas DP, Ramaswamy SV, Rosas II, Graviss EA, Vijg J, Quitugua TN. 2005. Detection by denaturing gradient gel electrophoresis of pncA mutations associated with pyrazinamide resistance in Mycobacterium tuberculosis isolates from the United States-Mexico border region. Antimicrob. Agents Chemother. 49:2210–2217. 10.1128/AAC.49.6.2210-2217.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirabal NC, Yzquierdo SL, Lemus D, Madruga M, Milián Y, Echemendía M, Takiff H, Martin A, Van der Stuyf P, Palomino JC, Montoro E. 2010. Evaluation of colorimetric methods using nicotinamide for rapid detection of pyrazinamide resistance in Mycobacterium tuberculosis. J. Clin. Microbiol. 48:2729–2733. 10.1128/JCM.00311-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muthaiah M, Jagadeesan S, Ayalusamy N, Sreenivasan M, Prabhu SS, Muthuraj U, Senthilkumar K, Veerappan S. 2010. Molecular epidemiological study of pyrazinamide-resistance in clinical isolates of Mycobacterium tuberculosis from South India. Int. J. Mol. Sci. 11:2670–2680. 10.3390/ijms11072670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimic M, Sheen P, Quiliano M, Gutierrez A, Gilman RH. 2010. Peruvian and globally reported amino acid substitutions on the Mycobacterium tuberculosis pyrazinamidase suggest a conserved pattern of mutations associated to pyrazinamide resistance. Infect. Genet. Evol. 10:346–349. 10.1016/j.meegid.2009.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui Z, Wang J, Lu J, Huang X, Zheng R, Hu Z. 2013. Evaluation of drug susceptibility testing methods of clinical Mycobacterium tuberculosis isolates to pyrazinamide. J. Clin. Microbiol. 51:1374–1380. 10.1128/JCM.03197-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marttila HJ, Marjamäki M, Vyshnevskaya E, Vyshnevskiy BI, Otten TF, Vasilyef AV, Viljanen MK. 1999. pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis isolates from northwestern Russia. Antimicrob. Agents Chemother. 43:1764–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SK, Lee JY, Chang CL, Lee MK, Son HC, Kim CM, Jang HJ, Park HK, Jeong SH. 2001. pncA mutations in clinical Mycobacterium tuberculosis isolates from Korea. BMC Infect. Dis. 1:4. 10.1186/1471-2334-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scorpio A, Lindholm-Levy P, Heifets L, Gilman R, Siddiqi S, Cynamon M, Zhang Y. 1997. Characterization of pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 41:540–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonmalung J, Prammananan T, Leechawengwongs M, Chaiprasert A. 2010. Surveillance of pyrazinamide susceptibility among multidrug-resistant Mycobacterium tuberculosis isolates from Siriraj Hospital, Thailand. BMC Microbiol. 10:223–228. 10.1186/1471-2180-10-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mphahlele M, Syre H, Valvatne H, Stavrum R, Mannsåker T, Muthivhi T, Weyer K, Fourie PB, Grewal HM. 2008. Pyrazinamide resistance among South African multidrug-resistant Mycobacterium tuberculosis isolates. J. Clin. Microbiol. 46:3459–3464. 10.1128/JCM.00973-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simons SO, Van Ingen J, Van der Laan T, Mulder A, Dekhuijzen PN, Boeree MJ, Van Soolingen D. 2012. Validation of pncA gene sequencing in combination with the mycobacterial growth indicator tube method to test susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Clin. Microbiol. 50:428–434. 10.1128/JCM.05435-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louw GE, Warren RM, Donald PR, Murray MB, Bosman M, Van Helden PD, Young DB, Victor TC. 2006. Frequency and implications of pyrazinamide resistance in managing previously treated tuberculosis patients. Int. J. Tuberc. Lung Dis. 10:802–807 [PubMed] [Google Scholar]
- 30.Sekiguchi JI, Nakamura T, Miyoshi-Akiyama T, Kirikae F, Kobayashi I, Augustynowicz-Kopec E, Zwolska Z, Morita K, Suetake T, Yoshida H, Kato S, Mori T, Kirikae T. 2007. Development and evaluation of a line probe assay for rapid identification of pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis strains. J. Clin. Microbiol. 45:2802–2807. 10.1128/JCM.00352-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheen P, Méndez M, Gilman RH, Peña L, Caviedes L, Zimic MJ, Zhang Y, Moore DAJ, Evans CA. 2009. Sputum PCR–single-strand conformational polymorphism test for same-day detection of pyrazinamide resistance in tuberculosis patients. J. Clin. Microbiol. 47:2937–2943. 10.1128/JCM.01594-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelly S, Bishai WR, Lamichhane G. 2012. A screen for non-coding RNA in Mycobacterium tuberculosis reveals a cAMP-responsive RNA that is expressed during infection. Gene 500:85–92. 10.1016/j.gene.2012.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmad Z, Tyagi S, Minkowski A, Peloquin CA, Grosset JH, Nuermberger EL. 2013. Contribution of moxifloxacin or levofloxacin in second-line regimens with or without continuation of pyrazinamide in murine tuberculosis. Am. J. Respir. Crit. Care Med. 188:97–102. 10.1164/rccm.201212-2328OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simons SO, Mulder A, Van Ingen J, Boeree MJ, Van Soolingen D. 2013. Role of rpsA gene sequencing in diagnosis of pyrazinamide resistance. J. Clin. Microbiol. 51:382 (Letter.) 10.1128/JCM.02739-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tortoli E. 2012. Phylogeny of the genus Mycobacterium: many doubts, few certainties. Infect. Genet. Evol. 12:827–831. 10.1016/j.meegid.2011.05.025 [DOI] [PubMed] [Google Scholar]
- 36.Guo M, Sun ZH, Zhang Y. 2000. Mycobacterium smegmatis has two pyrazinamidase enzymes, PncA and PzaA. J. Bacteriol. 182:3881–3884. 10.1128/JB.182.13.3881-3884.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ilina EN, Shitikov EA, Ikryannikova LN, Alekseev DG, Kamashev DE, Malakhova MV, Parfenova TV, Afanas'ev MV, Ischenko DS, Bazaleev NA, Smirnova TG, Larionova EE, Chernousova LN, Beletsky AV, Mardanov AV, Ravin NV, Skryabin KG, Govorun VM. 2013. Comparative genomic analysis of Mycobacterium tuberculosis drug resistant strains from Russia. PLoS One 8:e56577. 10.1371/journal.pone.0056577 [DOI] [PMC free article] [PubMed] [Google Scholar]