Abstract
Lactobacillus spp. are part of the normal human flora and are generally assumed to be nonpathogenic. We determined the genotypic identification of >100 Lactobacillus isolates from clinical specimens in the context of presumed pathogenic potential (e.g., recovered as the single/predominant isolate from a sterile site or at ≥105 CFU/ml from urine). This study assessed the clinical significance and the frequency of occurrence of each Lactobacillus sp. We identified 16 species of Lactobacillus by 16S rRNA gene sequence analysis, 10 of which could not be associated with disease. While Lactobacillus rhamnosus, Lactobacillus gasseri, and Lactobacillus paracasei were associated with infections, L. gasseri was also a common colonizing/contaminating species. Lactobacillus casei, Lactobacillus johnsonii, and Lactobacillus delbrueckii were associated with at least one infection. Species commonly used in probiotic products (e.g., L. rhamnosus and L. casei) were identical, by 16S rRNA gene sequencing, to our isolates associated with disease. Human isolates of Lactobacillus spp. have differing site associations and levels of clinical significance. Knowing the niche and pathogenic potential of each Lactobacillus sp. can be of importance to both clinical microbiology and the food and probiotic supplement industry.
INTRODUCTION
The genus Lactobacillus consists of a large and diverse spectrum of bacterial species, which in general are nonpathogenic and are considered part of the normal human microbiota. Lactobacilli are non-spore-forming Gram-positive rods that are microaerophilic and catalase negative. In the clinical microbiology laboratory, the proper identification of lactobacilli by conventional methods can be difficult (1). Biochemical characteristics are insufficient to allow reliable identification of lactobacilli to the species level, making molecular diagnostic approaches such as 16S rRNA gene sequencing necessary (2). Because lactobacilli range in size and shape, even presumptive genus identification can be difficult, as lactobacilli may be confused with other Gram-positive, catalase-negative organisms such as Streptococcus (3, 4) and Leuconostoc (5). Since lactobacilli rarely cause disease, many laboratories choose not to determine the species and simply report the isolated organism as “Lactobacillus sp.” Apart from the known pathogenic association between lactobacilli and dental caries, several clinical case reports describe the opportunistic nature by which lactobacilli cause disease. Reported infections include endocarditis, bacteremia, peritonitis, and localized soft-tissue infections (1–3, 6–8). Most infections caused by Lactobacillus spp. are associated with underlying diseases (6).
Lactobacilli have been recognized as commensal flora that function to inhibit pathogenic bacteria by producing antimicrobial substances such as lactic acid, hydrogen peroxide, and bacteriocins (9–11). They are found as normal flora in the oral cavity, gastrointestinal (GI) tract, and female urogenital (UG) tract. At the time of birth, these lactic acid-producing bacteria are ingested and soon colonize the intestines. As individuals age, the lactobacillus profile changes over time (9, 12, 13). Moreover, changes in the species composition of commensal lactobacilli can suggest disease processes such as periodontitis (14) and bacterial vaginosis (11).
Exogenous sources of lactobacilli include fermented food products such as yogurt and cheeses, and lactobacilli are vital components of probiotics (e.g., foods and dietary supplements containing live bacteria). The role of Lactobacillus spp. in food products dates back more than 100 years, and beneficial effects on health and longevity have long been proposed (13, 15). Probiotics, often containing members of the genera Lactobacillus and Bifidobacterium, have been accorded a number of beneficial effects in a variety of disorders, often with little or no scientific support. Recent studies have identified clinical applications in which probiotics are reported as being effective for UG infections (9), nosocomial rotavirus gastroenteritis in infants (16), antibiotic-associated diarrhea (17), acute infectious diarrhea, irritable bowel syndrome, and inflammatory bowel disease (13, 18).
With the increasing use of probiotic food products and dietary supplements by both healthy individuals and patients with various underlying diseases, it is important to distinguish the sites from which Lactobacillus may be recovered and the settings in which Lactobacillus spp. can behave as pathogens. The aim of this study was to determine the frequency of occurrence, in a routine clinical laboratory, of identified species of Lactobacillus in relation to the site of isolation and associated diseases.
MATERIALS AND METHODS
Patients and bacterial isolates.
In this retrospective study of laboratory records between 1998 and 2013 at the Veterans Affairs medical centers in Houston, TX, and Seattle, WA, we identified a total of 107 Lactobacillus isolates that were found in clinical specimens in the context of presumed pathogenic potential (e.g., recovered as the single/predominant isolate from a sterile site or at ≥105 CFU/ml from urine) and could be assigned unambiguously to a species by 16S rRNA sequencing. Four strains were sent from other Veterans Affairs medical centers for identification (3). All of the available isolates were included in the study, with one exception; since isolates from male UG specimens were the most numerous, a convenience sample of about 30% of the total was chosen to match the number of female UG specimens. The patient group consisted of 22 women (age, 25 to 77 years [mean, 45 years]) and 59 men (age, 40 to 81 years [mean, 48 years]).
Assessment of clinical significance.
For all strains, we determined the sites from which they were isolated. We were able to determine the age and gender for 95% of the patients. To assess the clinical significance of isolates from urine, we used urinalysis results, the concentrations of both Lactobacillus and other organisms that might have been present, and, if available, clinical notes documenting symptoms of a urinary tract infection (UTI). The significance of non-UG cultures was determined by the quantity of lactobacilli recovered (for blood cultures, the number of positive bottles), the presence of other organisms, the presence of inflammatory cells, and the description of the specimen. For the purposes of this study, we regarded localized infections in closed spaces as abscesses.
DNA preparation and strain identification by 16S rRNA sequencing.
Strains were subcultured from freezer stocks onto blood agar plates and were incubated aerobically for 24 to 72 h. The DNA was extracted using Prepman reagent (PE Applied Biosystems, Foster City, CA), following the instructions of the manufacturer, from a loopful of bacteria either directly or by suspension in 500 μl distilled H2O and centrifugation at 15,000 × g for 5 min, with the pellet being suspended in 300 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA). The sequence for the first 500 bp of 16S rRNA was edited and compared with the reference database that is part of the MicroSEQ 16S rDNA bacterial identification system (Applied Biosystems). BIBI and GenBank databases were also used (19, 20). The accession numbers for sequences from GenBank used in the dendrogram are listed in Table S1 in the supplemental material. Phylogenetic trees were constructed using sequences aligned with MUSCLE, a program for generating multiple alignments (21), with MEGA5 version 1 software (http://www.megasoftware.net).
Isolation of Lactobacillus from food products and probiotic dietary capsules.
We sequenced isolates from food products and probiotic dietary capsules to determine whether the food strains differed genetically from human strains of the same species. Two hundred microliters of yogurt and kefir was spread onto blood agar (Remel, Lenexa, KS) and Schaedler blood agar (Remel, Lenexa, KS) plates and incubated at 35°C both aerobically (with added CO2) and anaerobically. One capsule of an acidophilus dietary supplement was mixed into 5 ml of brain heart infusion broth. Two hundred microliters was spread onto blood agar plates and incubated as previously mentioned. The plates were examined after 48 and 72 h. Multiple colonies of different morphological types were subcultured for isolation both aerobically (with added CO2) and anaerobically. Gram-positive isolates (3 isolates from the yogurt, 4 bacterial isolates from the kefir, and 3 isolates from the probiotic capsules) were selected for 16S rRNA gene sequence analysis.
This study was completed with institutional review board approval (MIRB no. 01012).
RESULTS
The overall relationships between the Lactobacillus species and the site from which the species were recovered are shown in Table 1. The specimen types are categorized as UG (95% urine and 5% vaginal specimens), blood, respiratory (lung tissue, bronchoalveolar lavage [BAL] fluid, bronchial brush, pleural fluid, endotracheal aspirate, and sputum specimens), deep wounds (abscesses and tissue samples from normally sterile sites, including kidney, liver, and aortic graft), superficial wounds (swabs and ulcers), and body fluids (bile, gallbladder, peritoneal, and pericardial specimens). We found 16 species of Lactobacillus, of which Lactobacillus gasseri and Lactobacillus rhamnosus were most frequently isolated (Table 1). Fifty-three percent of the L. gasseri isolates were isolated from urine, with superficial and deep isolates accounting for 11% and 19%, respectively, of the L. gasseri isolates. The second most commonly isolated species in this study, L. rhamnosus, was recovered from all sites, with significant proportions of isolates obtained from blood (29%), respiratory specimens (29%), and deep wounds (21%). All except one of the Lactobacillus crispatus strains were isolated from vaginal specimens or female urine samples. The majority (57%) of Lactobacillus paracasei strains were isolated from blood. In contrast, the majority (57%) of the Lactobacillus fermentum isolates were from respiratory sources. Only one strain each of Lactobacillus acidophilus, Lactobacillus cantenaformis, Lactobacillus iners, Lactobacillus plantarum, and Lactobacillus reuteri were isolated.
TABLE 1.
Distribution of Lactobacillus species among different specimen types
| Lactobacillus sp. | No. of isolates ina: |
Total no. of isolates | |||||
|---|---|---|---|---|---|---|---|
| Urine/UG specimens | Blood | Respiratory tract specimens | Deep wounds | Superficial wounds | Fluids | ||
| L. gasseri | 19 | 3 | 3 | 7 | 4 | 36 | |
| L. rhamnosus | 1 | 7 | 7 | 5 | 2 | 2 | 24 |
| L. crispatus | 10 | 10 | |||||
| L. paracasei | 4 | 1 | 2 | 7 | |||
| L. fermentum | 4 | 2 | 1 | 7 | |||
| L. johnsonii | 1 | 1 | 1 | 2 | 5 | ||
| L. jensenii | 4 | 4 | |||||
| L. casei | 1 | 1 | 1 | 3 | |||
| L. delbrueckii | 1 | 1 | 2 | ||||
| L. salivarius | 1 | 1 | 2 | ||||
| L. vaginalis | 1 | 1 | 2 | ||||
| L. acidophilus | 1 | 1 | |||||
| L. cantenaformis | 1 | 1 | |||||
| L. iners | 1 | 1 | |||||
| L. plantarum | 1 | 1 | |||||
| L. reuteri | 1 | 1 | |||||
| Total | 40 | 19 | 17 | 19 | 6 | 6 | 107 |
The specimen types included urine and urogenital (UG) specimens (vaginal secretion and mucous membrane specimens), blood, respiratory tract specimens (lung tissue, pleural fluid, BAL fluid, bronchial brush, endotracheal aspirate, and sputum specimens), deep wounds (sterile tissue, abscesses, soft-tissue infections, and pericardial grafts), superficial wounds (ulcers and swabs), and sterile body fluids (bile, pericardial, peritoneal, and gallbladder fluids).
Lactobacilli were most frequently isolated from UG specimens; the proportion would have been higher, as only about one-third of all male UG specimens were included. Lactobacillus jensenii, L. iners, and Lactobacillus vaginalis isolates were all isolated from female UG specimens and were not associated with UTIs, which is not surprising since these species are reported to be common vaginal microbiota (22, 23). There were only two of 39 evaluable cases (all male subjects) in which lactobacilli were present as the only isolate at ≥105 CFU/ml, and polymorphonuclear leukocytes (PMNs) were present. One isolate of L. gasseri and one of Lactobacillus casei were associated with urinary retention and dysuria with inguinal hernia and recurrent UTIs with renal carcinoma, respectively. Lactobacillus delbrueckii was isolated from the blood and urine from a patient, but clinical details were not available for these referred specimens.
Details of non-UG cases are shown in Tables 2, 3, and 4. Table 2 summarizes data for isolates recovered from deep wounds, abscesses, or sterile sites. Additional organisms were cultivated from eight of 14 specimens, often Candida albicans, Staphylococcus aureus, or viridans group streptococci. L. rhamnosus, L. paracasei, L. gasseri, and Lactobacillus johnsonii were all recovered as single isolates. The most common single-type specimen was an abscess at a drug injection site. We recovered L. johnsonii twice, 2 years apart, from the same patient, both times from an assumed injection site in his arm; in each case, PMNs and Gram-positive organisms were seen on Gram stains. L. rhamnosus and L. gasseri were both recovered from two injection sites. In addition, L. gasseri was recovered from two diabetic ulcers with other organisms and from an infected aortic graft.
TABLE 2.
Lactobacillus spp. and deep wound associations
| Patient no. | Organism | Specimen typea | Patient sex | Patient age (yr) | Quantity | Other significant organism(s) | Underlying disease(s)/condition(s)b |
|---|---|---|---|---|---|---|---|
| 1 | L. gasseri | Abscess | M | 46 | Few | None | Diverticulitis |
| 2 | L. gasseri | Wound, fistula after esophageal surgery | M | 63 | Moderate | Moderate Candida albicans, Streptococcus mitis | Small cell carcinoma, leak from fistula |
| 3 | L. gasseri | Abdominal abscess | M | 61 | NA | C. albicans and Staphylococcus aureus | Crohn's disease, bowel obstruction |
| 4 | L. gasseri | Pericolic abscess, extension from duodenum | M | NA | NA | Streptococcus constellatus | DM, peptic ulcer disease |
| 5a | L. johnsonii | Abscess on arm | M | 55 | Many | None | IVDA, DM, hepatitis C |
| 5b | L. johnsonii | Abscess on arm 2 yr later | M | 57 | Many | None | IVDA, DM, hepatitis C |
| 6 | L. paracasei | Bile fluid | M | 63 | Moderate | None | Cholangiocarcinoma, obstruction |
| 7 | L. paracasei | Liver tissue | M | 84 | Moderate | Many Escherichia coli | Cholecystitis, gallbladder rupture |
| 8 | L. reuteri | Peritoneal fluid | M | 64 | Few | Rare Streptococcus anginosus | Perforated diverticulum, DM |
| 9 | L. rhamnosus | Abscess in spleen | M | NA | Many | Many C. albicans | Unknown |
| 10 | L. rhamnosus | Abscess at site of perforated viscus | M | 70 | Moderate | Clostridium disporicum | Perforated viscus |
| 11 | L. rhamnosus and L. gasseri | Abscess and cellulitis in hand | M | 52 | Many | None | IVDA, DM |
| 12a | L. rhamnosus | Tissue from stump after lower limb removal | M | 60 | Moderate | None | IVDA, DM |
| 12b | L. rhamnosus and L. gasseri | Abscess at injection site in upper extremity | M | 60 | Moderate | Moderate S. aureus | IVDA, DM |
Specimens from deep wounds consisted of sterile tissue, abscess, soft tissue infection, and pericardial graft specimens.
DM, diabetes mellitus; IVDA, intravenous drug abuse; BKA, below-the-knee amputation; NA, not applicable.
TABLE 3.
Lactobacillus spp. and blood associationsa
| Patient no. | Organism | Patient sex | Patient age (yr) | No. positive/no. of bottles | Other significant organism(s) | WBC count (CFU/ml) | Underlying disease(s)/condition(s)b |
|---|---|---|---|---|---|---|---|
| 1 | L. johnsonii | M | 79 | 1/3 | None | 7.6 | Bowel obstruction |
| 2 | L. gasseri | M | 68 | 1/2 | Enterococcus faecalis | 10.1 | Neurogenic bladder |
| 3 | L. paracasei | M | 59 | 3/3 | None | 11.5 | Perforated diverticulum |
| 4 | L. rhamnosus | M | 60 | 1/3 | None | 22.3 | Esophageal adenocarcinoma, pleural effusions |
| 5 | L. rhamnosus | M | 38 | 1/4 | None | 11.4 | Pancreatitis, hepatitis B, DM |
| 6 | L. rhamnosus | M | 57 | 1/2 | Staphylococcus sp. | 5.5 | HIV, IVDA, hepatitis C, recurrent abscesses |
| 7 | L. rhamnosus | M | 57 | 8/8 | None | 10.9 | Crohn's disease |
| 8 | L. rhamnosus | M | 49 | 4/6 | α-Hemolytic streptococci, E. coli | 23.7 | Necrotizing pancreatitis |
| 9 | L. rhamnosus | M | 57 | 2/4 | C. albicans, Pseudomonas aeruginosa, Staphylococcus sp. | 9.8 | Total hip arthroplasty, infection of hardware |
| 10 | L. casei | M | 65 | 2/2 | None | NA | Pneumonectomy, lung cancer, sepsis |
Significance was determined by (i) the number of positive cultures, (ii) whether other organisms were present (polymicrobial or monomicrobial), and (iii) the patient's WBC count at the time of blood drawing.
DM, diabetes mellitus; HIV, human immunodeficiency virus; IVDA, intravenous drug abuse; NA, not applicable.
TABLE 4.
Lactobacillus spp. and respiratory associations
| Patient no. | Organism | Patient sex | Patient age (yr) | Specimen typea | Other significant organism(s) | Underlying disease(s)/condition(s)b | Assessment |
|---|---|---|---|---|---|---|---|
| 1 | L. fermentum | F | 50 | Bronchial biopsy | None, in broth only | Eosinophilic pneumonia, acute or chronic | Contaminant |
| 2 | L. fermentum (few) | M | 58 | Sputum | Aspergillus fumigatus | Aspergillus pneumonia, myelodysplastic syndrome with myeloproliferative disease | Probable contaminant |
| 3 | L. gasseri | M | 70 | Lung tissue | Staphylococcus epidermidis | S. epidermidis sepsis | Probable contaminant |
| 4 | L. gasseri (moderate) | M | 58 | Pleural fluid | Many α-hemolytic streptococci, not S. pneumoniae, many PMNs | IR drainage of empyema, Crohn's disease | Contributed to infection |
| 5 | L. acidophilus and L. johnsonii (few) | M | 54 | Lung tissue | Candida glabrata, Enterobacter cloacae, Corynebacterium sp. (all few) | Pulmonary infiltrates with no histological evidence of infection or neoplastic etiology, patient has non-Hodgkin's lymphoma and is planning to undergo BMT | Contamination, tissue was handled in a nonsterile manner in histology department before being cultured |
| 6 | L. rhamnosus | M | 74 | Pleural fluid | C. albicans, E. faecalis, S. epidermidis | Gastrectomy, surgical leak | Leak from upper GI tract surgical site |
| 7 | L. rhamnosus (many) | M | 87 | Endotracheal aspirate | Few mixed normal flora, many PMNs | Pneumonia, COPD, gastrostomy tube, new multilobar infiltrates concerning for aspiration vs HAP | Infection or colonization |
| 8 | L. rhamnosus (25,000 CFU/ml) | M | 80 | BAL fluid | Klebsiella oxytoca (5,000 CFU/ml) | Endotracheal tube, MRSA septicemia, died | Infection or colonization |
| 9 | L. rhamnosus | M | 60 | Pleural fluid | None | Esophageal adenocarcinoma, pleural effusions | Infection or colonization |
| 10 | L. fermentum and L. gasseri | M | 54 | Bronchial brush | None | Chronic liver disease | Unknown |
Specimens from respiratory sites included lung tissue, pleural fluid, endotracheal aspirate, sputum, and bronchoalveolar lavage (BAL) fluid specimens.
BMT, bone marrow transplantation; IR, interventional radiological; COPD, chronic obstructive pulmonary disease; HAP, hospital-acquired pneumonia; MRSA, methicillin-resistant S. aureus.
Four of 10 cases of bacteremia were polymicrobial (Table 3). L. rhamnosus was the most common species, often associated with an underlying disease that had allowed bloodstream access to lower gastrointestinal flora. The white blood cell (WBC) count was rarely elevated; of the four cases in which there was a single species and the WBC count was above 10.5 × 103 cells/mm3, L. rhamnosus accounted for 3 cases and L. paracasei for one case. Also, a pure culture of L. vaginalis was recovered from a 70-year-old man at autopsy (data not shown).
Table 4 outlines cases in which Lactobacillus spp. were isolated from respiratory specimens such as lung tissue, sputum, pleural fluid, endotracheal aspirate, and bronchoalveolar lavage (BAL) fluid. Most respiratory cultures were polymicrobial, some with other organisms of high pathogenicity. As shown in the last column in Table 4, the Lactobacillus isolate was considered a contaminant or colonizing organism for most of the patients. L. fermentum and L. rhamnosus were the two most common respiratory isolates. The two examples of a Lactobacillus strain probably causing disease as a single agent involved L. rhamnosus. For example, for patient 7, L. rhamnosus was recovered from an endotracheal aspirate as a predominant isolate and was seen on Gram stains with many inflammatory cells. Additionally, L. rhamnosus was associated with pneumonia in patient 9, who is described below.
L. rhamnosus was isolated from one patient from two sites (patient 4 in Table 3 and patient 9 in Table 4). The patient had undergone an esophagectomy to remove an adenocarcinoma 12 days previously. The patient presented with severe acute shortness of breath and demonstrated pleural effusions, and the gastric conduit was distended with retained food. A pleural fluid sample was sent for culture. Direct Gram staining demonstrated 4+ WBCs and a rare Gram-positive organism; 16S rRNA gene sequencing identified the only organism from the pleural fluid as L. rhamnosus. L. rhamnosus was also isolated from blood cultures.
Table 5 summarizes the organisms listed as ingredients of three probiotics and the organisms actually isolated. Of the 5 possible Lactobacillus spp. (L. casei, L. plantarum, L. acidophilus, Lactobacillus delbruekii subsp. bulgaricus, and L. paracasei) listed, we were able to isolate only L. paracasei. The kefir preparation claimed to contain L. casei, but we recovered only L. rhamnosus. Importantly, L. casei and L. rhamnosus are difficult to distinguish from each other, as they are similar by both 16S rRNA gene sequence analysis (Fig. 1) and phenotyping. We hypothesize that the strain used in the probiotic may actually be L. rhamnosus, which may have been incorrectly identified. The 16S rRNA gene sequences of some of the clinical L. rhamnosus isolates are identical to that of the probiotic isolate (Fig. 1).
TABLE 5.
Lactobacillus spp. and probiotic associations
| Probiotic | Ingredients listed | Isolated organisms |
|---|---|---|
| Kefir | Streptococcus lactis | L. rhamnosus |
| Streptococcus cremoris | Streptococcus thermophilus | |
| S. lactis subsp. diacetylactis | ||
| L. casei | ||
| L. plantarum | ||
| Saccharomyces fragilis | ||
| Leuconostoc cremoris | ||
| Yogurt | L. acidophilus | Bifidobacterium animalis |
| Lactobacillus delbruekii subsp. bulgaricus | S. thermophilus | |
| Bifidobacterium bifidum | ||
| Capsule | L. acidophilus | L. paracasei |
| L. delbruekii subsp. bulgaricus | S. thermophilus | |
| L. paracasei | ||
| Bifidobacterium lactis |
FIG 1.
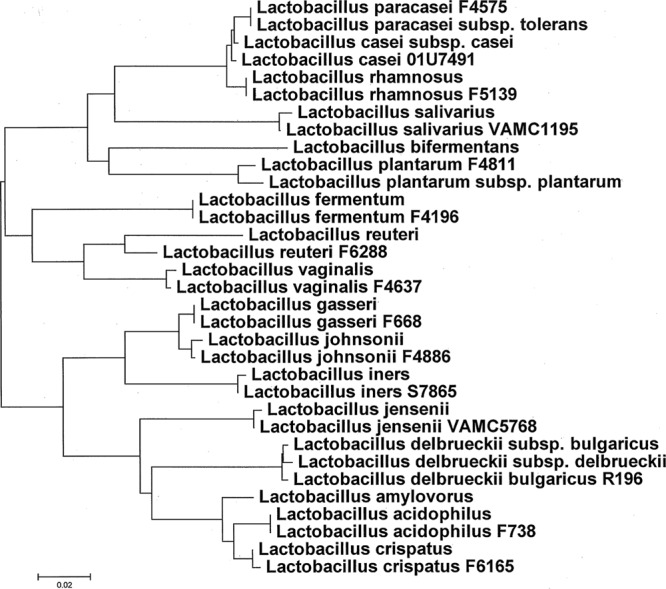
Dendrogram showing the relationships of the first ∼500 bp of the 16S rRNA gene sequences. Our strains are indicated with alphanumeric designations following their names; the other strains are type or reference strains and are listed in Table S1 in the supplemental material. The evolutionary distances were computed using the maximum composite likelihood method, and the units are the numbers of base substitutions per site (MEGA software, version 5.1 [http://www.megasoftware.net]).
DISCUSSION
In this study, we obtained isolates in the context of presumed pathogenic potential and found associations of Lactobacilli spp. with both the anatomical site and the clinical condition. Of the 16 species of lactobacilli identified, L. gasseri and L. rhamnosus were the most commonly isolated. Ten species could not be associated with disease. While L. rhamnosus, L. gasseri, and L. paracasei were associated with infections, L. gasseri was also a common colonizing/contaminating species. L. casei, L. johnsonii, and L. delbrueckii were associated with infections.
The problem of accurate identification of the Lactobacillus species affects the interpretation of their clinical significance; however, identification by reliable molecular methods such as 16S rRNA gene sequence analysis was not common until after 2005 (1, 24). In addition, changes in the taxonomy of Lactobacillus have expanded the number of species to more than 90 and changed the nomenclature for some species. For example, L. cantenaformis has been found to be more closely related to Erysipelothrix (24). The use of matrix-assisted laser desorption ionization–time of flight mass spectrometry for Lactobacillus identification holds promise (25, 26).
The problem of correct identification of isolates might have affected the large literature review of 200 cases, which included cases from as far back as 1950 (6). Misidentification of isolates is suggested by the high rate of vancomycin sensitivity and the frequent association with endocarditis, which is more commonly associated with viridians streptococci (which, when the cocci are elongated, can be confused with lactobacilli). None of the blood culture isolates in the present study was noted to be associated with endocarditis. The review by Cannon et al. (6) suggested that the two most common Lactobacillus species associated with disease were L. casei and L. rhamnosus. However, these species cannot be reliably distinguished by phenotypic methods (the methods used in most of the papers those authors reviewed) from each other (24). Similarly, L. acidophilus (their third most common strain) and L. gasseri are difficult to distinguish. It is thus possible that these organisms were misidentified and that, similar to what was observed in our study, L. rhamnosus and L. gasseri were more commonly isolated. The pathogenicity of L. rhamnosus has been confirmed by other reports (2, 7, 8, 27), particularly in association with respiratory infections (28). In addition, the Gram staining of lactobacilli from the original specimen can be confusing; several of our specimens were interpreted as Gram-positive cocci in chains and possible rods. For example, we initially considered several isolates to be Streptococcus based on the Gram staining, catalase test, and colony morphology results. However, 16S rRNA gene sequencing provided the correct identification.
Of 23 specimens from female patients, 22 isolates and six species (L. crispatus, L. gasseri, L. jensenii, L. iners, L. plantarum, and L. vaginalis) were obtained from UG sources and none was associated with disease. L. crispatus was the most common isolate, which confirms the site-specific propensity of L. crispatus and L. jensenii for the female UG tract. The most common lactobacilli in male urine have been reported as L. iners (29), whereas we found L. gasseri to be the predominant species isolated from male urine. Recent studies showed that the urine microbiota consists mainly of lactobacilli (29, 30); therefore, the organisms isolated from UG samples in this study likely represent commensal organisms. However, Lactobacillus has been associated with urinary infections in the literature (31) and was associated with a UTI with obstruction in one of our patients (L. rhamnosus in the urine of a male patient). A limitation of our study is that it was conducted within a primarily adult male population.
Our observed site-species relationship for lactobacilli suggests that probiotic efficacy may vary depending on the species and the specific niche targeted. For example, in order to reestablish normal vaginal flora, a preparation containing L. casei may not be appropriate. While our investigation of organisms isolated from probiotics obtained from a health food store and identified using 16S rRNA gene sequencing was limited, the isolation of L. rhamnosus, which was not listed as an ingredient in the probiotic preparation, is a significant finding highlighting the fact that, since there is no regulation of these products, the actual strains in commercial preparations may be different than claimed (15, 18).
This is the largest study, to our knowledge, involving Lactobacillus spp. identified by 16S rRNA gene sequencing in association with both the site of isolation and the clinical significance. Knowing the niche and pathogenic potential of each Lactobacillus sp. is of importance to both the field of clinical microbiology and the food industry.
Supplementary Material
ACKNOWLEDGMENT
We acknowledge the excellent support of personnel in the microbiology laboratory.
Footnotes
Published ahead of print 16 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02072-13.
REFERENCES
- 1.Wade WG, Kononen E, Dessein R, Armand S, Courcol RJ. 2011. Propionibacterium, Lactobacillus, Actinomyces, and other non-spore-forming anaerobic Gram-positive rods. In Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW. (ed), Manual of clinical microbiology, 10th ed. ASM Press, Washington, DC [Google Scholar]
- 2.Wallet F, Dessein R, Armand S, Courcol RJ. 2002. Molecular diagnosis of endocarditis due to Lactobacillus casei subsp. rhamnosus. Clin. Infect. Dis. 35:e117–e119. 10.1086/344181 [DOI] [PubMed] [Google Scholar]
- 3.Neef PA, Polenakovik H, Clarridge JE, Saklayen M, Bogard L, Bernstein JM. 2003. Lactobacillus paracasei continuous ambulatory peritoneal dialysis-related peritonitis and review of the literature. J. Clin. Microbiol. 41:2783–2784. 10.1128/JCM.41.6.2783-2784.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruoff KL, Kuritzkes DR, Wolfson JS, Ferraro MJ. 1988. Vancomycin-resistant Gram-positive bacteria isolated from human sources. J. Clin. Microbiol. 26:2064–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tommasi C, Equitani F, Masala M, Ballardini M, Favaro M, Meledandri M, Fontana C, Narciso P, Nicastri E. 2008. Diagnostic difficulties of Lactobacillus casei bacteraemia in immunocompetent patients: a case report. J. Med. Case Rep. 2:315. 10.1186/1752-1947-2-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon JP, Lee TA, Bolanos JT, Danziger LH. 2005. Pathogenic relevance of Lactobacillus: a retrospective review of over 200 cases. Eur. J. Clin. Microbiol. Infect. Dis. 24:31–40. 10.1007/s10096-004-1253-y [DOI] [PubMed] [Google Scholar]
- 7.Klein G, Zill E, Schindler R, Louwers J. 1998. Peritonitis associated with vancomycin-resistant Lactobacillus rhamnosus in a continuous ambulatory peritoneal dialysis patient: organism identification, antibiotic therapy, and case report. J. Clin. Microbiol. 36:1781–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salminen MK, Rautelin H, Tynkkynen S, Poussa T, Saxelin M, Valtonen V, Jarvinen A. 2006. Lactobacillus bacteremia, species identification, and antimicrobial susceptibility of 85 blood isolates. Clin. Infect. Dis. 42:e35–e44. 10.1086/500214 [DOI] [PubMed] [Google Scholar]
- 9.Cribby S, Taylor M, Reid G. 2008. Vaginal microbiota and the use of probiotics. Interdiscip. Perspect. Infect. Dis. 2008:256490. 10.1155/2008/256490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merk K, Borelli C, Korting HC. 2005. Lactobacilli: bacteria-host interactions with special regard to the urogenital tract. Int. J. Med. Microbiol. 295:9–18. 10.1016/j.ijmm.2004.11.006 [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan S, Fredricks DN. 2008. The human vaginal bacterial biota and bacterial vaginosis. Interdiscip. Perspect. Infect. Dis. 2008:750479. 10.1155/2008/750479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badet C, Thebaud NB. 2008. Ecology of lactobacilli in the oral cavity: a review of literature. Open Microbiol. J. 2:38–48. 10.2174/1874285800802010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verna EC, Lucak S. 2010. Use of probiotics in gastrointestinal disorders: what to recommend? Therap. Adv. Gastroenterol. 3:307–319. 10.1177/1756283X10373814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koll-Klais P, Mandar R, Leibur E, Marcotte H, Hammarstrom L, Mikelsaar M. 2005. Oral lactobacilli in chronic periodontitis and periodontal health: species composition and antimicrobial activity. Oral Microbiol. Immunol. 20:354–361. 10.1111/j.1399-302X.2005.00239.x [DOI] [PubMed] [Google Scholar]
- 15.Shortt C, Rautelin H, Tynkkynen S, Poussa T, Saxelin M, Valtonen V, Jarvinen A. 1999. The probiotic century: historical and current perspectives. Trends Food Sci. Technol. 10:411–417 [Google Scholar]
- 16.Szajewska H, Kotowska M, Mrukowicz JZ, Armanska M, Mikolajczyk W. 2001. Efficacy of Lactobacillus GG in prevention of nosocomial diarrhea in infants. J. Pediatr. 138:361–365. 10.1067/mpd.2001.111321 [DOI] [PubMed] [Google Scholar]
- 17.Johnston BC, Goldenberg JZ, Vandvik PO, Sun X, Guyatt GH. 2011. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev.(2):CD004827. 10.1002/14651858.CD004827.pub3 [DOI] [PubMed] [Google Scholar]
- 18.Alvarez-Olmos MI, Oberhelman RA. 2001. Probiotic agents and infectious diseases: a modern perspective on a traditional therapy. Clin. Infect. Dis. 32:1567–1576. 10.1086/320518 [DOI] [PubMed] [Google Scholar]
- 19.Clayton RA, Sutton G, Hinkle PSJ, Bult C, Fields C. 1995. Intraspecific variation in small-subunit rRNA sequences in GenBank: why single sequences may not adequately represent prokaryotic taxa. Int. J. Syst. Bacteriol. 45:595–599 [DOI] [PubMed] [Google Scholar]
- 20.Park KS, Ki CS, Kang CI, Kim YJ, Chung DR, Peck KR, Song JH, Lee NY. 2012. Evaluation of the GenBank, EzTaxon, and BIBI services for molecular identification of clinical blood culture isolates that were unidentifiable or misidentified by conventional methods. J. Clin. Microbiol. 50:1792–1795. 10.1128/JCM.00081-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jespers V, Menten J, Smet H, Poradosu S, Abdellati S, Verhelst R, Hardy L, Buve A, Crucitti T. 2012. Quantification of bacterial species of the vaginal microbiome in different groups of women, using nucleic acid amplification tests. BMC Microbiol. 12:83. 10.1186/1471-2180-12-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silvester ME, Dicks LM. 2003. Identification of lactic acid bacteria isolated from human vaginal secretions. Antonie Van Leeuwenhoek 83:117–123. 10.1023/A:1023373023115 [DOI] [PubMed] [Google Scholar]
- 24.Schleifer KH. 2009. Phylum XIII. Firmicutes Gibbons and Murray 1978, 5 (Firmacutes [sic] Gibbons and Murray 1978, 5), p 19–1317 In Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer K-H, Whitman WB.Bergey's manual of systematic bacteriology, 2nd ed, vol 3 Springer-Verlag, New York, NY. [Google Scholar]
- 25.Duskova M, Sedo O, Ksicova K, Zdrahal Z, Karpiskova R. 2012. Identification of lactobacilli isolated from food by genotypic methods and MALDI-TOF MS. Int. J. Food Microbiol. 159:107–114. 10.1016/j.ijfoodmicro.2012.07.029 [DOI] [PubMed] [Google Scholar]
- 26.McElvania Tekippe E, Shuey S, Winkler DW, Butler MA, Burnham CA. 2013. Optimizing identification of clinically relevant Gram-positive organisms by use of the Bruker Biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometry system. J. Clin. Microbiol. 51:1421–1427. 10.1128/JCM.02680-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robin F, Paillard C, Marchandin H, Demeocq F, Bonnet R, Hennequin C. 2010. Lactobacillus rhamnosus meningitis following recurrent episodes of bacteremia in a child undergoing allogeneic hematopoietic stem cell transplantation. J. Clin. Microbiol. 48:4317–4319. 10.1128/JCM.00250-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoji H, Yoshida K, Niki Y. 2010. Lung abscess and pleuritis caused by Lactobacillus rhamnosus in an immunocompetent patient. J. Infect. Chemother. 16:45–48 [DOI] [PubMed] [Google Scholar]
- 29.Nelson DE, Van Der Pol B, Dong Q, Revanna KV, Fan B, Easwaran S, Sodergren E, Weinstock GM, Diao L, Fortenberry JD. 2010. Characteristic male urine microbiomes associate with asymptomatic sexually transmitted infection. PLoS One 5:e14116. 10.1371/journal.pone.0014116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siddiqui H, Nederbragt AJ, Lagesen K, Jeansson SL, Jakobsen KS. 2011. Assessing diversity of the female urine microbiota by high throughput sequencing of 16S rDNA amplicons. BMC Microbiol. 11:244. 10.1186/1471-2180-11-244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickgiesser U, Weiss N, Fritsche D. 1984. Lactobacillus gasseri as the cause of septic urinary infection. Infection 12:14–16 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


