Abstract
Helcococcus kunzii was isolated from a brain abscess in a diabetic patient with cholesteatoma and demonstrated satellitism around Staphylococcus aureus in culture. This is the first reported case of severe central nervous system infection due to H. kunzii and the first description of a satelliting phenotypic variant of this organism.
CASE REPORT
An 83-year-old man was admitted with a worsening right earache and fever for 10 days. He had a background history of hypertension, diabetes mellitus, and prostate cancer and was on androgen blockade therapy. On admission, he had a temperature of 37.2°C and a fluctuating consciousness level. Otoscopic examination revealed an inflamed outer ear canal. Blood tests showed leukocytosis (13.93 × 109/liter) with neutrophilia (12.19 × 109/liter). Liver and renal function test results were unremarkable. Contrast-enhanced computed tomography scans of the head showed a right-sided cholesteatoma with abscess formation in the mastoid cavity. There was no definite evidence of intracranial extension. Excision of the cholesteatoma and mastoidectomy were performed. Histology showed the presence of keratinized squamous metaplasia consistent with the preoperative diagnosis of cholesteatoma. Cultures of pus swabs obtained from the mastoid abscess were negative for bacterial and fungal growth.
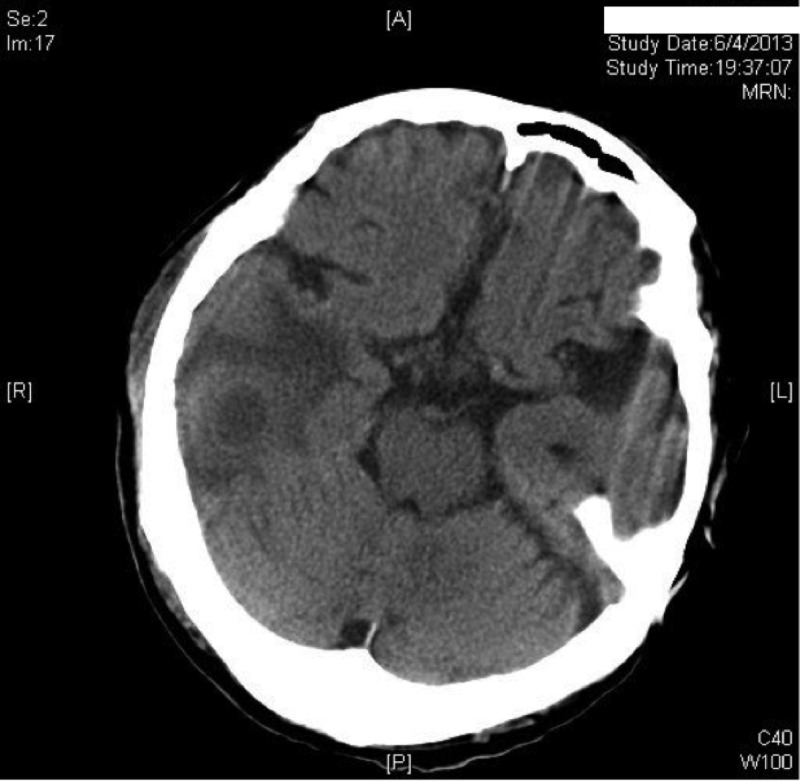
In view of the severe clinical infection with systemic upset, the head and neck surgeons in charge started the patient on intravenous ceftriaxone at 2 g every 12 h and intravenous metronidazole at 500 mg every 8 h for broad-spectrum coverage of Gram-negative pathogens, methicillin-sensitive Staphylococcus aureus, and anaerobes. Antipseudomonal coverage was to be added if the patient's condition did not improve. After 2 weeks of intravenous antibiotics, he showed clinical improvement and was switched to oral amoxicillin-clavulanate at 1 g twice daily. However, he developed generalized tonic-clonic convulsions and a high fever 3 weeks after the operation. Reassessment by computed tomography scans of the brain with contrast enhancement showed a right temporal brain abscess (Fig. 1). The abscess was drained surgically. Intravenous ceftriaxone at 2 g every 12 h and intravenous metronidazole at 500 mg every 8 h were restarted. In addition, intravenous vancomycin at 500 mg every 12 h (dose adjusted for estimated creatinine clearance) was commenced in view of breakthrough abscess formation despite previous broad-spectrum beta-lactam antibiotic use. Empirical local amikacin instillation was used to irrigate the abscess cavity through the abscess drain as described in the literature (1, 2). The patient's neurological status improved with no further seizure episodes. In view of the clinical improvement and the results of in vitro antimicrobial susceptibility testing of the bacterial isolate obtained from the brain abscess drainage at the second operation, he was kept on a regimen of ceftriaxone, vancomycin, and metronidazole. Local amikacin instillation through the abscess drain was discontinued. Subsequent computed tomography scans of the brain showed that the abscess had reduced in size. Three sets of blood cultures were negative after 5 days of incubation. The patient received a total of 12 weeks of antibiotic therapy postoperatively with complete resolution of abnormal biochemical parameters and radiological evidence of residual collections.
FIG 1.
Computed tomography scan of the brain with contrast showing a right temporal brain abscess.
Microbiology.
The Gram stain of the pus obtained from the brain abscess at the second operation showed Gram-positive cocci in chains and abundant polymorphs. The pus was inoculated into 5% horse blood agar, chocolate agar, and MacConkey agar under aerobic and anaerobic conditions, as well as hemin-vitamin K-supplemented blood agar under anaerobic conditions. The pus was also inoculated into aerobic and anaerobic blood culture broth (Bactec Plus Aerobic/F, Bactec Lytic/Anaerobic/F; Becton Dickinson) and incubated in the Bactec blood culture system (Becton Dickinson) to enhance the growth of fastidious organisms. The anaerobic blood culture broth turned positive after 20 h, but direct subculture of the broth to horse blood agar and chocolate agar under aerobic conditions and hemin-vitamin K-supplemented agar under anaerobic conditions showed no growth. In view of the possibility of nutritionally variant streptococci, the blood culture broth was also inoculated onto a horse blood agar plate streaked with a reference strain of S. aureus (ATCC 25923). Pinpoint, nonhemolytic colonies of Gram-positive bacteria demonstrating satellitism were observed the next day after incubation at 37°C in both room air and 5% CO2 (Fig. 2). The organism was catalase negative. Primary plates without S. aureus streaks remained negative for bacterial growth despite prolonged incubation under aerobic and anaerobic conditions. No other organisms were isolated. On subsequent subculture, the organism was able to grow on horse blood agar plates without S. aureus streaks. Older colonies (>72 h) were observed to be umbilicated with alpha-hemolysis on horse blood agar. The satelliting phenotype prompted an initial identification of Abiotropha defectiva/Granulicatella species, which are nutritionally variant species of Gram-positive cocci known for fastidious pyridoxal-dependent growth. However, using the Vitek 2 system (bioMérieux), an identity of Helcococcus kunzii was obtained with 99% probability. By matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Vitek MS [bioMérieux]), the isolate was also identified as H. kunzii at a confidence level of 99.9%.
FIG 2.
Blood agar plate showing satellitic pinpoint colonies of H. kunzii around an S. aureus ATCC 25923 streak.
16S rRNA gene sequencing.
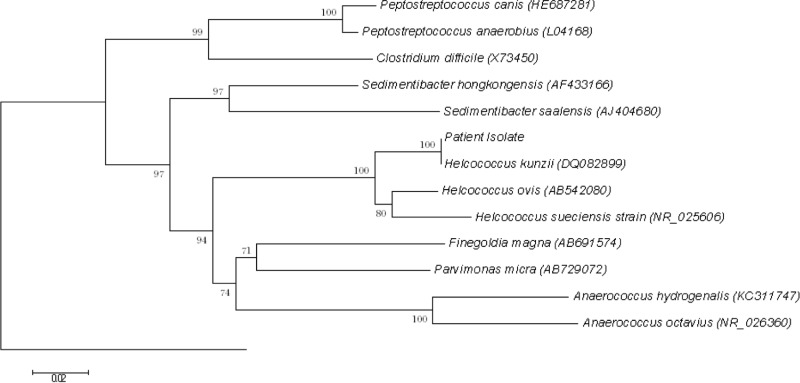
Bacterial DNA extraction, PCR amplification, and 16S rRNA gene sequencing were performed as described in our previous publications for other Gram-positive cocci (3, 4, 5, 6, 7, 8, 9, 10), with universal primers 1F (5′-AGTTTGATCMTGGCTCAG) and 2R (5′-GGACTACHAGGGTATCTAAT) as the PCR and sequencing primers. The sequences of the PCR products were compared with the sequences of closely related species in the GenBank database by multiple-sequence alignment with ClustalW (11). Phylogenetic relationships were determined by the neighbor-joining method. Sequencing of the 16S rRNA gene of the isolate showed that there was 1 (0.15%) base difference between the 16S rRNA gene sequence of the isolate and that of H. kunzii (GenBank accession no. DQ082899.1), 39 (5.69%) base differences between the 16S rRNA gene sequence of the isolate and that of Helcococcus ovis (GenBank accession no. AB542080.1), and 55 (7.99%) base differences between the 16S rRNA gene sequence of the isolate and that of Helcococcus sueciensis (GenBank accession no. NR025606.1), confirming that the isolate was H. kunzii (Fig. 3).
FIG 3.
Phylogenetic tree showing the relationships of the patient's isolate to related species. The tree was inferred from 16S rRNA data by the neighbor-joining method and rooted by using the 16S rRNA gene sequence of B. subtilis (accession no. AB065370). Bootstrap values were calculated from 1,000 trees. The scale bar indicates the estimated number of substitutions per 50 bases. Names and accession numbers are given as cited in the GenBank database.
Antimicrobial susceptibility testing.
Susceptibility to penicillin, ceftriaxone, and vancomycin was determined by the Etest assay (bioMérieux, Marcy l'Etoile, France) on blood Mueller-Hinton agar, and the results were expressed as susceptible, intermediate, or resistant according to the CLSI criteria for Gram-positive cocci (12). The MICs of penicillin, ceftriaxone, and vancomycin were 0.003 μg/ml (susceptible), 0.016 μg/ml (susceptible), and 0.25 μg/ml (susceptible), respectively. Disk diffusion testing on blood Mueller-Hinton agar was performed for amikacin and gentamicin with no discernible zone of inhibition, suggesting that the organism had reduced susceptibility to these aminoglycosides. This was confirmed by using the Etest (bioMérieux, France) method on blood Mueller-Hinton agar, which showed amikacin and gentamicin MICs of >256 and 32 μg/ml, respectively.
Discussion.
First described in 1993, H. kunzii is a catalase-negative Gram-positive coccus that has been recognized as a skin commensal, especially of the lower extremities (13, 14). It has been isolated from chronic venous ulcers, but its presence did not appear to be correlated with a poor healing outcome (15). Therefore, the significance of the isolation of this organism from clinical specimens is often uncertain, especially in cases where mixed cultures are obtained (16). However, the role of H. kunzii as an occasional monomicrobial opportunistic pathogen in immunocompetent patients has been firmly established in skin and soft tissue infections, including foot abscesses (17), breast abscesses (18), infected sebaceous cysts (19), and pacemaker pocket infections (20). Similar to other skin flora, H. kunzii has been implicated in chronic osteomyelitis and prosthetic joint infections (16, 21). Invasive and life-threatening infections due to H. kunzii are rarely reported. We have previously described two such cases in intravenous drug users where the organism resulted in bacteremia and empyema thoracis, respectively (22). One of the isolates demonstrated ermA gene-mediated erythromycin and clindamycin resistance. Our case is the first description of a monomicrobial brain abscess due to H. kunzii and further illustrates the pathogenic potential of this organism. The diabetic patient, with an extensive cholesteatoma and mastoiditis, was particularly vulnerable to invasive central nervous system abscess formation by colonizing skin flora. Following the initial cholesteatoma excision, there was breakthrough progression of infection by this susceptible organism while the patient was on ceftriaxone, an agent to which the isolate demonstrated in vitro susceptibility. This was likely due to poor penetration of the sizable abscess cavity by antibiotics. Thorough surgical drainage and prolonged administration of effective antibiotics ensured a good clinical outcome for this patient.
A number of interesting laboratory findings were illustrated in our case. First, the isolate demonstrated fastidious growth requirements and satellitism initially. Such a phenotype has never been described among isolates of H. kunzii in the literature before. However, a related species, H. ovis, is known to exhibit satellitism around S. aureus, as well as pyridoxal dependence, in a manner similar to that of so-called nutritionally variant streptococci (23). This phenomenon is lost when H. ovis is subcultured. H. ovis is an infrequent cause of pneumonia, lung abscesses, and infective endocarditis among ruminants (23, 24, 25) but has never been reported to cause human disease. Thus, satellitism and pyridoxal dependence may be phenomena that are demonstrated by organisms of the Helcococcus genus in nutritionally abundant environmental milieus such as areas of necrosis with extensive tissue destruction, as in this patient with an erosive cholesteatoma. Nutritionally deficient bacteria are significant pathogens that are often difficult to grow and identify in the clinical microbiology laboratory. Pyridoxal-dependent organisms such as Abiotropha defectiva and Granulicatella species are well-known examples of auxotrophism (26). Commonly encountered pathogens may also demonstrate growth factor dependence, resulting in unusual phenotypes; examples include S. aureus (small-colony variants with hemin and menadione dependence) (27) and Escherichia coli (cysteine dependence) (28). This illustrates the importance of having a high clinical suspicion of auxotrophic organisms, especially when organisms identified on Gram staining fail to grow on routine agar media within 24 h of inoculation. Inoculation of specimens from sterile sites into blood culture broth and S. aureus streak plates in selected circumstances may be useful. As this practice is not routine in many microbiology laboratories, we speculate that some infections due to fastidious isolates of H. kunzii may be missed, which underestimates the true incidence of disease caused by this skin commensal. Second, the API system (API20 STREP; bioMérieux) frequently yields a numerical profile that corresponds to a doubtful identification of Aerococcus viridans in the API database. The Vitek 2 system (bioMérieux) used in this case yielded an unambiguous identification of H. kunzii. 16S rRNA gene sequencing and MALDI-TOF are important in the identification of fastidious organisms with misleading phenotypes. Formal evaluations of the performance of MALDI-TOF with nonstreptococcal, nonenterococcal, catalase-negative, Gram-positive cocci are still forthcoming. However, expanding databases improve the ability of this technique to identify such infrequently encountered organisms rapidly and with reasonable confidence.
The isolate in our case demonstrated resistance to the aminoglycosides amikacin and gentamicin. To the best of our knowledge, this is the first documented case of resistance to aminoglycosides in a member of the Helcococcus genus. We postulate that such resistance may be due to the expression of aminoglycoside-modifying enzymes by this organism. Further study is required to determine the prevalence of such resistance among clinical isolates of H. kunzii.
In summary, our case shows that H. kunzii, a skin colonizer, is able to cause severe central nervous system infection and may exhibit fastidious growth, rendering diagnosis difficult. A battery of phenotypic kits, molecular techniques, and MS remains the optimal approach to the identification of H. kunzii in the clinical laboratory, especially in cases where an unusual phenotype is encountered.
Nucleotide sequence accession number.
The 16S rRNA gene sequence of the isolate described here has been deposited in the GenBank sequence database under accession number KF663569.
ACKNOWLEDGMENTS
This work was partly supported by the University Development Fund and the Committee for Research and Conference Grant, The University of Hong Kong.
Footnotes
Published ahead of print 30 October 2013
REFERENCES
- 1.Broggi G, Franzini A, Peluchetti D, Servello D. 1985. Treatment of deep brain abscesses by stereotactic implantation of an intracavitary device for evacuation and local application of antibiotics. Acta Neurochir. (Wien) 76:94–98. 10.1007/BF01418467 [DOI] [PubMed] [Google Scholar]
- 2.Radoi M, Ciubotaru V, Tataranu L. 2013. Brain abscesses: clinical experience and outcome of 52 consecutive cases. Chirurgia (Bucur.) 108:215–225 http://revistachirurgia.ro/pdfs/2013-2-215.pdf [PubMed] [Google Scholar]
- 3.To KK, Cheng VC, Chan JF, Wong AC, Chau S, Tsang FH, Curreem SO, Lau SK, Yuen KY, Woo PC. 2011. Molecular characterization of a catalase-negative Staphylococcus aureus subsp. aureus strain collected from a patient with mitral valve endocarditis and pericarditis revealed a novel nonsense mutation in the katA gene. J. Clin. Microbiol. 49:3398–3402. 10.1128/JCM.00849-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan JF, Lau SK, Curreem SO, To KK, Leung SS, Cheng VC, Yuen KY, Woo PC. 2012. First report of spontaneous intrapartum Atopobium vaginae bacteremia. J. Clin. Microbiol. 50:2525–2528. 10.1128/JCM.00212-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan JF, Woo PC, Teng JL, Lau SK, Leung SS, Tam FC, Yuen KY. 2011. Primary infective spondylodiscitis caused by Lactococcus garvieae and a review of human L. garvieae infections. Infection 39:259–264. 10.1007/s15010-011-0094-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan JF, Wong SS, Leung SS, Fan RY, Ngan AH, To KK, Lau SK, Yuen KY, Woo PC. 2012. First report of chronic implant-related septic arthritis and osteomyelitis due to Kytococcus schroeteri and a review of human K. schroeteri infections. Infection 40:567–573. 10.1007/s15010-012-0250-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo PC, Tam DM, Leung KW, Lau SK, Teng JL, Wong MK, Yuen KY. 2002. Streptococcus sinensis sp. nov., a novel species isolated from a patient with infective endocarditis. J. Clin. Microbiol. 40:805–810. 10.1128/JCM.40.3.805-810.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo PC, Tse H, Chan KM, Lau SK, Fung AM, Yip KT, Tam DM, Ng KH, Que TL, Yuen KY. 2004. “Streptococcus milleri” endocarditis caused by Streptococcus anginosus. Diagn. Microbiol. Infect. Dis. 48:81–88. 10.1016/j.diagmicrobio.2003.09.011 [DOI] [PubMed] [Google Scholar]
- 9.Woo PC, Fung AM, Lau SK, Wong SS, Yuen KY. 2001. Group G beta-hemolytic streptococcal bacteremia characterized by 16S ribosomal RNA gene sequencing. J. Clin. Microbiol. 39:3147–3155. 10.1128/JCM.39.9.3147-3155.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo PC, Tam DM, Lau SK, Fung AM, Yuen KY. 2004. Enterococcus cecorum empyema thoracis successfully treated with cefotaxime. J. Clin. Microbiol. 42:919–922. 10.1128/JCM.42.2.919-922.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson JD, Higgins DG, Gibson TJ. 1994. ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680. 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute 2013. Performance standards for antimicrobial susceptibility testing: twenty-third informational supplement. M100-S23 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 13.Collins MD, Facklam RR, Rodrigues UM, Ruoff KL. 1993. Phylogenetic analysis of some Aerococcus-like organisms from clinical sources: description of Helcococcus kunzii gen. nov., sp. nov. Int. J. Syst. Bacteriol. 43:425–429. 10.1099/00207713-43-3-425 [DOI] [PubMed] [Google Scholar]
- 14.Haas J, Jernick SL, Scardina RJ, Teruya J, Caliendo AM, Ruoff KL. 1997. Colonization of skin by Helcococcus kunzii. J. Clin. Microbiol. 35:2759–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore K, Hall V, Paull A, Morris T, Brown S, McCulloch D, Richardson MC, Harding KG. 2010. Surface bacteriology of venous leg ulcers and healing outcome. J. Clin. Pathol. 63:830–834. 10.1136/jcp.2010.077032 [DOI] [PubMed] [Google Scholar]
- 16.Stanger KM, Albert F, Kneser U, Bogdan C, Horch R. 16 July 2013. Management of chronic osteomyelitis of the tibia with life-threatening complications under negative pressure wound therapy and isolation of Helcococcus kunzii. Int. Wound J. (Epub ahead of print.) 10.1111/iwj.12133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riegel P, Lepargneur JP. 2003. Isolation of Helcococcus kunzii from a post-surgical foot abscess. Int. J. Med. Microbiol. 293:437–439. 10.1078/1438-4221-00284 [DOI] [PubMed] [Google Scholar]
- 18.Chagla AH, Borczyk AA, Facklam RR, Lovgren M. 1998. Breast abscess associated with Helcococcus kunzii. J. Clin. Microbiol. 36:2377–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peel MM, Davis JM, Griffin KJ, Freedman DL. 1997. Helcococcus kunzii as sole isolate from an infected sebaceous cyst. J. Clin. Microbiol. 35:328–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNicholas S, McAdam B, Flynn M, Humphreys H. 2011. The challenges of implantable cardiac device infection due to Helcococcus kunzii. J. Hosp. Infect. 78:337–338. 10.1016/j.jhin.2011.04.010 [DOI] [PubMed] [Google Scholar]
- 21.Pérez-Jorge C, Cordero J, Marin M, Esteban J. 2012. Prosthetic joint infection caused by Helcococcus kunzii. J. Clin. Microbiol. 50:528–530. 10.1128/JCM.01244-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woo PC, Tse H, Wong SS, Tse CW, Fung AM, Tam DM, Lau SK, Yuen KY. 2005. Life-threatening invasive Helcococcus kunzii infections in intravenous-drug users and ermA-mediated erythromycin resistance. J. Clin. Microbiol. 43:6205–6208. 10.1128/JCM.43.12.6205-6208.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kutzer P, Schulze C, Engelhardt A, Wieler LH, Nordhoff M. 2008. Helcococcus ovis, an emerging pathogen in bovine valvular endocarditis. J. Clin. Microbiol. 46:3291–3295. 10.1128/JCM.00867-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García A, Risco D, Benítez JM, Martínez R, García WL, Cuesta JM, Gómez L, Sánchez S. 2012. Helcococcus ovis isolated from a goat with purulent bronchopneumonia and pulmonary abscesses. J. Vet. Diagn. Invest. 24:235–237. 10.1177/1040638711425950 [DOI] [PubMed] [Google Scholar]
- 25.Rothschild CM, Oaks JL, Schaupp JK, Rurangirwa FR, Sellon DC, Hines MT. 2004. Helcococcus ovis isolated from a pulmonary abscess in a horse. J. Clin. Microbiol. 42:2224–2226. 10.1128/JCM.42.5.2224-2226.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woo PC, Fung AM, Lau SK, Chan BY, Chiu SK, Teng JL, Que TL, Yung RW, Yuen KY. 2003. Granulicatella adiacens and Abiotrophia defectiva bacteremia characterized by 16S rRNA sequencing. J. Med. Microbiol. 52:137–140. 10.1099/jmm.0.04950-0 [DOI] [PubMed] [Google Scholar]
- 27.von Eiff C, Peters G, Becker K. 2006. The small colony variant (SCV) concept—the role of staphylococcal SCVs in persistent infections. Injury 37:S26–33. 10.1016/j.injury.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 28.Yuen KY, Seto WH, Tsui KH, Hui WT. 1990. Septicemia caused by cysteine-dependent Escherichia coli. J. Clin. Microbiol. 28:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]