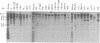Abstract
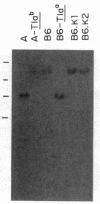
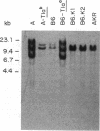
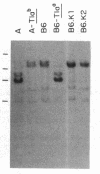
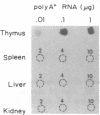
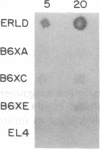
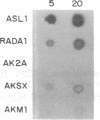
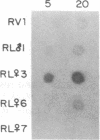
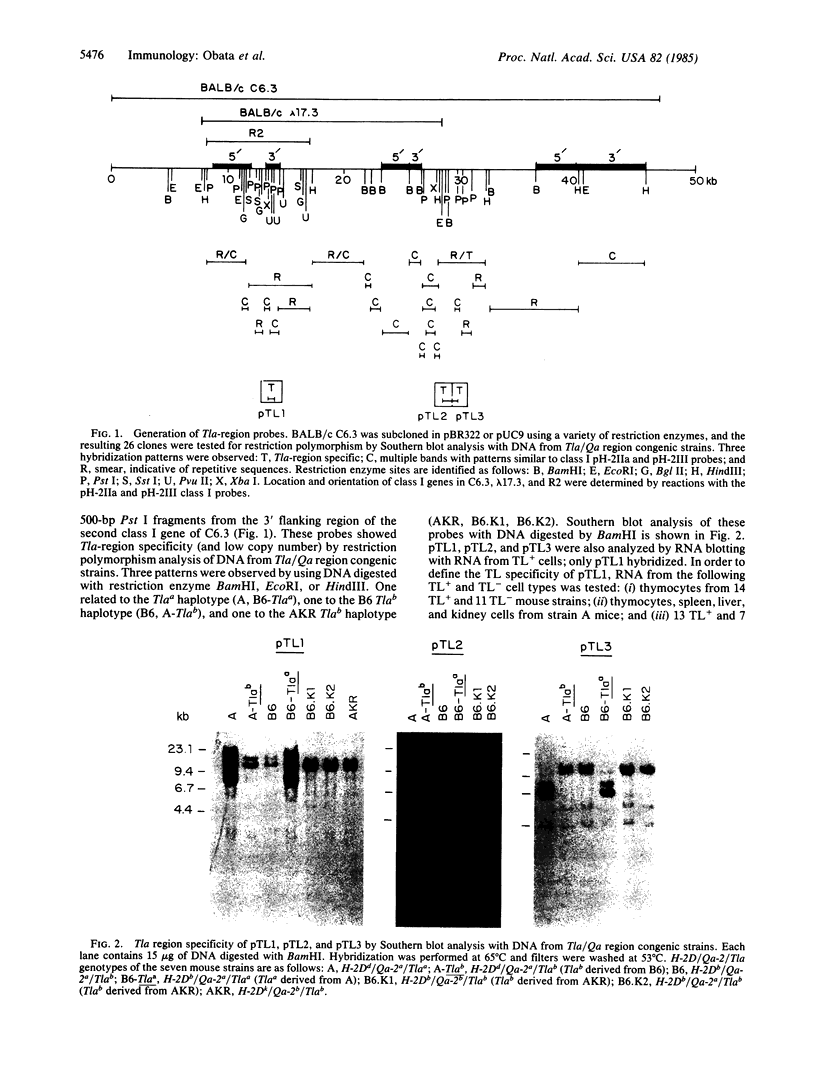
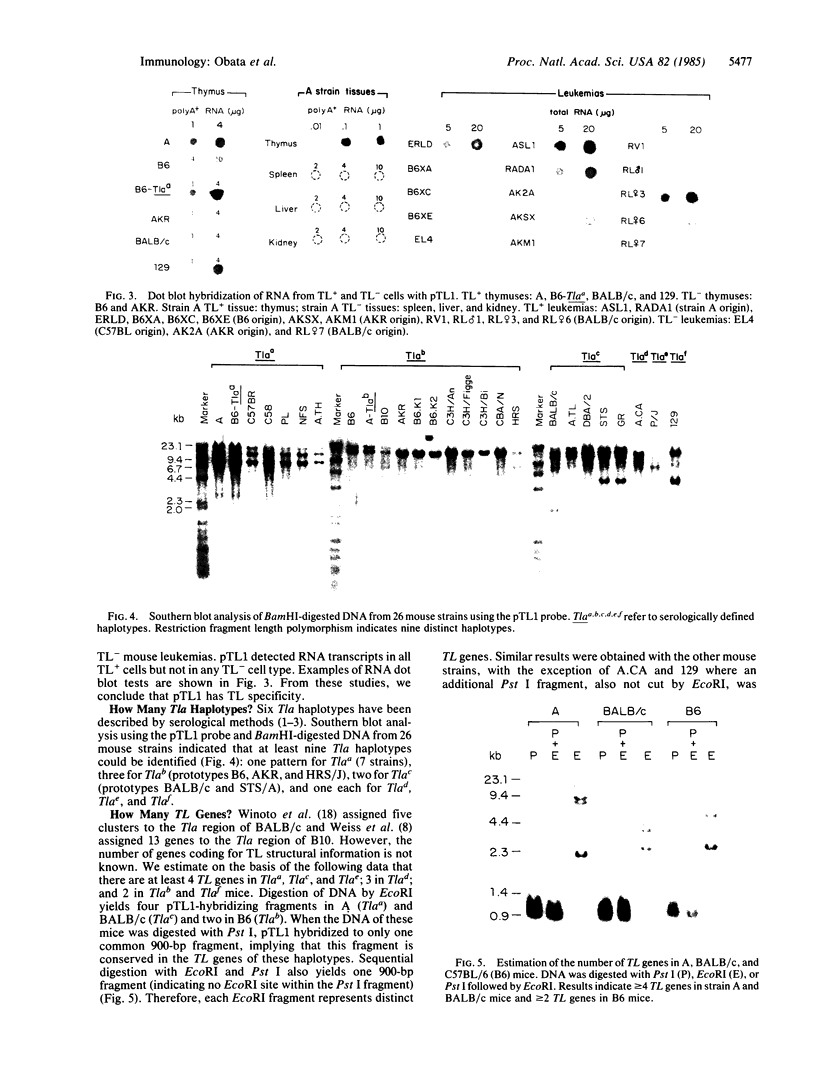
Three Tla region-specific probes have been generated from the BALB/c genomic cosmid clone C6.3. One probe, pTL1, corresponds to 3' sequences of a thymus leukemia (TL)-encoding gene, whereas pTL2 and pTL3 detect noncoding flanking sequences. The TL specificity of pTL1 was demonstrated by studies of RNA from thymocytes of TL+ and TL- mouse strains and from TL+ and TL- leukemias; presence/absence of pTL1+ transcripts correlated with presence/absence of TL antigens detected serologically. Nine Tla haplotypes were defined by restriction fragment polymorphism with pTL1, and the number of TL genes has been estimated to be greater than or equal to 4 in Tlaa, Tlac and Tlae mice, greater than or equal to 3 in Tlad mice, and greater than or equal to 2 in Tlab and Tlaf mice. A TL-encoding gene (C25.1) from the C57BL/6TL+ leukemia ERLD has been cloned and sequenced, and the exon/intron organization of C25.1 has been deduced from the structure of pTL1+ cDNA clones and from the known organization of H-2 genes. The major structural differences between TL and H-2 genes are in exons coding for the cytoplasmic domain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- Flaherty L., Rinchik E. A new allele and antigen at the Tla locus. Immunogenetics. 1980;11(2):205–208. doi: 10.1007/BF01567786. [DOI] [PubMed] [Google Scholar]
- Goodenow R. S., McMillan M., Nicolson M., Sher B. T., Eakle K., Davidson N., Hood L. Identification of the class I genes of the mouse major histocompatibility complex by DNA-mediated gene transfer. Nature. 1982 Nov 18;300(5889):231–237. doi: 10.1038/300231a0. [DOI] [PubMed] [Google Scholar]
- Hood L., Steinmetz M., Malissen B. Genes of the major histocompatibility complex of the mouse. Annu Rev Immunol. 1983;1:529–568. doi: 10.1146/annurev.iy.01.040183.002525. [DOI] [PubMed] [Google Scholar]
- Krayev A. S., Kramerov D. A., Skryabin K. G., Ryskov A. P., Bayev A. A., Georgiev G. P. The nucleotide sequence of the ubiquitous repetitive DNA sequence B1 complementary to the most abundant class of mouse fold-back RNA. Nucleic Acids Res. 1980 Mar 25;8(6):1201–1215. doi: 10.1093/nar/8.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krayev A. S., Markusheva T. V., Kramerov D. A., Ryskov A. P., Skryabin K. G., Bayev A. A., Georgiev G. P. Ubiquitous transposon-like repeats B1 and B2 of the mouse genome: B2 sequencing. Nucleic Acids Res. 1982 Dec 11;10(23):7461–7475. doi: 10.1093/nar/10.23.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund T., Grosveld F. G., Flavell R. A. Isolation of transforming DNA by cosmid rescue. Proc Natl Acad Sci U S A. 1982 Jan;79(2):520–524. doi: 10.1073/pnas.79.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre K. R., Hämmerling U., Uhr J. W., Vitetta E. S. Structural analysis of thymus-leukemia (TL) antigens in the mouse. J Immunol. 1982 Apr;128(4):1712–1717. [PubMed] [Google Scholar]
- Nathenson S. G., Uehara H., Ewenstein B. M., Kindt T. J., Coligan J. E. Primary structural: analysis of the transplantation antigens of the murine H-2 major histocompatibility complex. Annu Rev Biochem. 1981;50:1025–1052. doi: 10.1146/annurev.bi.50.070181.005113. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- OLD L. J., BOYSE E. A. ANTIGENIC PROPERTIES OF EXPERIMENTAL LEUKEMIAS. I. SEROLOGICAL STUDIES IN VITRO WITH SPONTANEOUS AND RADIATION-INDUCED LEUKEMIAS. J Natl Cancer Inst. 1963 Oct;31:977–995. [PubMed] [Google Scholar]
- Old L. J., Stockert E. Immunogenetics of cell surface antigens of mouse leukemia. Annu Rev Genet. 1977;11:127–160. doi: 10.1146/annurev.ge.11.120177.001015. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F. W., Chorney M. J., Boyse E. A. Further polymorphism of the Tla locus defined by monoclonal TL antibodies. Immunogenetics. 1982;15(6):573–578. doi: 10.1007/BF00347051. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Frelinger J. G., Fisher D., Hunkapiller T., Pereira D., Weissman S. M., Uehara H., Nathenson S., Hood L. Three cDNA clones encoding mouse transplantation antigens: homology to immunoglobulin genes. Cell. 1981 Apr;24(1):125–134. doi: 10.1016/0092-8674(81)90508-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Winoto A., Minard K., Hood L. Clusters of genes encoding mouse transplantation antigens. Cell. 1982 Mar;28(3):489–498. doi: 10.1016/0092-8674(82)90203-3. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. H., Golden L., Fahrner K., Mellor A. L., Devlin J. J., Bullman H., Tiddens H., Bud H., Flavell R. A. Organization and evolution of the class I gene family in the major histocompatibility complex of the C57BL/10 mouse. Nature. 1984 Aug 23;310(5979):650–655. doi: 10.1038/310650a0. [DOI] [PubMed] [Google Scholar]
- Weiss E., Golden L., Zakut R., Mellor A., Fahrner K., Kvist S., Flavell R. A. The DNA sequence of the H-2kb gene: evidence for gene conversion as a mechanism for the generation of polymorphism in histocompatibilty antigens. EMBO J. 1983;2(3):453–462. doi: 10.1002/j.1460-2075.1983.tb01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Winoto A., Steinmetz M., Hood L. Genetic mapping in the major histocompatibility complex by restriction enzyme site polymorphisms: most mouse class I genes map to the Tla complex. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3425–3429. doi: 10.1073/pnas.80.11.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K., Stockert E., Old L. J., Nathenson S. G. Structural evidence that the small subunit found associated with the TL antigen is beta 2-microglobulin. Immunogenetics. 1982;15(6):543–549. doi: 10.1007/BF00347048. [DOI] [PubMed] [Google Scholar]