Abstract
The seroprevalence of the recently discovered human Malawi polyomavirus (MWPyV) was determined by virus-like particle-based enzyme-linked immunosorbent assay (ELISA) in age-stratified Italian subjects. The findings indicated that MWPyV infection occurs early in life, and seroprevalence was shown to reach 42% in adulthood.
TEXT
Twelve human polyomaviruses have been described to date, including JC and BK polyomaviruses (JCPyV and BKPyV), Karolinska Institute polyomavirus (KIPyV), Washington University polyomavirus (WUPyV), Merkel cell polyomavirus (MCPyV), human polyomaviruses 6 and 7 (HPyV6 and HPyV7), trichodysplasia spinulosa-associated polyomavirus (TSPyV), human polyomavirus 9 (HPyV9) (1), and three recently identified polyomaviruses, i.e., Malawi polyomavirus/human polyomavirus 10 (MWPyV/HPyV10), Saint Louis polyomavirus (STLPyV) isolated from stools (2–4), and human polyomavirus 12 (HPyV12) identified in the liver (5).
Polyomavirus infections are ubiquitous, with seroprevalence ranging from 40 to 90% in adults, and asymptomatic infections occur early in childhood (6–12).
Four human polyomaviruses are associated with disease. JCPyV causes progressive multifocal leukoencephalopathy, BKPyV is the cause of hemorrhagic cystitis in allogeneic hematopoietic stem cell transplant patients and induced nephropathy frequently followed by graft loss in renal transplant patients. MCPyV is responsible for Merkel cell carcinoma (MCC), which occurs mainly in elderly individuals and immunocompromised subjects, and TSPyV is associated with trichodysplasia spinulosa. JCPyV-, BKPyV-, and TSPyV-associated diseases have been observed only in immunocompromised subjects (1, 13).
In this study, we investigated the seroepidemiology of the recently identified Malawi polyomavirus in an Italian population using a virus-like particle (VLP)-based enzyme-linked immunosorbent assay (ELISA) and evaluated the existence of cross-reactivity with MCPyV, HPyV6, HPyV7, TSPyV, and HPyV9 polyomaviruses.
MWPyV seroprevalence was determined in 825 Italian subjects 1 to 100 years of age. This study population has previously been investigated for reactivity against five other polyomaviruses (12), and the approach here was similar. Antibodies against MWPyV were determined using a VLP-based ELISA (14). The MWPyV VP1 coding sequence (GenBank accession no. NC_018102.1) was codon optimized for expression in Spodoptera frugiperda cells (Genscript, Piscataway, NJ) and used to generate a recombinant baculovirus. The presence of 45- to 50-nm VLPs and smaller particles was observed by electron microscopy (Fig. 1). Purified MWPyV VLPs (100 ng/well in phosphate-buffered saline [PBS]) were used to sensitize microplates (Maxisorp; Nunc) overnight at 4°C. Briefly, sera were diluted 1:100, and peroxidase-conjugated anti-human IgG (Southern Biotech, Clinisciences, Nanterre, France) diluted 1:20,000 was used to detect human IgG binding (14). A histogram of the ELISA optical density (OD) values in 1- to 10-year-old children (data not shown) revealed a bimodal distribution of seroreactivity. The cutoff point for MWPyV positivity was therefore set at 0.199 (mean of the lowest distribution of OD values plus 2 standard deviations).
FIG 1.
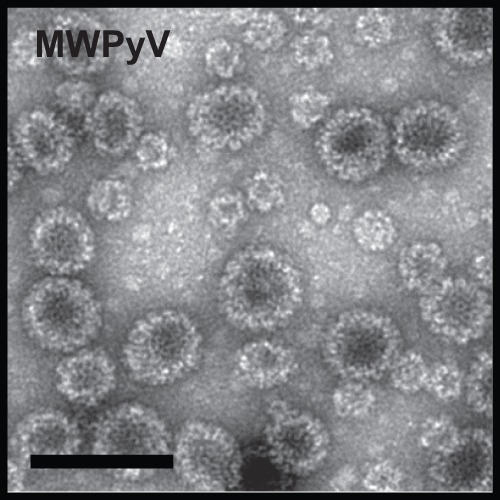
Electron microscopy of Malawi polyomavirus VP1 VLPs produced in insect cells (scale bar,100 nm).
Cross-reactivity between MWPyV and MCPyV, HPyV6, HPyV7, HPyV9, and TSPyV was evaluated by determining the Spearman coefficient correlation (RS) between OD values for MWPyV and OD values for the five other polyomaviruses (XLStat software; Addinsoft, Paris, France). The results clearly indicated the absence of cross-reactivity between MWPyV and MCPyV (RS = 0.012, P = 0.002), HPyV6 (RS = 3.10−5, P = 0.871), HPyV7 (RS = 6.10−5, P = 0.828), HPyV9 (RS = 0.024, P < 1.10−4), and TSPyV (RS = 0.027, P < 1.10−4).
Figure 2 summarizes MWPyV age-specific seroprevalence. MWPyV antibodies were detected in 26.9% of 1- to 2-year-old children and then increased to 68.2% in the 3- to 4-year-old age group. After this peak, seroprevalence slowly decreased with age to 31.5% in the 30- to 39-year-old age group. The mean seroprevalence was 41.8% and was relatively stable in adulthood (>20 years) through to old age (range, 31.5% to 47.3%). No difference in MWPyV seroprevalence according to gender was observed (data not shown).
FIG 2.
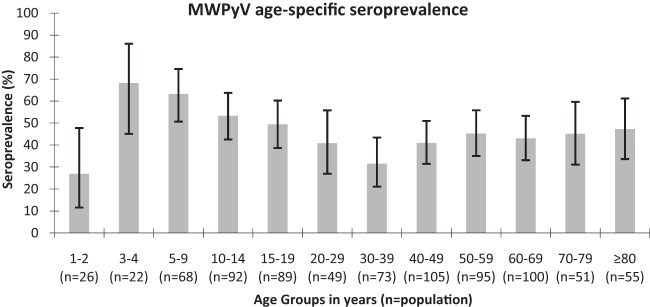
Age-specific seroprevalence of Malawi polyomavirus (error bars represent 95% CI).
In order to investigate variations in antibody level according to age, as observed for other polyomaviruses (12), samples were considered as having high levels of antibodies when the OD value was greater than that for the third quartile of seropositive samples (OD = 0.776). Age- and sex-adjusted odds ratio estimates (OR*) with 95% confidence intervals were calculated (XLStat software) to assess the association between high reactivity, gender, and age (Table 1). High reactivity was not associated with gender (OR* = 1.175, P = 0.514). However, high reactivity was negatively associated with age, since the percentage of high OD values decreased significantly with age from 38.5% in 1- to 9-year-old children to 18.6% in the 20- to 39-year-old adult group (OR* = 0.325, P = 0.018). Moderate variations in the percentage of high-level reactivity were observed after 20 years of age, since high OD values were detected in 19.8% and 22.8% of 40- to 59-year-olds and of those ≥60 years old, respectively.
TABLE 1.
Levels of Malawi polyomavirus reactivity according to age and gender in seropositive subjectsa
| Parameter | No. of subjects with high antibody levels/no. of antibody-positive subjects (%) | OR* (95% CI)b | P value |
|---|---|---|---|
| Gender | |||
| Female | 54/229 (23.6) | 1 | |
| Male | 42/150 (28.0) | 1.175 (0.725–1.904) | 0.514 |
| Age | |||
| 1–9 yr | 25/65 (38.5) | 1 | |
| 10–19 yr | 25/93 (26.9) | 0.610 (0.306–1.217) | 0.161 |
| 20–39 yr | 8/43 (18.6) | 0.325 (0.124–0.854) | 0.018 |
| 40–59 yr | 17/86 (19.8) | 0.400 (0.192–0.833) | 0.013 |
| ≥60 yr | 21/92 (22.8) | 0.480 (0.238–0.966) | 0.039 |
OR*, adjusted OR (adjusted for age and gender). The third quartile of OD values of seropositive samples (0.776) was used as the cutoff for high levels of antibodies.
Boldface values indicate significant OR* at P ≤ 0.05.
MWPyV infection is relatively common, since a seroprevalence of 41.8% was observed in adulthood. This seroprevalence was similar to the seroprevalence of 39.4% reported for HPyV9 (12) but lower than the seroprevalence reported for MCPyV, HPyV6, HPyV7, and TSPyV (63.6% to 87.0%) (12). The relatively low seroprevalence observed for MWPyV correlates with the detection of its DNA in only 2.3% of human stools (2), similar to levels observed for HPyV9, for which seroprevalence and DNA prevalence are low (12, 15, 16).
MWPyV seroconversion occurs early in life, as observed with other polyomaviruses (8, 12), since 26.9% of 1- to 2-year-old children were seropositive, followed by a seroprevalence peak of 68.2% in 2- to 4-year-old children.
The OD value at a 1:100 dilution of the sera can be used as a surrogate for antibody titer, and it has been shown that titers increase with age for MCPyV and decrease with age for TSPyV (12). A decrease in the percentage of high reactivity with age was also observed for MWPyV, suggesting that MWPyV clearance occurs or that there is an absence of reactivation with age, as reported for TSPyV (12).
Correlation analysis suggested an absence of cross-reactivity between MWPyV and the five human polyomaviruses tested. Nevertheless, the recently discovered Saint Louis polyomavirus (4) is closely-related to MWPyV and further studies are needed to investigate any cross-reactivity between these two polyomaviruses.
In this study, we determined that MWPyV seroprevalence was 41.8% in our adult population and that infection occurred early in life, as observed for other human polyomaviruses. Further studies are required to elucidate the transmission mode of Malawi polyomavirus in humans and its potential involvement in human diseases and cancers in both immunocompetent and immunocompromised patients.
ACKNOWLEDGMENTS
We thank Pierre-Yves Sizaret for help with electron microscopy of VLPs.
J.T.J.N. was supported by a doctoral grant from the Région Centre, France, and E.M. is a postdoctoral fellow of the Veronesi Foundation(Milan, Italy) at the University of Ferrara, Ferrara, Italy. This study was also funded by grants to P.C. from the Ligue Contre le Cancer (Comité du Cher and Comité de l'Indre; 2011) and to M.T. from the University of Ferrara.
We have no conflicts of interest.
Footnotes
Published ahead of print 30 October 2013
REFERENCES
- 1.Feltkamp MCW, Kazem S, van der Meijden E, Lauber C, Gorbalenya AE. 2013. From Stockholm to Malawi: recent developments in studying human polyomaviruses. J. Gen. Virol. 94:482–496. 10.1099/vir.0.048462-0 [DOI] [PubMed] [Google Scholar]
- 2.Siebrasse EA, Reyes A, Lim ES, Zhao G, Mkakosya RS, Manary MJ, Gordon JI, Wang D. 2012. Identification of MW polyomavirus, a novel polyomavirus in human stool. J. Virol. 86:10321–10326. 10.1128/JVI.01210-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck CB, Phan GQ, Raiji MT, Murphy PM, McDermott DH, McBride AA. 2012. Complete genome sequence of a tenth human polyomavirus. J. Virol. 86:10887. 10.1128/JVI.01690-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim ES, Reyes A, Antonio M, Saha D, Ikumapayi UN, Adeyemi M, Stine OC, Skelton R, Brennan DC, Mkakosya RS, Manary MJ, Gordon JI, Wang D. 2013. Discovery of STL polyomavirus, a polyomavirus of ancestral recombinant origin that encodes a unique T antigen by alternative splicing. Virology 436:295–303. 10.1016/j.virol.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korup S, Rietscher J, Calvignac-Spencer S, Trusch F, Hofmann J, Moens U, Sauer I, Voigt S, Schmuck R, Ehlers B. 2013. Identification of a novel human polyomavirus in organs of the gastrointestinal tract. PLoS One 8:e58021. 10.1371/journal.pone.0058021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, Gosert R, Hirsch HH. 2009. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J. Infect. Dis. 199:837–846. 10.1086/597126 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen NL, Le BM, Wang D. 2009. Serologic evidence of frequent human infection with WU and KI polyomaviruses. Emerg. Infect. Dis. 15:1199–1205. 10.3201/eid1508.090270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kean JM, Rao S, Wang M, Garcea RL. 2009. Seroepidemiology of human polyomaviruses. PLoS Pathog. 5:e1000363. 10.1371/journal.ppat.1000363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schowalter RM, Pastrana DV, Pumphrey KA, Moyer AL, Buck CB. 2010. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe 7:509–515. 10.1016/j.chom.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T, Mattila PS, Jartti T, Ruuskanen O, Söderlund-Venermo M, Hedman K. 2011. Seroepidemiology of the newly found trichodysplasia spinulosa-associated polyomavirus. J. Infect. Dis. 204:1523–1526. 10.1093/infdis/jir614 [DOI] [PubMed] [Google Scholar]
- 11.Nicol JTJ, Touzé A, Robinot R, Arnold F, Mazzoni E, Tognon M, Coursaget P. 2012. Seroprevalence and cross-reactivity of human polyomavirus 9. Emerg. Infect. Dis. 18:1329–1332. 10.3201/eid1808.111625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicol JT, Robinot R, Carpentier A, Carandina G, Mazzoni E, Tognon M, Touzé A, Coursaget P. 2013. Age-specific seroprevalence of Merkel cell polyomavirus, human polyomaviruses 6, 7, and 9 and trichodysplasia spinulosa-associated polyomavirus. Clin. Vaccine Immunol. 20:363–368. 10.1128/CVI.00438-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicol JT, Touzé A. 2011. Prevalence of polyomaviruses and links with human diseases. Future Virol. 6:1187–1197. 10.2217/fvl.11.96 [DOI] [Google Scholar]
- 14.Touzé A, Gaitan J, Arnold F, Cazal R, Fleury MJ, Combelas N, Sizaret P-Y, Guyetant S, Maruani A, Baay M, Tognon M, Coursaget P. 2010. Generation of Merkel cell polyomavirus (MCV)-like particles and their application to detection of MCV antibodies. J. Clin. Microbiol. 48:1767–1770. 10.1128/JCM.01691-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scuda N, Hofmann J, Calvignac-Spencer S, Ruprecht K, Liman P, Kühn J, Hengel H, Ehlers B. 2011. A novel human polyomavirus closely related to the African green monkey-derived lymphotropic polyomavirus. J. Virol. 85:4586–4590. 10.1128/JVI.02602-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trusch F, Klein M, Finsterbusch T, Kühn J, Hofmann J, Ehlers B. 2012. Seroprevalence of human polyomavirus 9 and cross-reactivity to African green monkey-derived lymphotropic polyomavirus. J. Gen. Virol. 93:698–705. 10.1099/vir.0.039156-0 [DOI] [PubMed] [Google Scholar]


