Abstract
Rhodotorula is an emerging opportunistic fungal pathogen that is rarely reported to cause endocarditis. We describe a case involving a patient who developed endocarditis due to Rhodotorula mucilaginosa and Staphylococcus epidermidis, proven by culture and histopathology. The case illustrates the unique diagnostic and therapeutic challenges relevant to Rhodotorula spp.
CASE REPORT
A 54-year-old woman presented to New York Presbyterian Hospital (NYPH) with a 3-week history of fatigue and anorexia. Her past medical history was significant for Staphylococcus aureus aortic valve endocarditis requiring bioprosthetic aortic valve replacement 9 years prior, as well as end-stage renal disease status following renal transplantation which had failed several years previously. She was on hemodialysis at the time of admission. Among other medications, she was taking 5 mg of prednisone daily. After an electrocardiogram revealed bradycardia with complete heart block, she was admitted to the cardiac care unit. On physical examination, the patient appeared nontoxic but chronically ill. She was afebrile and bradycardic with a pulse rate in the 30s and a 2/6 systolic murmur at the right upper sternal border. The site of her tunneled hemodialysis catheter was clean and without purulence. Her admission laboratory tests, including complete blood count, basic metabolic panel, and liver function tests, were unremarkable. Four peripheral blood culture sets were drawn on admission in BacT/Alert standard aerobic (SA) and standard anaerobic (SN) bottles (bioMérieux, Durham, NC) prior to the initiation of antimicrobial therapy. The aerobic and anaerobic bottles of all four sets grew Staphylococcus epidermidis. Antibiotic therapy with vancomycin, gentamicin, and rifampin was initiated. A transesophageal echocardiogram demonstrated a 0.4-cm thickening of the aortic valve with a 2-cm extension involving the aortic root, suggestive of abscess. A 0.5-cm by 0.6-cm erratically moving echodensity was also seen on the ventricular side of the aortic valve leaflets, consistent with a vegetation. Single-photon-emission computed tomography (SPECT) with indium 111-labeled leukocytes revealed increased uptake of indium around the aortic root, consistent with an infectious focus.
On hospital day 2, three sets of follow-up peripheral blood cultures were drawn. The aerobic bottle of one set flagged positive on day 5 (day 7 of hospitalization). Gram stain from the bottle revealed oval budding yeast forms with occasional rudimentary pseudohyphae (Fig. 1A). The remaining blood cultures (both routine aerobic/anaerobic and fungal Isolator tubes [Alere, Waltham, MA]) from hospital day 7 were negative for growth. Three subsequent blood culture sets submitted over days 8 and 9 were also negative for growth. The patient was started on empirical micafungin (100 mg per day intravenously) on hospital day 7.
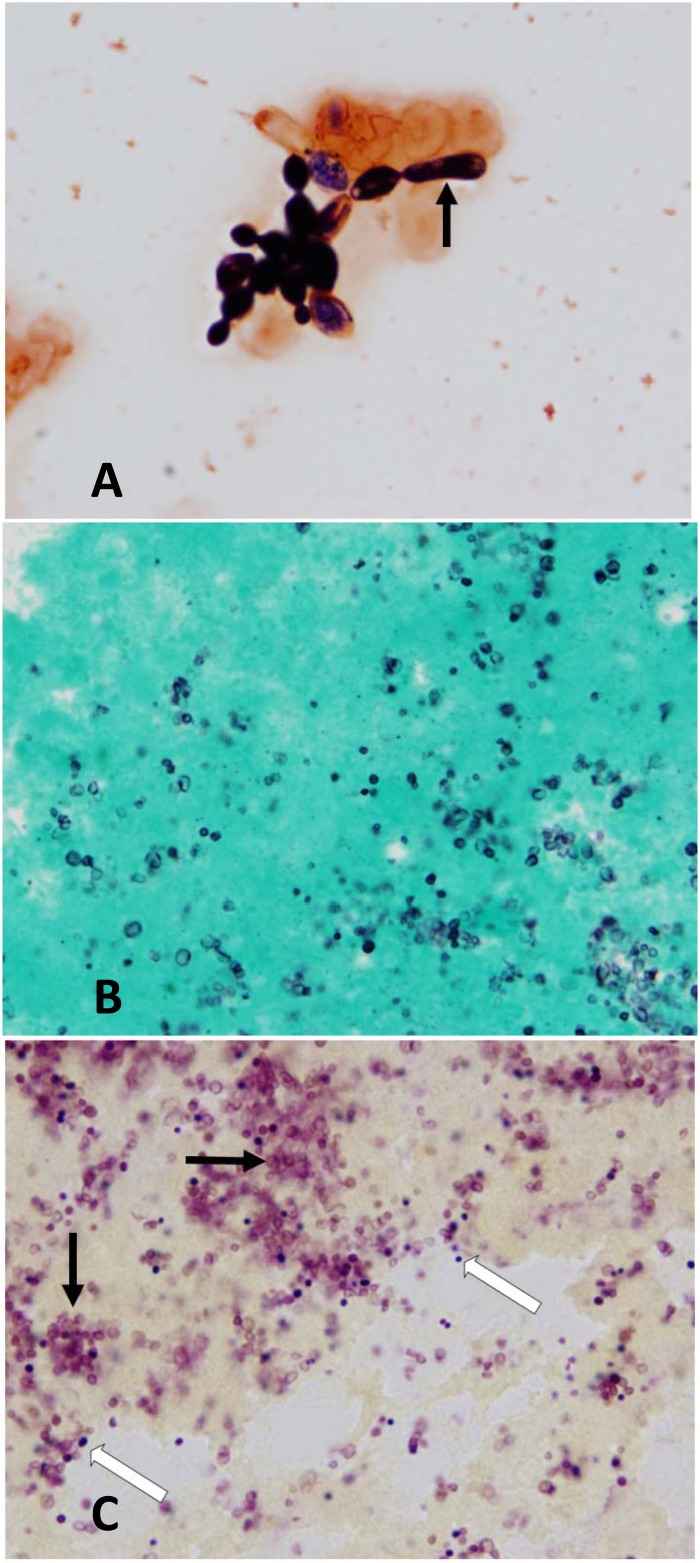
FIG 1.
(A) Gram stain of the blood culture bottle broth, revealing oval to elongate budding yeast forms with rudimentary pseudohyphae (black arrow) (1,000×). (B) Grocott methenamine silver (GMS) stain of valvular tissue demonstrating numerous yeast forms measuring approximately 3 μm (1,000×). (C) Brown and Hopps Gram stain of valvular tissue demonstrating light-pink yeast forms (black arrows) and dark-purple Gram-positive cocci (white arrows) (1,000×).
Yeast grew rapidly on mycologic media at 30°C and 37°C as smooth, pink to coral colonies. Urease testing was positive. The Vitek2 YST card (code number 21343; bioMérieux) identified the yeast as Rhodotorula mucilaginosa. The isolate was sent to the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio (UTHSCSA) for molecular identification and susceptibility testing. Molecular identification was performed as previously described, using the ITS (internal transcribed spacer) region and D1/D2 region of the 28s ribosomal subunit as the sequencing targets (1). The top three hits from a GenBank search using the BLASTn algorithm for both loci corresponded to Rhodotorula mucilaginosa, at 99.81% and 99.66% identity for the ITS and D1/D2 regions, respectively.
On hospital day 10, after the yeast was confirmed as a Rhodotorula species, the antifungal therapy was changed to liposomal amphotericin B at 370 mg intravenously daily. The results of susceptibility testing on the isolate from three independent laboratories varied using three different susceptibility testing methods (Table 1), the YeastOne Y09 panel (TREK Diagnostic Systems, Thermo Fisher Scientific, Inc., Waltham, MA) performed by the NYPH laboratory, broth microdilution (BMD) testing following the Clinical and Laboratory Standards Institute (CLSI) methods performed at UTHSCSA, and the Etest (bioMérieux) performed at the New York State Department of Health Wadsworth Center. Susceptibility testing for all three methods revealed elevated MICs to echinocandins and fluconazole and low MICs to amphotericin B and 5-fluorocytosine. Wide discrepancies between the MICs for posaconazole (5 or more doubling dilutions) and voriconazole (2 or more doubling dilutions) were evident with different testing methodologies. Specifically, the Etest demonstrated lower MICs than the two broth microdilution testing methods (YeastOne and CLSI BMD). Both the YeastOne and the CLSI BMD resulted in elevated MICs to posaconazole and voriconazole, while the Etest demonstrated low MICs.
TABLE 1.
Comparison of MICs of the Rhodotorula mucilaginosa isolate determined by different susceptibility testing methods
| Antifungal agent | MIC (μg/ml) determined bya: |
||
|---|---|---|---|
| YeastOne | CLSI broth microdilution | Etest | |
| Amphotericin B | 0.5 | 0.125 | 0.25 |
| Anidulafungin | ≥8 | NP | NP |
| Caspofungin | ≥8 | >32 | 8 |
| Micafungin | ≥8 | NP | NP |
| Fluconazole | ≥256 | >256 | > 64 |
| Itraconazole | ≥8 | NP | 0.5 |
| Posaconazole | ≥8 | >32 | 0.25 |
| Voriconazole | 4 | >32 | 1 |
| 5-Fluorocytosine | 0.06 | 0.008 | ≤0.125 |
YeastOne Y09 panel (TREK Diagnostic Systems, Thermo Fisher Scientific, Inc., Waltham, MA); Clinical and Laboratory Standards Institute (CLSI) broth microdilution testing; Etest (bioMérieux, Durham, NC). NP, not performed.
The patient underwent successful pacemaker implantation and aortic valve replacement, during which aortic vegetations were noted intraoperatively. Histopathology of the resected valvular tissue demonstrated acute inflammation with focal areas of necrosis and organizing fibrin with an acute inflammatory infiltrate consistent with vegetations. Small round to oval yeast forms approximately 3 μm in diameter which were morphologically consistent with Rhodotorula spp. were seen on both Grocott methenamine silver (GMS) and Gram stain of the valvular tissue (Fig. 1B and C). Gram stain also demonstrated Gram-positive cocci, consistent with S. epidermidis isolated from prior blood cultures. Bacterial and fungal cultures from aortic valve tissue and the hemodialysis catheter did not grow any microorganisms. The patient completed a 6-week course of liposomal amphotericin B, as well as vancomycin, gentamicin, and rifampin. She was discharged to home on hospital day 55. Since discharge, she has been maintained on chronic voriconazole suppression therapy at a dose of 400 mg by mouth every 12 h, which she tolerated for over a year without recurrence.
To assess the potential for more-rapid identification of Rhodotorula from blood culture, we retrospectively tested whether the blood culture broth would demonstrate urease activity when inoculated directly on a urea slant. We performed an experiment with seeded bottles using Bactec Plus aerobic/F bottles (BD, Franklin Lakes, NJ). In short, we inoculated 2 ml of brain heart infusion (BHI) broth (BD) with 10 μl of the patient's Rhodotorula isolate. Once the broth was turbid, we seeded a blood bottle which had been negative for growth with 3 ml of the BHI broth. After the bottle flagged positive on the instrument, a urea slant was inoculated with three drops of broth from the blood bottle. The experiment was repeated with a separate R. mucilaginosa isolate from another patient. The urea slants were incubated at 37°C and reexamined each shift until the slant turned positive. The urea slants from the seeded Rhodotorula bottles were positive for urease activity at between 36 and 48 h from inoculation. Negative urease controls were run with slants inoculated with broth from no-growth blood bottles and also with broth from a bottle seeded with a urease-negative Candida albicans isolate.
Rhodotorula species, previously considered to be nonpathogenic, are increasingly reported to cause invasive disease, including endocarditis, catheter-associated bloodstream infection, peritonitis, and endophthalmitis, particularly in immunocompromised hosts (2, 3). Recent reports have concentrated on catheter-associated fungemia or examined the consequences of infection in specific patient populations, but none have examined published endocarditis cases in detail (4, 5). We report our laboratory and clinical experience involving a patient who developed coinfection with Rhodotorula mucilaginosa and Staphylococcus epidermidis aortic valve endocarditis proven by culture and histopathology and associated with aortic root abscess and complete heart block. Multiple risk factors increased this patient's susceptibility to Rhodotorula infection, including the presence of an indwelling central venous hemodialysis catheter, a bioprosthetic aortic valve, and chronic low-dose prednisone therapy. We present this case and a review of the previously published literature to highlight several of the unique diagnostic and management challenges associated with endocarditis involving this pathogen and to serve as a guide for clinicians and clinical microbiologists who may encounter this infection in practice.
Rhodotorula was first described in 1960 as a cause of endocarditis, and a total of 7 cases have been detailed in the literature (Table 2) (6–12). Several features of this patient's case are instructive. First, it was only the patient's fifth routine blood culture set which was positive for Rhodotorula spp., with the prior sets being negative for yeast. The bottle with Rhodotorula became positive on day five, the last day of incubation. However, the previous four blood culture sets were all positive at earlier intervals for S. epidermidis, potentially preventing recognition of late growth of the yeast in the bottle. Upon pathological examination of aortic valve tissue, however, numerous yeast forms were evident, indicating a high burden of valvular infection despite a negative culture. There is a paucity of literature on the yield of blood cultures for Rhodotorula species. Of concern is the possible poor sensitivity of routine blood cultures for the diagnosis of Rhodotorula endocarditis, despite technological advancements in the detection of other common yeasts. However, the gold standard of culture of valvular tissue used in such studies may not be appropriate, as the yield of bacterial and fungal culture was shown to be 25.4% in one large infective endocarditis study (13). During the management of this patient's infection, there was initial uncertainty as to whether the single blood culture positive for yeast represented contamination versus true infection. Thus, the presence of even a single blood culture positive for yeast in a patient with a compatible clinical history, particularly in immunocompromised hosts, those with central venous catheters, or those with a history of cardiac valvular abnormalities, should heighten concern for the diagnosis of endocarditis involving this pathogen. Our literature review showed that six (75%) of eight reported cases involved patients with either concomitant bacterial endocarditis or a history of endocarditis requiring prosthetic valve replacement, highlighting the opportunistic nature of the pathogen.
TABLE 2.
Published reports of Rhodotorula spp. endocarditis
| Yr (reference) | Species of Rhodotorula | Risk factor | Valve | Coinfection or extracardiac involvement | Blood culture result | Antifungal treatment | Outcome |
|---|---|---|---|---|---|---|---|
| 1958 (6) | Not specified | Rheumatic heart disease | Aortic | Dental abscess | Positive | None | Died |
| 1962 (7) | Not specified | Rheumatic heart disease | Not specified | Staphylococcus aureus endocarditis | Positive | Amphotericin B | Recovered |
| 1965 (8) | Not specified | Endocarditis | Not specified | Enterococcus faecalis endocarditis | Positive | Amphotericin B | Recovered |
| 1975 (9) | R. pilimanae | Presumed bacterial endocarditis | Not specified | None identified | Positive | 5-Fluorocytosine | Recovered |
| 2003 (10) | R. mucilaginosa | History of endocarditis/aortic homograft | Not specified | None identified | Negative | Fluconazole, amphotericin B | Recovered |
| 2005 (11) | R. glutinis | Cardiac transplant recipient (donor had traumatic brain injury) | Left atrial appendage | None identified | Negative | Fluconazole, amphotericin B | Recovered |
| 2005 (12) | Not specified | ALL, CVCa | Right atrium | None identified | CVC culture positive | Amphotericin B, rifampin, 5-fluorocytosine | Recovered |
| 2011, our case | R. mucilaginosa | Prosthetic aortic valve, corticosteroids, CVC | Aortic | Staphylococcus epidermidis | Positive | Micafungin, amphotericin B, voriconazole | Recovered |
CVC, central venous catheter; ALL, acute lymphocytic leukemia.
A second key feature of this case demonstrates the importance of early identification of Rhodotorula, because empirical antifungal therapy for non-Rhodotorula fungemia typically involves a triazole or echinocandin, two agents to which Rhodotorula is characteristically resistant. Morphology on Gram stain of the bottle can be helpful in differentiating Candida glabrata from non-glabrata Candida spp., but the Gram stain morphology of Rhodotorula spp. has not been studied sufficiently to warrant a preliminary diagnosis from the bottle Gram stain (14). The bottle Gram stain in our case demonstrated oval, somewhat elongated budding yeasts with rudimentary pseudohyphae that were similar to many Candida species. The Clinical Microbiology Procedures Handbook notes that inoculation of a urea slant may be performed directly with broth from a positive blood culture bottle once a yeast suspicious for Cryptococcus (also urease positive) is seen (15). The differential diagnosis of urease-positive yeasts includes the basidiomycetous organisms Cryptococcus spp., Rhodotorula spp., Malassezia spp., and Trichosporon spp. Although other yeasts, including most Candida spp., are urease negative, Candida lipolytica and some strains of Candida krusei may be urease positive (16). We demonstrated that Rhodotorula can turn the urease slant positive when it is inoculated directly with broth from seeded blood bottles. Inoculation of a urea slant with broth directly from the blood bottle may be helpful as a potential preliminary identification of urease-positive yeast.
It is important, however, to recognize that the reaction of a urea slant directly from blood is dependent upon several factors, including organism load and strain, ingredients in the commercial blood culture bottles (such as yeast extractions) which may potentially cause the urea slant to turn positive, the type of blood culture system used, and possibly even urease-producing bacteria. Therefore, the laboratory should be aware of the limitations of such a practice and discuss the limitations with the clinical team. Additionally, it took 36 to 48 h for the urea slants to become positive, but most yeasts would be identified at that point. Although our approach of inoculation of a urea slant with blood culture broth for early detection of Rhodotorula spp. is preliminary and not sufficient to recommend routine adoption in the clinical laboratory, it lays the foundation for further study. Effective communication between the clinical microbiology team and infectious disease practitioners is critical in guiding appropriate antifungal therapy when Rhodotorula endocarditis is a diagnostic possibility.
Although previous studies have compared susceptibility results across the literature, ours is the first report to our knowledge comparing various susceptibility testing methods on the same isolate. We demonstrated low MICs to amphotericin B and 5-fluorocytosine and elevated MICs to echinocandins, fluconazole, and other azole agents. These data are supported by previous susceptibility studies of Rhodotorula. Diekema et al. used the CLSI BMD to test 64 Rhodotorula spp. isolates, 24 of which were R. mucilaginosa (17). The authors found a lack of activity of echinocandins against Rhodotorula spp., which is consistent with Rhodotorula as a member of the family Cryptococcaceae (18). They concluded that fluconazole was not active in vitro and also noted no significant difference in activities of tested antifungals by species of Rhodotorula. Likewise, Gomez-Lopez and colleagues reported similar susceptibility profiles for 29 Rhodotorula spp. tested by BMD following CLSI recommendations with minor modifications (19). Interestingly, the MICs of our isolate varied by testing method. Lower MICs to posaconazole and voriconazole were seen by the Etest method, as opposed to the high MICs obtained when testing by YeastOne and CLSI BMD. Gomez-Lopez et al. also found discrepant susceptibility results when comparing the MIC values of 102 Rhodotorula strains from nine published studies using three different susceptibility testing methods, including Etest, YeastOne, and BMD (19). They reported that, regardless of method, amphotericin B and 5-fluorocytosine showed the lowest MICs. However, lower MICs to azole agents were obtained for itraconazole with YeastOne, as opposed to the MICs obtained with Etest and BMD. The upper MIC limits for itraconazole in the three YeastOne studies were 0.125, 0.25, and 1 μg/ml. The itraconazole upper MIC limits for the remaining six studies utilizing Etest and BMD ranged from 1 to ≥32 μg/ml (19). This finding is in contrast to our data, which point to a lack of agreement of Etest with CLSI BMD and YeastOne. The reason for this discrepancy is not known, as susceptibility testing was confirmed repeatedly by each different methodology and laboratory. Given these uncertainties, we suggest that laboratories that recover Rhodotorula from blood perform susceptibility testing using CLSI BMD as at least one of the methods, since it is considered the current gold standard for susceptibility testing. If consensus cannot be reached among methods, CLSI BMD appears to be the method which obtains the highest MICs and is thus the most conservative.
In summary, we report a case of culture- and histopathology-proven endocarditis caused by Rhodotorula mucilaginosa and Staphylococcus epidermidis. Blood cultures in this case became positive for Rhodotorula on the fifth set, delaying recognition of fungemia. We demonstrated that once yeast is seen on Gram stain of the blood culture broth, direct inoculation from the blood culture of a urea slant may aid in narrowing the differential diagnosis. Finally, the susceptibility testing results of the Rhodotorula isolate varied according to different testing methodologies, emphasizing the need to include CLSI BMD as a standard susceptibility testing method.
Nucleotide sequence accession numbers.
Sequences obtained for the ITS and D1/D2 regions of our isolate were deposited in GenBank under accession numbers KF726105 (ITS) and KF726104 (D1/D2).
ACKNOWLEDGMENTS
T.J.W. receives support from the SOS Kids Foundation, Henry Schueler Foundation (Scholar in Mucormycosis), and Sharpe Family Foundation (Scholar in Pediatric Infectious Diseases), as well as research grants for experimental and clinical antimicrobial pharmacotherapeutics from Astellas, Novartis, Merck, ContraFect, and Pfizer. He has served as consultant to Astellas, ContraFect, Drais, iCo, Novartis, Pfizer, Methylgene, SigmaTau, and Trius. The work in this paper was not supported financially.
A.N.S. would like to acknowledge Adrienne Brudie for her help with the urease testing.
Footnotes
Published ahead of print 6 November 2013
REFERENCES
- 1.Romanelli AM, Sutton DA, Thompson EH, Rinaldi MG, Wickes BL. 2010. Sequence-based identification of non-sporulating basidiomycetous fungi from clinical specimens, a cautionary note. J. Clin. Microbiol. 48:741–752. 10.1128/JCM.01948-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lunardi LW, Aquino VR, Zimerman RA, Goldani LZ. 2006. Epidemiology and outcome of Rhodotorula fungemia in a tertiary care hospital. Clin. Infect. Dis. 43:e60–e63. 10.1086/507036 [DOI] [PubMed] [Google Scholar]
- 3.Tuon FF, Costa SF. 2008. Rhodotorula infection. A systematic review of 128 cases from literature. Rev. Iberoam. Micol. 25:135–140. 10.1016/S1130-1406(08)70032-9 [DOI] [PubMed] [Google Scholar]
- 4.Mori T, Nakamura Y, Kato J, Sugita K, Murata M, Kamei K, Okamoto S. 2012. Fungemia due to Rhodotorula mucilaginosa after allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 14:91–94. 10.1111/j.1399-3062.2011.00647.x [DOI] [PubMed] [Google Scholar]
- 5.Tuon FF, de Almeida GM, Costa SF. 2007. Central venous catheter-associated fungemia due to Rhodotorula spp.—a systematic review. Med. Mycol. 45:441–447. 10.1080/13693780701381289 [DOI] [PubMed] [Google Scholar]
- 6.Louria DB, Greenberg SM, Molander DW. 1960. Fungemia caused by certain nonpathogenic strains of the family Cryptococcaceae: report of two cases due to Rhodotorula and Torulopsis glabrata. N. Engl. J. Med. 263:1281–1284. 10.1056/NEJM196012222632504 [DOI] [PubMed] [Google Scholar]
- 7.Shelburne PF, Carey RJ. 1962. Rhodotorula fungemia complicating staphylococcal endocarditis. JAMA 180:38–42. 10.1001/jama.1962.03050140040009 [DOI] [PubMed] [Google Scholar]
- 8.Leeber DA, Scher I. 1969. Rhodotorula fungemia presenting as endotoxic shock. Arch. Intern. Med. 123:78–81. 10.1001/archinte.1969.00300110080016 [DOI] [PubMed] [Google Scholar]
- 9.Naveh Y, Friedman A, Merzbach D, Hashman N. 1975. Endocarditis caused by Rhodotorula successfully treated with 5-fluorocytosine. Br. Heart J. 37:101–104. 10.1136/hrt.37.1.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeder M, Vogt PR, Schaer G, von Graevenitz A, Gunthard HF. 2003. Aortic homograft endocarditis caused by Rhodotorula mucilaginosa. Infection 31:181–183. 10.1007/s15010-002-3155-1 [DOI] [PubMed] [Google Scholar]
- 11.Gamma R, Carrel T, Schmidli J, Zimmerli S, Tanner H, Hullin R, Mohacsi PJ. 2005. Transplantation of yeast-infected cardiac allografts: a report of 2 cases. J. Heart Lung Transplant. 24:1159–1162. 10.1016/j.healun.2004.07.015 [DOI] [PubMed] [Google Scholar]
- 12.Pasqualotto GC, Copetti FA, Meneses CF, Machado AR, Brunetto AL. 2005. Infection by Rhodotorula sp. in children receiving treatment for malignant diseases. J. Pediatr. Hematol. Oncol. 27:232–233. 10.1097/01.mph.0000158970.27196.c5 [DOI] [PubMed] [Google Scholar]
- 13.Munoz P, Bouza E, Marin M, Alcala L, Rodriguez Creixems M, Valerio M, Pinto A, Group for the Management of Infective Endocarditis of the Gregorio Maranon Hospital 2008. Heart valves should not be routinely cultured. J. Clin. Microbiol. 46:2897–2901. 10.1128/JCM.02173-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington A, McCourtney K, Nowowiejski D, Limaye A. 2007. Differentiation of Candida albicans from non-albicans yeast directly from blood cultures by Gram stain morphology. Eur. J. Clin. Microbiol. Infect. Dis. 26:325–329. 10.1007/s10096-007-0291-7 [DOI] [PubMed] [Google Scholar]
- 15.York MK, Henry M, Gilligan P. 2010. Blood cultures---general detection and interpretation, p 3.4.1.1–3.4.1.20 In Garcia LS. (ed), Clinical microbiology procedures handbook, 3rd ed, vol 1 ASM Press, Washington, DC [Google Scholar]
- 16.Howell SA, Hazen KC. 2011. Candida, Cryptococcus, and other yeasts of medical importance, p 1793–1821 In Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW. (ed), Manual of clinical microbiology, 10th ed. ASM Press, Washington, DC [Google Scholar]
- 17.Diekema DJ, Petroelje B, Messer SA, Hollis RJ, Pfaller MA. 2005. Activities of available and investigational antifungal agents against Rhodotorula species. J. Clin. Microbiol. 43:476–478. 10.1128/JCM.43.1.476-478.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espinel-Ingroff A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez-Lopez A, Mellado E, Rodriguez-Tudela JL, Cuenca-Estrella M. 2005. Susceptibility profile of 29 clinical isolates of Rhodotorula spp. and literature review. J. Antimicrob. Chemother. 55:312–316. 10.1093/jac/dki020 [DOI] [PubMed] [Google Scholar]