Abstract
The QuantiFERON-TB Gold In-Tube (QFT-G IT) test (Cellestis Inc., Valencia, CA) is one of the gamma interferon release assays (IGRAs) that are promising tools for diagnosing active or latent Mycobacterium tuberculosis infections. We investigated the clinical and laboratory factors that affect the rate of indeterminate QFT-G IT test results. We also suggest a workflow strategy for achieving optimized test results using the QFT-G IT test for the diagnosis of active tuberculosis (TB) or latent TB infection. We performed statistical analysis using data from a retrospective review of medical records. The first phase included 683 QFT-G IT test results from 676 patients tested between January 2008 and May 2008, and the second phase included an additional 663 QFT-G IT test results from 653 patients tested between January 2008 and December 2008 at Samsung Medical Center, a tertiary care hospital in South Korea. Immunosuppressive drug therapy, underlying diseases, bedridden status, and hypoalbuminemia were significantly associated with indeterminate QFT-G IT test results. With reduction of the incubation delay during the test procedure from an average of 9.82 h to an average of 2.70 h with changes in the workflow, the frequency of indeterminate QFT-G IT test results was significantly reduced from 11.4% to 2.7%. With >6 h of incubation delay, however, the frequency of indeterminate QFT-G IT test results was increased in a statistically significant manner. This study demonstrates that not only clinicopathological factors but also laboratory factors, such as incubation delay, significantly affect the rate of indeterminate QFT-G IT test results; therefore, optimization of the test procedure may contribute to reductions in the rate of indeterminate QFT-G IT test results, which delay the diagnosis of TB.
INTRODUCTION
Tuberculosis (TB) is one of the most common infectious diseases causing morbidity and death worldwide (1, 2). Moreover, population aging and increased use of immunosuppressive treatments highlight the need for additional strategies to maintain appropriate TB control (2, 3). Particularly in countries with low or intermediate prevalence rates, the diagnosis and treatment of latent TB infection (LTBI) are essential for the control of TB. The tuberculin skin test (TST) has been the standard diagnostic tool for screening for LTBI because it is inexpensive and easy to perform; however, it has a major limitation in diagnosing LTBI in BCG-vaccinated populations, including the Korean population, due to cross-reactivity between BCG and purified protein derivative (PPD) antigens (4, 5).
Recently introduced gamma interferon (IFN-γ) release assays (IGRAs), including QuantiFERON TB-Gold (QFT-G) (Cellestis Ltd., Carnegie, Victoria, Australia) and TSPOT.TB (Oxford Immunotec, Oxford, United Kingdom), have an advantage over the TST with respect to the aforementioned limitation and have shown promise as alternative diagnostic tools for LTBI in BCG-vaccinated populations (5, 6). IGRAs detect and quantify IFN-γ secreted from T cells in response to Mycobacterium tuberculosis-specific antigens. However, immunological impairment may affect the performance of lymphocyte-based assays. Indeterminate results (i.e., results that are interpreted as unreliable, mainly due to inappropriate values for the negative and/or positive controls, regardless of the patient's results) are a limitation of these new methods, particularly in immunocompromised individuals (7). Rates of indeterminate results of 5% to 40% for all participants and approximately 6% for health care workers have been reported (8–10). Rates of indeterminate results of 15.6% and 16.1% have been reported for two large tertiary care hospitals in South Korea (11, 12). Considering the composition of the high-risk populations and the therapeutic approaches used at the hospital, the frequency of indeterminate results may vary depending on hospital conditions. One of the IGRAs, the QuantiFERON-TB Gold In-Tube (QFT-G IT) test, has been performed for the diagnosis of active TB or LTBI among patients in our hospital since 2007. The rate of indeterminate QFT-G IT test results, which was monitored regularly, was about 11% until 2008. Hence, we decided to investigate both the clinical and laboratory factors that cause indeterminate QFT-G IT results and thus to reduce the rate of these indeterminate results.
In this study, we found that not only clinical factors that are known to affect QFT-G IT test results, such as immunosuppressive drug therapy, underlying diseases, bedridden status, and hypoalbuminemia, but also laboratory factors, such as prolonged preincubation storage (incubation delay), are significantly associated with indeterminate QFT-G IT test results. To our knowledge, this study is one of the largest investigating the factors affecting indeterminate results with the QFT-G IT method and the first to investigate the allowable incubation delay based on clinical data.
MATERIALS AND METHODS
Study population.
A total of 1,346 QFT-G IT test results consecutively acquired from 1,329 patients who had visited Samsung Medical Center between January 2008 and October 2008 were investigated retrospectively. We analyzed the data in two phases, one for the investigation of clinical factors affecting QFT-G IT test results and the other for the evaluation of laboratory factors. In the first phase, a total of 683 results from 676 patients who had undergone testing with the QFT-G IT test between January 2008 and May 2008 were investigated. The specimens were collected from Samsung Medical Center, and the tests were performed at the Green Cross Reference Laboratory in South Korea. The median age of the patients was 54 years (range, 0 to 92 years). Thirty-six patients (5.3%) were under 20 years of age, and 121 patients (17.9%) were over 70 years of age. The male/female ratio was 1:0.8 (Table 1). The QFT-G IT test results and related laboratory findings (including acid-fast bacillus [AFB] stain, culture, and M. tuberculosis PCR results), radiological findings, and clinical information were thoroughly reviewed from the electronic medical records. The collected clinical information included demographic data, underlying diseases (e.g., malignancies, diabetes mellitus, chronic renal failure, autoimmune diseases, infections, major surgery, or trauma), clinical courses, and medications, including immunosuppressive drugs such as anticancer chemotherapy, glucocorticosteroids, or anti-tumor necrosis factor alpha (TNF-α) agents, which are known to possibly influence QFT-G IT test results. A total of 157 patients had a history of TB disease. All patients except 4 patients for whom the diagnosis was confirmed with Western blot analysis had negative serological tests for HIV. Diagnoses of TB disease were made based on the combination of laboratory findings, radiological findings, and clinical features.
TABLE 1.
Clinical characteristics and laboratory findings of the groups with indeterminate and determinate test results analyzed between January 2008 and May 2008 (n = 683)a
| Characteristicb | Data for indicated results (n) |
Pc | |
|---|---|---|---|
| Indeterminate (78) | Determinate (605) | ||
| Age (median [range]) (yr) | 54 (39–56) | 53 (36–69) | 0.51 |
| Age ≥ 70 yr (n [%]) | 18 (23.1) | 103 (17.2) | 0.27 |
| Age < 20 yr (n [%]) | 7 (9.0) | 29 (4.8) | 0.21 |
| Taking immunosuppressive drugs (n [%]) | 26 (33.3) | 34 (5.7) | <0.05 |
| Underlying diseases (n [%]) | 65 (83.3) | 281 (47.0) | <0.05 |
| Malignant diseases | 17 (21.8) | 127 (21.2) | 0.97 |
| DM | 10 (12.8) | 57 (9.5) | 0.48 |
| CRF | 8 (8.9) | 11 (1.8) | <0.05 |
| HIV infection | 0 (0.0) | 4 (0.7) | 0.77 |
| Bedridden (n [%]) | 10 (12.8) | 3 (0.5) | <0.05 |
| Lymphocyte count (median [range]) (cells/μl) | 1,224 (730–1,782) | 1,746 (1,288–2,271) | <0.05 |
| Lymphocytopenia (<1,500 cells/μl) (n [%]) | 47 (60.3) | 221 (36.5) | <0.05 |
| Albumin level (median [range]) (g/dl) | 3.0 (2.6–3.4) | 3.9 (3.5–4.1) | <0.05 |
| Hypoalbuminemia (<3.5 g/dl) (n [%]) | 57 (73.1) | 129 (21.3) | <0.05 |
The positivity rate was 47.4% (324/683 tests) in this period.
DM, diabetes mellitus; CRF, chronic renal failure; HIV, human immunodeficiency virus.
P values were calculated using the chi-square test and the Mann-Whitney U test.
In the second phase, the results of an additional 663 QFT-G IT tests that were performed for 653 patients between June 2008 and October 2008 at Samsung Medical Center were compared with the previous 683 QFT-G IT test results. During this period, all of the procedures, including specimen collection, incubation, and testing, were conducted at Samsung Medical Center.
The first 683 QFT-G IT test results and the second 663 QFT-G IT test results were generated using the same test kit and method, according to the manufacturer's instructions; however, there were changes in the workflow of sample collection and processing. During the first period of 683 QFT-G IT tests, the blood samples were collected at the patient's premises and then delivered to the laboratory to be transported to the reference laboratory; the incubation and measurements were performed at the Green Cross Reference Laboratory, which is located at a 1-hour distance. Since June 2008, all procedures, including sample collection, incubation, and measurements, were performed at Samsung Medical Center. The study was approved by the institutional review board of Samsung Medical Center (Seoul, South Korea).
QuantiFERON-TB Gold In-Tube test.
The QFT-G IT tests were performed according to the manufacturer's recommendations (Cellestis Ltd., Carnegie, Australia). In brief, 1 ml of blood was drawn directly into three blood collection tubes, one containing heparin alone (the nil tube, i.e., the negative control), one containing the T cell mitogen phytohemagglutinin (the mitogen tube, i.e., the positive control), and one containing the M. tuberculosis-specific antigens ESAT-6, CFP-10, and TB7.7 (the TB antigen tube). After thorough mixing, the tubes were transferred to the incubator within 16 h and then incubated upright at 37°C for 20 to 24 h before being centrifuged for plasma harvesting. The plasma samples were stored at 4°C until further analysis, within 2 days. The concentration of IFN-γ in each plasma sample was determined using the enzyme-linked immunosorbent assay (ELISA) method. Interpretations were performed according to the guidelines proposed by the Centers for Disease Control and Prevention (CDC). A test result was defined as indeterminate if the value in the positive-control well was <0.5 IU/ml, irrespective of the IFN-γ level in the TB antigen well (low mitogen response, i.e., positive-control failure), or if the value in the negative-control well was >8 IU/ml, irrespective of the IFN-γ level in the TB antigen well or mitogen well (high background response, i.e., negative-control failure).
Statistical analysis.
The prevalence of indeterminate and determinate QFT-G IT test results was calculated. Clinicopathological factors for patients in the groups with indeterminate and determinate QFT-G IT test results were compared using the chi-square test and Mann-Whitney U test. We performed logistic regression analyses to analyze the relationships between indeterminate QFT-G IT test results and various factors and between the frequency of indeterminate QFT-G IT test results and the incubation delay of the test. P values of <0.05 were considered statistically significant. SPSS Statistics version 20 and Stata version 12.0 were used for statistical analyses.
RESULTS
Clinicopathological factors affecting the rate of indeterminate QFT-G IT test results.
Among the total of 683 QFT-G IT results of the first phase, there were 78 indeterminate results (11.4%). There were 324 positive (47.4%) and 281 negative (41.1%) QFT-G IT test results in 605 determinate results (88.6%). Indeterminate QFT-G IT test results were considered to be due to low IFN-γ levels of the positive-control sample in 76 of 78 tests (97.5%) and due to high IFN-γ levels of the negative-control sample in the remaining two tests. The four HIV-infected patients showed negative determinate results. They were suspected to be in the clinically latent phase after acute HIV infection and had CD4+ lymphocyte counts of 200, 240, 340, and 360 cells/μl.
The median ages for the indeterminate and determinate groups were not significantly different (indeterminate group, 54 years; determinate group, 53 years) (Table 1). In the indeterminate group, the proportions of patients who were receiving immunosuppressive drugs, had underlying diseases including chronic renal failure, and were bedridden were significantly larger than those in the determinate group (P < 0.05). In the indeterminate group, the patients had a significantly lower median lymphocyte count and the number of patients with lymphocyte counts of <1,500 cells/μl was significantly higher than that in the determinate group (1,224 cells/μl versus 1,746 cells/μl and 65.3% versus 36.1%; P < 0.05, Pearson's chi-square test). Also, the median albumin level and the number of patients with hypoalbuminemia (albumin levels of <3.5 g/dl) in the indeterminate group were significantly different from those in the determinate group (3.0 g/dl versus 3.9 g/dl and 78.1% versus 23.5%; P < 0.05). In multivariate logistic regression analysis, immunosuppressive drug therapy, underlying diseases, bedridden status, and hypoalbuminemia were still significantly associated with indeterminate QFT-G IT test results (Table 2) but a lymphocyte count of <1,500 cells/μl was not.
TABLE 2.
Results of multivariate logistic regression analysis for the factors associated with increases in the frequency of indeterminate QFT-G IT test results
| Factor | Odds ratio (95% CI)a | Pb |
|---|---|---|
| Taking immunosuppressive drugs | 3.9 (1.9–8.1) | <0.05 |
| Underlying diseases | 4.6 (1.0–4.6) | <0.05 |
| Bedridden | 7.6 (1.9–30.5) | <0.05 |
| Lymphocytopenia (<1,500 cells/μl) | 1.6 (0.9–2.9) | 0.15 |
| Hypoalbuminemia (<3.5 g/dl) | 7.0 (3.6–13.6) | <0.05 |
CI, confidence interval.
Adjusted for sex and gender.
Laboratory factors that affect the rate of indeterminate QFT-G IT test results.
The frequency of indeterminate results of QFT-G IT tests that were performed from June 2008 to October 2008 became remarkably low at 2.7% (18/663 tests), and this frequency of indeterminate results was significantly lower than that during the previous 5 months, although the frequency of positive results remained stable. Among the 645 determinate QFT-G IT test results (97.3%), there were 360 positive (54.3%) and 285 negative (43.0%) results. Indeterminate QFT-G IT test results were due to low IFN-γ levels of the positive-control sample in 17 of 18 tests (94.4%) and to high IFN-γ levels of the negative-control sample in one test.
In the second phase of analysis, the factors that caused considerable reductions in the frequency of indeterminate QFT-G IT test results between the two periods were explored. There were a few differences in test procedures between the two periods. The tests were performed at an outside reference laboratory, the Green Cross Reference Laboratory, in January to May 2008, and they started to be performed at Samsung Medical Center in June 2008. Also, there was a substantial change in the processing of samples. During the first period, the samples collected from patients between 8 a.m. and 5 p.m. were stored at room temperature and were transported to the reference laboratory in the evening of the same day, in a batch. Then at midnight the samples began to be incubated simultaneously at 37°C for 20 to 24 h; they underwent ELISAs with plasma on the next day. It has been estimated that there is an incubation delay of 6 to 16 h during this process. After the whole test procedure was started at the clinical laboratory of Samsung Medical Center, phlebotomy for the QFT-G IT test was performed in the blood collection room of the laboratory except for patients in intensive care units. The batches of samples were processed and incubated twice per day, at noon and at 6 p.m., and plasma samples from each batch were harvested the next day after 16 to 24 h. All of the other laboratory processes were exactly the same in both laboratories, in a detailed comparison of all processes. The one significant difference between the two periods was the incubation delay at room temperature before the samples were brought to the incubator, which was markedly shortened from an average of 9.82 h to an average of 2.70 h.
The association between the incubation delay and the rate of indeterminate QFT-G IT test results is shown in Table 3. The results are presented according to the extent of incubation delay, in 2-h intervals from 0 to 12 h, and are categorized into groups from 1 to 7. Logistic regression analysis was performed with the reference that had a <2-h incubation delay. In groups 2 and 3, in which the incubation delay was <6 h, the odds ratio was not significantly increased. In groups 4, 5, 6, and 7, however, the odds ratio for each group was statistically significantly increased; the odds ratios were 18.60 (95% confidence interval [CI], 4.32 to 80.14; P < 0.001), 8.73 (95% CI, 2.70 to 28.22; P < 0.001), 6.78 (95% CI, 1.70 to 27.01; P = 0.007), and 4.77 (95% CI, 1.27 to 17.98; P = 0.021), respectively. These results suggested that a >6-h incubation delay caused a statistically significant increase in the frequency of indeterminate QFT-G IT test results. The significant reduction in the frequency of indeterminate QFT-G IT test results from 11.4% to 2.7% between the two periods could be mainly due to decreased incubation delay.
TABLE 3.
Influence of incubation delay on the frequency of indeterminate QFT-G IT test results using logistic regression analysis
| Group no. | Incubation delay (h) | % of indeterminate results (no. of indeterminate results/total no. tested)a |
Odds ratio (95% CI)b | P | |
|---|---|---|---|---|---|
| Between January 2008 and May 2008 | Between June 2008 and October 2008 | ||||
| 1 | 0–2 | 0 (0/0) | 1.8 (3/169) | Reference | |
| 2 | 2–4 | 0 (0/0) | 3.0 (12/403) | 1.8 (0.5–6.3) | 0.38 |
| 3 | 4–6 | 0 (0/0) | 3.3 (3/91) | 1.9 (0.4–9.6) | 0.44 |
| 4 | 6–8 | 23.1 (6/26) | 0 (0/0) | 18.6 (4.3–80.1) | <0.05 |
| 5 | 8–10 | 12.2 (56/460) | 0 (0/0) | 8.7 (2.7–28.2) | <0.05 |
| 6 | 10–12 | 9.9 (7/71) | 0 (0/0) | 6.8 (1.7–27.0) | <0.05 |
| 7 | >12 | 7.1 (9/126) | 0 (0/0) | 4.8 (1.3–18.0) | <0.05 |
| Total | 11.4 (78/683) | 2.7 (18/663) | |||
The positivity rates were 47.4% (324/683 tests) in the first period and 54.3% (360/663 tests) in the second period.
CI, confidence interval.
DISCUSSION
For the diagnosis of tuberculosis, the role of IGRAs, including the ELISA-based QFT-G IT test (Cellestis Ltd., Carnegie, Australia) and the ELISPOT-based T.SPOT TB test (Oxford Immunotec, Marlborough, MA), has been increasing, especially for BCG-vaccinated populations. However, a major concern regarding these tests is that the rates of indeterminate results are not negligible, which leads to delays in the initiation of treatment by masking positive results. To date, several studies have been performed to investigate the factors related to the frequency of indeterminate QFT-G IT test results.
Using the QuantiFERON TB-2G test, Kobashi et al. performed a large-scale study to investigate clinical factors affecting the frequency of indeterminate results (13). In that study, highly advanced age, immunosuppressive treatment, lymphocytopenia (including CD4+ lymphocytopenia), and hypoproteinemia could promote indeterminate QFT-2G test results. Those authors also showed that only a small proportion of results (16.7%) became determinate even at the repeated test within 1 month. Using the QFT-G IT test, Kim et al. performed a similar study with 211 patients and reported that immunosuppressive conditions, lymphocytopenia, hypoproteinemia, hypoalbuminemia, low hemoglobin levels, and leukocytosis were more frequently observed in patients with indeterminate results versus determinate results (12). In our study, based on the multivariate logistic regression analysis, taking immunosuppressive drugs, previously existing underlying diseases, bedridden status, and hypoalbuminemia were associated with increases in the frequency of indeterminate QFT-G IT test results. Regarding lymphocytopenia, it was observed that lymphocyte counts of <1,500 cells/μl were associated with increased frequency of indeterminate results in univariate analysis by Pearson's chi-square test. However, we found that immunosuppressive treatment was associated with lymphocyte counts; if we removed the effect of immunosuppressive treatment in the multivariate logistic regression analysis, then the association between the frequency of indeterminate results and lymphocytopenia was no longer statistically significant (Tables 1 and 2).
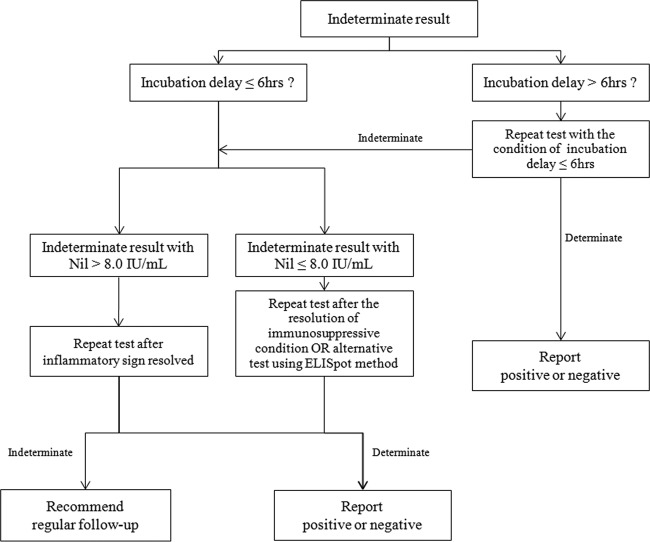
The reported frequencies of indeterminate QFT-G IT test results range from 5% to 40% for all participants, with 6% for health care workers (8–10). To investigate the interlaboratory differences, a small study evaluating the association between incubation delay and frequency of indeterminate QFT-G IT test results was performed in 2010 (14). In that study, Herrera et al. reported that incubation delay is an important factor in the increase in the frequency of indeterminate QFT-G IT test results, and immediate incubation is the best way to reduce the frequency of indeterminate QFT-G IT test results; however, those authors commented that the small sample size of the study did not allow for complete evaluation (14). Another limitation of the study was that the three times for incubation delay, i.e., 0 h, 6 h, and 12 h, were selected based on the time point and not on the time interval. Hence, it was not easy to determine how much delay is acceptable before the start of incubation, since there was no statistically significant increase in the frequency of indeterminate results in the study by Herrera et al. In this study, as described previously, it seems that a maximum of a 6-h incubation delay is acceptable for controlling the frequency of indeterminate QFT-G IT test results. To achieve the best accuracy of the QFT-G IT test, immediate incubation may be ideal (14). However, it is not feasible to perform immediate incubation at every center. Therefore, an effective strategy that balances feasibility and test accuracy needs to be developed. If the incubation delay cannot be reduced to <6 h by making changes in the workflow, then we suggest a new diagnostic algorithm for facilities at which immediate incubation or incubation within 6 h cannot be routinely performed (Fig. 1). With the application of the suggested algorithm, other preanalytical errors, such as storage of tubes outside the recommended temperature range, overfilling or underfilling of tubes with blood, and insufficient mixing, need to be evaluated if the test performance is not satisfactory, based on the manufacturer's instructions (http://www.cellestis.com/irm/content/PI/QFT/2PK/US.pdf).
FIG 1.
New diagnostic algorithm for indeterminate results of the QuantiFERON-TB Gold In-Tube test.
After changes were made to the workflow, the frequencies of indeterminate QFT-G IT test results at our institution were estimated as 1.5% (31/2,080 tests), 3.6% (64/1,777 tests), 4.8% (65/1,342 tests), and 3.5% (65/1,888 tests) in 2009, 2010, 2011, and 2012, respectively. Considering the reported frequency of indeterminate QFT-G IT test results that was previously observed in South Korea (11, 12), the frequency of indeterminate QFT-G IT test results at our institution can be considered to be reasonably low.
In summary, we described the statistically significant clinical and laboratory factors that affect the accuracy of QFT-G IT tests. In particular, the laboratory factor of incubation delay needs to be monitored closely to achieve optimized test results, and it seems that it is important to control the incubation delay to <6 h to reduce the frequency of indeterminate QFT-G IT test results.
ACKNOWLEDGMENTS
We thank Jae Moon Yun, Department of Family Medicine, Seoul National University Hospital, for support in statistical analysis.
We have no potential conflicts of interest that are relevant to this article.
Footnotes
Published ahead of print 23 October 2013
REFERENCES
- 1.Ralph AP, Anstey NM, Kelly PM. 2009. Tuberculosis into the 2010s: is the glass half full? Clin. Infect. Dis. 49:574–583. 10.1086/600889 [DOI] [PubMed] [Google Scholar]
- 2.Maartens G, Wilkinson RJ. 2007. Tuberculosis. Lancet 370:2030–2043. 10.1016/S0140-6736(07)61262-8 [DOI] [PubMed] [Google Scholar]
- 3.Horsburgh CR., Jr 2004. Priorities for the treatment of latent tuberculosis infection in the United States. N. Engl. J. Med. 350:2060–2067. 10.1056/NEJMsa031667 [DOI] [PubMed] [Google Scholar]
- 4.Mazurek GH, Weis SE, Moonan PK, Daley CL, Bernardo J, Lardizabal AA, Reves RR, Toney SR, Daniels LJ, LoBue PA. 2007. Prospective comparison of the tuberculin skin test and 2 whole-blood interferon-gamma release assays in persons with suspected tuberculosis. Clin. Infect. Dis. 45:837–845. 10.1086/521107 [DOI] [PubMed] [Google Scholar]
- 5.Pai M, Riley LW, Colford JM., Jr 2004. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect. Dis. 4:761–776. 10.1016/S1473-3099(04)01206-X [DOI] [PubMed] [Google Scholar]
- 6.Ferrara G, Losi M, D'Amico R, Roversi P, Piro R, Meacci M, Meccugni B, Dori IM, Andreani A, Bergamini BM, Mussini C, Rumpianesi F, Fabbri LM, Richeldi L. 2006. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet 367:1328–1334. 10.1016/S0140-6736(06)68579-6 [DOI] [PubMed] [Google Scholar]
- 7.Kobashi Y, Mouri K, Obase Y, Fukuda M, Miyashita N, Oka M. 2007. Clinical evaluation of QuantiFERON TB-2G test for immunocompromised patients. Eur. Respir. J. 30:945–950. 10.1183/09031936.00040007 [DOI] [PubMed] [Google Scholar]
- 8.Cummings KJ, Smith TS, Shogren ES, Khakoo R, Nanda S, Bunner L, Smithmyer A, Soccorsi D, Kashon ML, Mazurek GH, Friedman LN, Weissman DN. 2009. Prospective comparison of tuberculin skin test and QuantiFERON-TB Gold In-Tube assay for the detection of latent tuberculosis infection among healthcare workers in a low-incidence setting. Infect. Control Hosp. Epidemiol. 30:1123–1126. 10.1086/644754 [DOI] [PubMed] [Google Scholar]
- 9.Lalvani A, Pareek M. 2010. Interferon gamma release assays: principles and practice. Enferm. Infecc. Microbiol. Clin. 28:245–252. 10.1016/j.eimc.2009.05.012 [DOI] [PubMed] [Google Scholar]
- 10.Miranda C, Yen-Lieberman B, Terpeluk P, Tomford JW, Gordon S. 2009. Reducing the rates of indeterminate results of the QuantiFERON-TB Gold In-Tube test during routine preemployment screening for latent tuberculosis infection among healthcare personnel. Infect. Control Hosp. Epidemiol. 30:296–298. 10.1086/595732 [DOI] [PubMed] [Google Scholar]
- 11.Cho K, Cho E, Kwon S, Im S, Sohn I, Song S, Kim H, Kim S. 2012. Factors associated with indeterminate and false negative results of QuantiFERON-TB Gold In-Tube test in active tuberculosis. Tuberc. Respir. Dis. (Seoul) 72:416–425. 10.4046/trd.2012.72.5.416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim EY, Lim JE, Jung JY, Son JY, Lee KJ, Yoon YW, Park BH, Moon JW, Park MS, Kim YS, Kim SK, Chang J, Kang YA. 2009. Performance of the tuberculin skin test and interferon-gamma release assay for detection of tuberculosis infection in immunocompromised patients in a BCG-vaccinated population. BMC Infect. Dis. 9:207. 10.1186/1471-2334-9-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobashi Y, Sugiu T, Mouri K, Obase Y, Miyashita N, Oka M. 2009. Indeterminate results of QuantiFERON TB-2G test performed in routine clinical practice. Eur. Respir. J. 33:812–815. 10.1183/09031936.00075008 [DOI] [PubMed] [Google Scholar]
- 14.Herrera V, Yeh E, Murphy K, Parsonnet J, Banaei N. 2010. Immediate incubation reduces indeterminate results for QuantiFERON-TB Gold in-tube assay. J. Clin. Microbiol. 48:2672–2676. 10.1128/JCM.00482-10 [DOI] [PMC free article] [PubMed] [Google Scholar]