Abstract
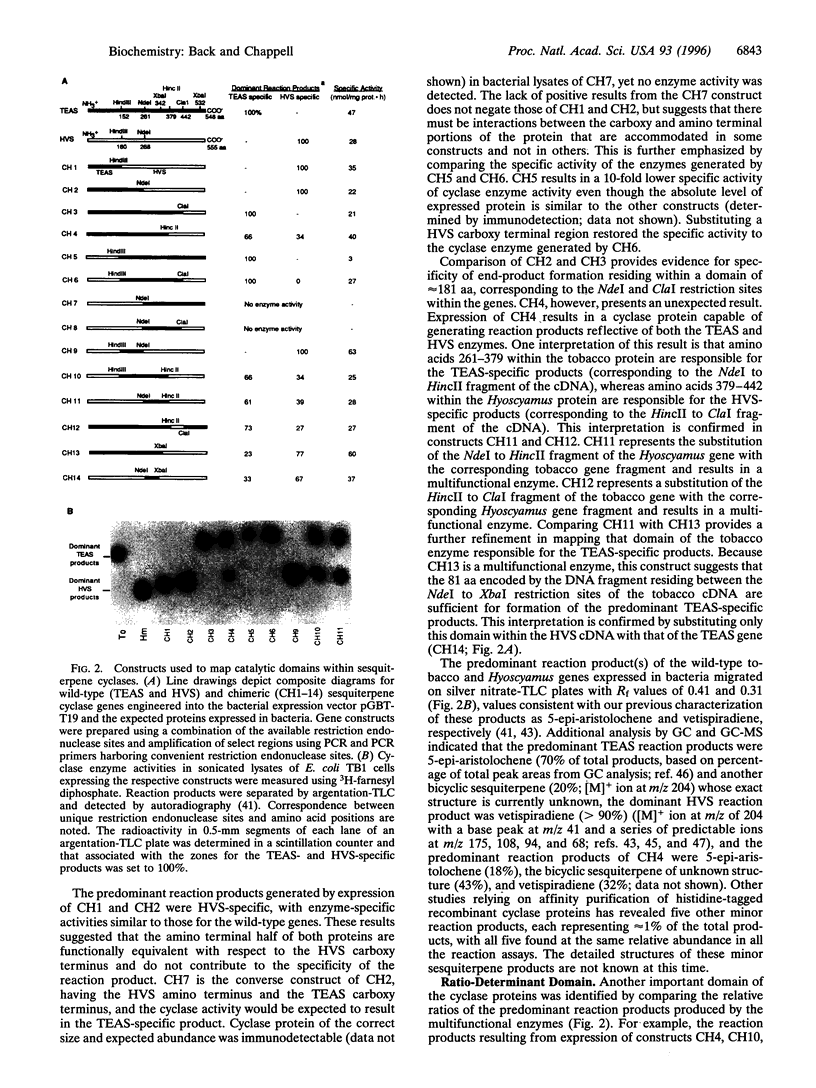
Cyclic terpenes and terpenoids are found throughout nature. They comprise an especially important class of compounds from plants that mediate plant- environment interactions, and they serve as pharmaceutical agents with antimicrobial and anti-tumor activities. Molecular comparisons of several terpene cyclases, the key enzymes responsible for the multistep cyclization of C10, C15, and C20 allylic diphosphate substrates, have revealed a striking level of sequence similarity and conservation of exon position and size within the genes. Functional domains responsible for a terminal enzymatic step were identified by swapping regions approximating exons between a Nicotiana tabacum 5-epi-aristolochene synthase (TEAS) gene and a Hyoscyamus muticus vetispiradiene synthase (HVS) gene and by characterization of the resulting chimeric enzymes expressed in bacteria. While exon 4 of the TEAS gene conferred specificity for the predominant reaction products of the tobacco enzyme, exon 6 of the HVS gene conferred specificity for the predominant reaction products of the Hyoscyamus enzyme. Combining these two functional domains of the TEAS and HVS genes resulted in a novel enzyme capable of synthesizing reaction products reflective of both parent enzymes. The relative ratio of the TEAS and HVS reaction products was also influenced by the source of exon 5 present in the new chimeric enzymes. The association of catalytic activities with conserved but separate exonic domains suggests a general means for generating additional novel terpene cyclases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso W. R., Rajaonarivony J. I., Gershenzon J., Croteau R. Purification of 4S-limonene synthase, a monoterpene cyclase from the glandular trichomes of peppermint (Mentha x piperita) and spearmint (Mentha spicata). J Biol Chem. 1992 Apr 15;267(11):7582–7587. [PubMed] [Google Scholar]
- Anke H., Sterner O. Comparison of the antimicrobial and cytotoxic activities of twenty unsaturated sesquiterpene dialdehydes from plants and mushrooms. Planta Med. 1991 Aug;57(4):344–346. doi: 10.1055/s-2006-960114. [DOI] [PubMed] [Google Scholar]
- Back K., Chappell J. Cloning and bacterial expression of a sesquiterpene cyclase from Hyoscyamus muticus and its molecular comparison to related terpene cyclases. J Biol Chem. 1995 Mar 31;270(13):7375–7381. doi: 10.1074/jbc.270.13.7375. [DOI] [PubMed] [Google Scholar]
- Back K., Yin S., Chappell J. Expression of a plant sesquiterpene cyclase gene in Escherichia coli. Arch Biochem Biophys. 1994 Dec;315(2):527–532. doi: 10.1006/abbi.1994.1533. [DOI] [PubMed] [Google Scholar]
- Cane D. E., Pargellis C. Partial purification and characterization of pentalenene synthase. Arch Biochem Biophys. 1987 May 1;254(2):421–429. doi: 10.1016/0003-9861(87)90120-2. [DOI] [PubMed] [Google Scholar]
- Cane D. E., Shim J. H., Xue Q., Fitzsimons B. C., Hohn T. M. Trichodiene synthase. Identification of active site residues by site-directed mutagenesis. Biochemistry. 1995 Feb 28;34(8):2480–2488. doi: 10.1021/bi00008a011. [DOI] [PubMed] [Google Scholar]
- Cane D. E., Sohng J. K., Lamberson C. R., Rudnicki S. M., Wu Z., Lloyd M. D., Oliver J. S., Hubbard B. R. Pentalenene synthase. Purification, molecular cloning, sequencing, and high-level expression in Escherichia coli of a terpenoid cyclase from Streptomyces UC5319. Biochemistry. 1994 May 17;33(19):5846–5857. doi: 10.1021/bi00185a024. [DOI] [PubMed] [Google Scholar]
- Cane D. E., Yang G., Xue Q., Shim J. H. Trichodiene synthase. Substrate specificity and inhibition. Biochemistry. 1995 Feb 28;34(8):2471–2479. doi: 10.1021/bi00008a010. [DOI] [PubMed] [Google Scholar]
- Chappell J. The Biochemistry and Molecular Biology of Isoprenoid Metabolism. Plant Physiol. 1995 Jan;107(1):1–6. doi: 10.1104/pp.107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. Y., Chen Y., Heinstein P., Davisson V. J. Cloning, expression, and characterization of (+)-delta-cadinene synthase: a catalyst for cotton phytoalexin biosynthesis. Arch Biochem Biophys. 1995 Dec 20;324(2):255–266. doi: 10.1006/abbi.1995.0038. [DOI] [PubMed] [Google Scholar]
- Colby S. M., Alonso W. R., Katahira E. J., McGarvey D. J., Croteau R. 4S-limonene synthase from the oil glands of spearmint (Mentha spicata). cDNA isolation, characterization, and bacterial expression of the catalytically active monoterpene cyclase. J Biol Chem. 1993 Nov 5;268(31):23016–23024. [PubMed] [Google Scholar]
- Croteau R., Alonso W. R., Koepp A. E., Johnson M. A. Biosynthesis of monoterpenes: partial purification, characterization, and mechanism of action of 1,8-cineole synthase. Arch Biochem Biophys. 1994 Feb 15;309(1):184–192. doi: 10.1006/abbi.1994.1101. [DOI] [PubMed] [Google Scholar]
- Croteau R., Alonso W. R., Koepp A. E., Shim J. H., Cane D. E. Irreversible inactivation of monoterpene cyclases by a mechanism-based inhibitor. Arch Biochem Biophys. 1993 Dec;307(2):397–404. doi: 10.1006/abbi.1993.1606. [DOI] [PubMed] [Google Scholar]
- Croteau R. Evidence for the ionization steps in monoterpene cyclization reactions using 2-fluorogeranyl and 2-fluorolinalyl pyrophosphates as substrates. Arch Biochem Biophys. 1986 Dec;251(2):777–782. doi: 10.1016/0003-9861(86)90390-5. [DOI] [PubMed] [Google Scholar]
- Facchini P. J., Chappell J. Gene family for an elicitor-induced sesquiterpene cyclase in tobacco. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11088–11092. doi: 10.1073/pnas.89.22.11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambliel H., Croteau R. Pinene cyclases I and II. Two enzymes from sage (Salvia officinalis) which catalyze stereospecific cyclizations of geranyl pyrophosphate to monoterpene olefins of opposite configuration. J Biol Chem. 1984 Jan 25;259(2):740–748. [PubMed] [Google Scholar]
- Guo Z., Severson R. F., Wagner G. J. Biosynthesis of the diterpene cis-abienol in cell-free extracts of tobacco trichomes. Arch Biochem Biophys. 1994 Jan;308(1):103–108. doi: 10.1006/abbi.1994.1015. [DOI] [PubMed] [Google Scholar]
- Habtemariam S., Gray A. I., Waterman P. G. A new antibacterial sesquiterpene from Premna oligotricha. J Nat Prod. 1993 Jan;56(1):140–143. doi: 10.1021/np50091a022. [DOI] [PubMed] [Google Scholar]
- Hohn T. M., Beremand P. D. Isolation and nucleotide sequence of a sesquiterpene cyclase gene from the trichothecene-producing fungus Fusarium sporotrichioides. Gene. 1989 Jun 30;79(1):131–138. doi: 10.1016/0378-1119(89)90098-x. [DOI] [PubMed] [Google Scholar]
- Hohn T. M., Plattner R. D. Purification and characterization of the sesquiterpene cyclase aristolochene synthase from Penicillium roqueforti. Arch Biochem Biophys. 1989 Jul;272(1):137–143. doi: 10.1016/0003-9861(89)90204-x. [DOI] [PubMed] [Google Scholar]
- Hohn T. M., Vanmiddlesworth F. Purification and characterization of the sesquiterpene cyclase trichodiene synthetase from Fusarium sporotrichioides. Arch Biochem Biophys. 1986 Dec;251(2):756–761. doi: 10.1016/0003-9861(86)90386-3. [DOI] [PubMed] [Google Scholar]
- Kubo I., Himejima M. Potentiation of antifungal activity of sesquiterpene dialdehydes against Candida albicans and two other fungi. Experientia. 1992 Dec 1;48(11-12):1162–1164. doi: 10.1007/BF01948015. [DOI] [PubMed] [Google Scholar]
- Lee K. H., Hall I. H., Mar E. C., Starnes C. O., ElGebaly S. A., Waddell T. G., HADGRAFT R. I., Ruffner C. G., Weidner I. Sesquiterpene antitumor agents: inhibitors of cellular metabolism. Science. 1977 Apr 29;196(4289):533–536. doi: 10.1126/science.191909. [DOI] [PubMed] [Google Scholar]
- Mau C. J., West C. A. Cloning of casbene synthase cDNA: evidence for conserved structural features among terpenoid cyclases in plants. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8497–8501. doi: 10.1073/pnas.91.18.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moesta P., West C. A. Casbene synthetase: regulation of phytoalexin biosynthesis in Ricinus communis L. seedlings. Purification of casbene synthetase and regulation of its biosynthesis during elicitation. Arch Biochem Biophys. 1985 Apr;238(1):325–333. doi: 10.1016/0003-9861(85)90171-7. [DOI] [PubMed] [Google Scholar]
- Munck S. L., Croteau R. Purification and characterization of the sesquiterpene cyclase patchoulol synthase from Pogostemon cablin. Arch Biochem Biophys. 1990 Oct;282(1):58–64. doi: 10.1016/0003-9861(90)90086-e. [DOI] [PubMed] [Google Scholar]
- Proctor R. H., Hohn T. M. Aristolochene synthase. Isolation, characterization, and bacterial expression of a sesquiterpenoid biosynthetic gene (Ari1) from Penicillium roqueforti. J Biol Chem. 1993 Feb 25;268(6):4543–4548. [PubMed] [Google Scholar]
- Pyun H. J., Wagschal K. C., Jung D. I., Coates R. M., Croteau R. Stereochemistry of the proton elimination in the formation of (+)- and (-)-alpha-pinene by monoterpene cyclases from sage (Salvia officinalis). Arch Biochem Biophys. 1994 Feb 1;308(2):488–496. doi: 10.1006/abbi.1994.1069. [DOI] [PubMed] [Google Scholar]
- Rajaonarivony J. I., Gershenzon J., Croteau R. Characterization and mechanism of (4S)-limonene synthase, a monoterpene cyclase from the glandular trichomes of peppermint (Mentha x piperita). Arch Biochem Biophys. 1992 Jul;296(1):49–57. doi: 10.1016/0003-9861(92)90543-6. [DOI] [PubMed] [Google Scholar]
- Rajaonarivony J. I., Gershenzon J., Miyazaki J., Croteau R. Evidence for an essential histidine residue in 4S-limonene synthase and other terpene cyclases. Arch Biochem Biophys. 1992 Nov 15;299(1):77–82. doi: 10.1016/0003-9861(92)90246-s. [DOI] [PubMed] [Google Scholar]
- Savage T. J., Croteau R. Biosynthesis of monoterpenes: regio- and stereochemistry of (+)-3-carene biosynthesis. Arch Biochem Biophys. 1993 Sep;305(2):581–587. doi: 10.1006/abbi.1993.1464. [DOI] [PubMed] [Google Scholar]
- Savage T. J., Hatch M. W., Croteau R. Monoterpene synthases of Pinus contorta and related conifers. A new class of terpenoid cyclase. J Biol Chem. 1994 Feb 11;269(6):4012–4020. [PubMed] [Google Scholar]
- Savage T. J., Hatch M. W., Croteau R. Monoterpene synthases of Pinus contorta and related conifers. A new class of terpenoid cyclase. J Biol Chem. 1994 Feb 11;269(6):4012–4020. [PubMed] [Google Scholar]
- Vögeli U., Freeman J. W., Chappell J. Purification and characterization of an inducible sesquiterpene cyclase from elicitor-treated tobacco cell suspension cultures. Plant Physiol. 1990 May;93(1):182–187. doi: 10.1104/pp.93.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagschal K. C., Pyun H. J., Coates R. M., Croteau R. Monoterpene biosynthesis: isotope effects associated with bicyclic olefin formation catalyzed by pinene synthases from sage (Salvia officinalis). Arch Biochem Biophys. 1994 Feb 1;308(2):477–487. doi: 10.1006/abbi.1994.1068. [DOI] [PubMed] [Google Scholar]
- Wheeler C. J., Croteau R. B. Direct demonstration of the isomerization component of the monoterpene cyclase reaction using a cyclopropylcarbinyl pyrophosphate substrate analog. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4856–4859. doi: 10.1073/pnas.84.14.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]



