Abstract
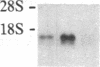
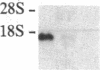
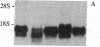
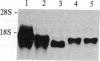
To further characterize sequential events involved in activation of genes encoding the T-cell receptor (a complex of T3 molecules and a disulfide-linked heterodimer designated Ti, T3-Ti) for antigen and the major histocompatibility complex during intrathymic ontogeny, cDNA probes specific for Ti alpha and Ti beta subunits were used for transcriptional analysis. Ti beta transcript levels were minimal in stage I thymocytes, maximal in stage II thymocytes, and intermediate in state III thymocytes. In contrast, Ti alpha transcriptional activity was virtually undetectable in stage I, was low in stage II, and achieved high levels only in the stage III compartment. Analysis of tumor populations derived from individual stages of thymic differentiation confirmed these observations and demonstrated that clonal stage I-II cells often express Ti beta RNA in the absence of Ti alpha RNA. The latter was not a consequence of functional Ti alpha-subunit isotypy because each of 13 interleukin 2-dependent T-cell clones, including inducer, suppressor, and cytotoxic T cells, contain transcripts that hybridize with the Ti alpha probe. From these data it is concluded that Ti beta gene activation precedes Ti alpha gene activation. Moreover, the high level of Ti beta mRNA preceding Ti alpha mRNA expression implies that Ti beta mRNA and/or its protein products may regulate Ti alpha gene transcription.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuto O., Campen T. J., Royer H. D., Hussey R. E., Poole C. B., Reinherz E. L. Molecular analysis of T cell receptor (Ti) variable region (V) gene expression. Evidence that a single Ti beta V gene family can be used in formation of V domains on phenotypically and functionally diverse T cell populations. J Exp Med. 1985 Jun 1;161(6):1326–1343. doi: 10.1084/jem.161.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuto O., Fabbi M., Smart J., Poole C. B., Protentis J., Royer H. D., Schlossman S. F., Reinherz E. L. Purification and NH2-terminal amino acid sequencing of the beta subunit of a human T-cell antigen receptor. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3851–3855. doi: 10.1073/pnas.81.12.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuto O., Hussey R. E., Fitzgerald K. A., Protentis J. P., Meuer S. C., Schlossman S. F., Reinherz E. L. The human T cell receptor: appearance in ontogeny and biochemical relationship of alpha and beta subunits on IL-2 dependent clones and T cell tumors. Cell. 1983 Oct;34(3):717–726. doi: 10.1016/0092-8674(83)90528-7. [DOI] [PubMed] [Google Scholar]
- Acuto O., Meuer S. C., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Peptide variability exists within alpha and beta subunits of the T cell receptor for antigen. J Exp Med. 1983 Oct 1;158(4):1368–1373. doi: 10.1084/jem.158.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Enea V., Bothwell A. L., Baltimore D. Activity of multiple light chain genes in murine myeloma cells producing a single, functional light chain. Cell. 1980 Aug;21(1):1–12. doi: 10.1016/0092-8674(80)90109-9. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Bensussan A., Acuto O., Hussey R. E., Milanese C., Reinherz E. L. T3-Ti receptor triggering of T8+ suppressor T cells leads to unresponsiveness to interleukin-2. Nature. 1984 Oct 11;311(5986):565–567. doi: 10.1038/311565a0. [DOI] [PubMed] [Google Scholar]
- Bensussan A., Meuer S. C., Schlossman S. F., Reinherz E. L. Delineation of an immunoregulatory amplifier population recognizing autologous Ia molecules. Analysis with human T cell clones. J Exp Med. 1984 Feb 1;159(2):559–576. doi: 10.1084/jem.159.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bernard O., Gough N. M., Adams J. M. Plasmacytomas with more than one immunoglobulin kappa mRNA: implications for allelic exclusion. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5812–5816. doi: 10.1073/pnas.78.9.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Burrows P., LeJeune M., Kearney J. F. Evidence that murine pre-B cells synthesise mu heavy chains but no light chains. Nature. 1979 Aug 30;280(5725):838–840. doi: 10.1038/280838a0. [DOI] [PubMed] [Google Scholar]
- Chien Y., Becker D. M., Lindsten T., Okamura M., Cohen D. I., Davis M. M. A third type of murine T-cell receptor gene. Nature. 1984 Nov 1;312(5989):31–35. doi: 10.1038/312031a0. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Fabbi M., Acuto O., Smart J. E., Reinherz E. L. Homology of Ti alpha-subunit of a T-cell antigen-MHC receptor with immunoglobulin. Nature. 1984 Nov 15;312(5991):269–271. doi: 10.1038/312269a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Chien Y., Becker D. M., Kavaler J., Davis M. M. Genomic organization and sequence of T-cell receptor beta-chain constant- and joining-region genes. Nature. 1984 Aug 2;310(5976):387–391. doi: 10.1038/310387a0. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Beveridge R. P., Schlossman S. F. Isolation of myeloid progenitor cells from peripheral blood of chronic myelogenous leukemia patients. Blood. 1982 Jul;60(1):30–37. [PubMed] [Google Scholar]
- Hannum C. H., Kappler J. W., Trowbridge I. S., Marrack P., Freed J. H. Immunoglobulin-like nature of the alpha-chain of a human T-cell antigen/MHC receptor. Nature. 1984 Nov 1;312(5989):65–67. doi: 10.1038/312065a0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Nielsen E. A., Kavaler J., Cohen D. I., Davis M. M. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984 Mar 8;308(5955):153–158. doi: 10.1038/308153a0. [DOI] [PubMed] [Google Scholar]
- Kwan S. P., Max E. E., Seidman J. G., Leder P., Scharff M. D. Two kappa immunoglobulin genes are expressed in the myeloma S107. Cell. 1981 Oct;26(1 Pt 1):57–66. doi: 10.1016/0092-8674(81)90033-7. [DOI] [PubMed] [Google Scholar]
- Levitt D., Cooper M. D. Mouse pre-B cells synthesize and secrete mu heavy chains but not light chains. Cell. 1980 Mar;19(3):617–625. doi: 10.1016/s0092-8674(80)80038-9. [DOI] [PubMed] [Google Scholar]
- Maki R., Kearney J., Paige C., Tonegawa S. Immunoglobulin gene rearrangement in immature B cells. Science. 1980 Sep 19;209(4463):1366–1369. doi: 10.1126/science.6774416. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Acuto O., Hussey R. E., Hodgdon J. C., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Evidence for the T3-associated 90K heterodimer as the T-cell antigen receptor. Nature. 1983 Jun 30;303(5920):808–810. doi: 10.1038/303808a0. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Cooper D. A., Hodgdon J. C., Hussey R. E., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Identification of the receptor for antigen and major histocompatibility complex on human inducer T lymphocytes. Science. 1983 Dec 16;222(4629):1239–1242. doi: 10.1126/science.6606228. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Cooper D. A., Hodgdon J. C., Hussey R. E., Morimoto C., Schlossman S. F., Reinherz E. L. Immunoregulatory human T lymphocytes triggered as a consequence of viral infection: clonal analysis of helper, suppressor inducer and suppressor effector cell populations. J Immunol. 1983 Sep;131(3):1167–1172. [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Hodgdon J. C., Hercend T., Schlossman S. F., Reinherz E. L. Surface structures involved in target recognition by human cytotoxic T lymphocytes. Science. 1982 Oct 29;218(4571):471–473. doi: 10.1126/science.6981845. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Schlossman S. F., Reinherz E. L. Clonal analysis of human cytotoxic T lymphocytes: T4+ and T8+ effector T cells recognize products of different major histocompatibility complex regions. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4395–4399. doi: 10.1073/pnas.79.14.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M. G., Alt F. W. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. 1984 Nov 29-Dec 5Nature. 312(5993):418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- Ritchie K. A., Brinster R. L., Storb U. Allelic exclusion and control of endogenous immunoglobulin gene rearrangement in kappa transgenic mice. Nature. 1984 Dec 6;312(5994):517–520. doi: 10.1038/312517a0. [DOI] [PubMed] [Google Scholar]
- Royer H. D., Acuto O., Fabbi M., Tizard R., Ramachandran K., Smart J. E., Reinherz E. L. Genes encoding the Ti beta subunit of the antigen/MHC receptor undergo rearrangement during intrathymic ontogeny prior to surface T3-Ti expression. Cell. 1984 Dec;39(2 Pt 1):261–266. doi: 10.1016/0092-8674(84)90003-5. [DOI] [PubMed] [Google Scholar]
- Royer H. D., Bensussan A., Acuto O., Reinherz E. L. Functional isotypes are not encoded by the constant region genes of the beta subunit of the T cell receptor for antigen/major histocompatibility complex. J Exp Med. 1984 Sep 1;160(3):947–952. doi: 10.1084/jem.160.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984 Nov 1;312(5989):36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Siden E. J., Baltimore D., Clark D., Rosenberg N. E. Immunoglobulin synthesis by lymphoid cells transformed in vitro by Abelson murine leukemia virus. Cell. 1979 Feb;16(2):389–396. doi: 10.1016/0092-8674(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Sim G. K., Yagüe J., Nelson J., Marrack P., Palmer E., Augustin A., Kappler J. Primary structure of human T-cell receptor alpha-chain. Nature. 1984 Dec 20;312(5996):771–775. doi: 10.1038/312771a0. [DOI] [PubMed] [Google Scholar]
- Sims J. E., Tunnacliffe A., Smith W. J., Rabbitts T. H. Complexity of human T-cell antigen receptor beta-chain constant- and variable-region genes. Nature. 1984 Dec 6;312(5994):541–545. doi: 10.1038/312541a0. [DOI] [PubMed] [Google Scholar]
- Siu G., Clark S. P., Yoshikai Y., Malissen M., Yanagi Y., Strauss E., Mak T. W., Hood L. The human T cell antigen receptor is encoded by variable, diversity, and joining gene segments that rearrange to generate a complete V gene. Cell. 1984 Jun;37(2):393–401. doi: 10.1016/0092-8674(84)90369-6. [DOI] [PubMed] [Google Scholar]
- Siu G., Kronenberg M., Strauss E., Haars R., Mak T. W., Hood L. The structure, rearrangement and expression of D beta gene segments of the murine T-cell antigen receptor. 1984 Sep 27-Oct 3Nature. 311(5984):344–350. doi: 10.1038/311344a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Umiel T., Daley J. F., Bhan A. K., Levey R. H., Schlossman S. F., Reinherz E. L. Acquisition of immune competence by a subset of human cortical thymocytes expressing mature T cell antigens. J Immunol. 1982 Sep;129(3):1054–1060. [PubMed] [Google Scholar]
- Weiss M. J., Daley J. F., Hodgdon J. C., Reinherz E. L. Calcium dependency of antigen-specific (T3-Ti) and alternative (T11) pathways of human T-cell activation. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6836–6840. doi: 10.1073/pnas.81.21.6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S. L. A sensitive and rapid method for recombinant phage screening. Methods Enzymol. 1979;68:389–395. doi: 10.1016/0076-6879(79)68028-x. [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Anatoniou D., Clark S. P., Yanagi Y., Sangster R., Van den Elsen P., Terhorst C., Mak T. W. Sequence and expression of transcripts of the human T-cell receptor beta-chain genes. Nature. 1984 Dec 6;312(5994):521–524. doi: 10.1038/312521a0. [DOI] [PubMed] [Google Scholar]