Abstract
Viruses with spindle-shaped virions are abundant in diverse environments. Over the years, such viruses have been isolated from a wide range of archaeal hosts. Evolutionary relationships between them remained enigmatic, however. Here, using structural proteins as markers, we define familial ties among these “dark horses” of the virosphere and segregate all spindle-shaped viruses into two distinct evolutionary lineages, corresponding to Bicaudaviridae and Fuselloviridae. Our results illuminate the utility of structure-based virus classification and bring additional order to the virosphere.
TEXT
Recent environmental studies have revealed that viruses with spindle-shaped virions are widespread and abundant in diverse habitats, including deep sea hydrothermal vents (1–3), hypersaline environments (4–7), anoxic freshwaters (8), cold Antarctic lakes (9), terrestrial hot springs (10–15), and acidic mines (16, 17), where these viruses often outnumber the ubiquitous head-tailed viruses and are likely to play an important ecological role. All spindle-shaped viruses that have been isolated so far exclusively infect archaeal hosts (18); none are associated with the two other cellular domains, the Bacteria or Eukarya. The virus species are classified by the International Committee on Taxonomy of Viruses (ICTV) into two families (Fuselloviridae and Bicaudaviridae) and one unassigned genus (Salterprovirus). Notably, there is certain flexibility in virion morphology among spindle-shaped viruses, even for members of the same family. For example, genetically close members of the genera Alphafusellovirus and Betafusellovirus (family Fuselloviridae) (19) are very different morphologically (compare Fig. 1B1 and B2). Importantly, virion flexibility might represent an inherent, biologically relevant property common to all spindle-shaped viruses. It has been demonstrated recently that under certain conditions, virions of halophilic salterprovirus His1 (5, 20) and hyperthermophilic virus Pyrococcus abysii virus 1 (PAV1) (2) also undergo structural transformation from regular spindles into elongated particles (2, 21, 22).
FIG 1.
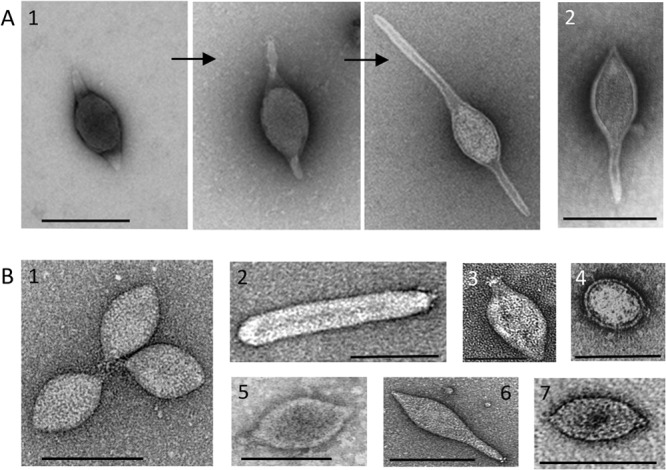
Negative-contrast electron micrographs of viral species with spindle-shaped virions. (A1) Three stages of extracellular tail development of ATV, the type species of the family Bicaudaviridae (35); (A2) STSV1 (27). (B1) SSV1, the type species of the genus Alphafusellovirus, family Fuselloviridae; (B2) Sulfolobus spindle-shaped virus 6 (SSV6), the type species of the genus Betafusellovirus, family Fuselloviridae (19); (B3) TPV1 (3); (B4), A3-VLP (25); (B5) PAV1 (2); (B6) APSV1 (26); (B7) His1 (20), the type species of the genus Salterprovirus. Scale bars, 100 nm.
Over the years, a number of spindle-shaped viruses that could not be assigned to the existing taxa based on genome similarity have been isolated from phylogenetically distant archaeal lineages, including Thermococcales (Thermococcus prieurii virus 1 [TPV1] [3] and PAV1 [2, 23]), Methanococcales (Methanococcus voltae A3 VLP [A3-VLP] [24, 25]), Desulfurococcales (Aeropyrum pernix spindle-shaped virus 1 [APSV1] [26]), and Sulfolobales (Sulfolobus tengchongensis spindle-shaped viruses 1 and 2 [STSV1 and -2] [27, 28]). For a long time, these viruses remained “dark horses” of the archaeal virosphere, with their origins and relationships to other archaeal viruses being untraceable. Here, we assess the morphological and genomic diversity of this prominent virus group, reveal the evolutionary relationships between different spindle-shaped viruses, and refine their classification.
Viral proteins underlying the key principles of virion assembly and architecture provide a valuable marker for tracing deep evolutionary connections between distantly related viruses (29–31). Major capsid proteins (MCP) have been experimentally characterized for Sulfolobus spindle-shaped virus 1 (SSV1), a type species of the Fuselloviridae (32, 33), Acidianus two-tailed virus (ATV), a type species of the Bicaudaviridae (34), and, more recently, for His1 virus, a type species of the genus Salterprovirus (22). We used this information to perform an in-depth genome analysis of all known unclassified spindle-shaped viruses.
Spindle-shaped viruses with tails.
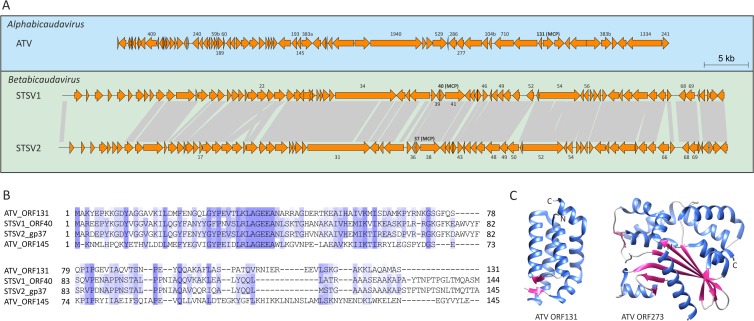
Acidianus two-tailed virus (ATV) is the sole member of the Bicaudaviridae family. A remarkable characteristic of this virus is that it can develop long tails at both pointed ends of the spindle-shaped virion outside the host cell (34, 35). The ATV virion consists of several structural proteins; high-resolution structures for two of these proteins are known (36, 37). Among the unclassified viruses, only STSV1 (27) and STSV2 (28) were found to encode homologues of the major structural protein gp131 of ATV (Fig. 2A and B). Notably, the same protein was indeed identified as the MCP of STSV1 (27). In addition, comparative genome analysis revealed that ATV has 18 genes in common with STSV1 and STSV2 (Fig. 2A), suggesting an evolutionary relationship between these viruses. Unlike ATV, STSV1 and STSV2 each have a single long tail emanating from one of the pointed ends of the virion (Fig. 1). Furthermore, extracellular morphogenesis has not been demonstrated for these viruses (27). STSV1 and STSV2 apparently possess more simple virions than ATV: (i) they do not encode homologues of the structural ATV protein gp273 (Fig. 2C), and (ii) a paralog of the ATV MCP, gp145 (Fig. 2B), also a structural component of the ATV virion, is not encoded by STSV1 or STSV2. Based on the shared gene content and similarities between their MCPs, we propose to classify STSV1 and STSV2 into a new genus, Betabicaudavirus, within the family Bicaudaviridae.
FIG 2.
Evolutionary relationship between spindle-shaped viruses with tails. (A) Genome maps of Acidianus two-tailed virus (ATV) and Sulfolobus tengchongensis spindle-shaped viruses 1 and 2 (STSV1 and -2). Homologous regions shared between STSV1 and STSV2 are connected by gray shading. Names of ATV genes that have homologs in STSV1 and/or STSV2 are indicated; the names of corresponding STSV1 and STSV2 genes are also shown. The new genus “Betabicaudavirus” within the family Bicaudaviridae is proposed for classification of STSV1 and STSV2. (B) Multiple alignment of major capsid protein sequences of ATV, STSV1, and STSV2. Note that products of ATV ORF145 and ORF131 are paralogs. GenBank identification (GI) numbers: ATV ORF131, 75750454; ATV ORF145, 75750440; STSV1 ORF40, 51980166; STSV2 gp37, 448260184. (C) Available X-ray structures of two ATV structural proteins, ORF131 (PDB ID no. 3FAJ) and ORF273 (PDB ID no. 4ATS), both displaying unique folds. Whereas a homologue of ORF131 is encoded by both STSV1 and STSV2 (A and B), ORF273 is unique to ATV.
Tailless spindle-shaped viruses.
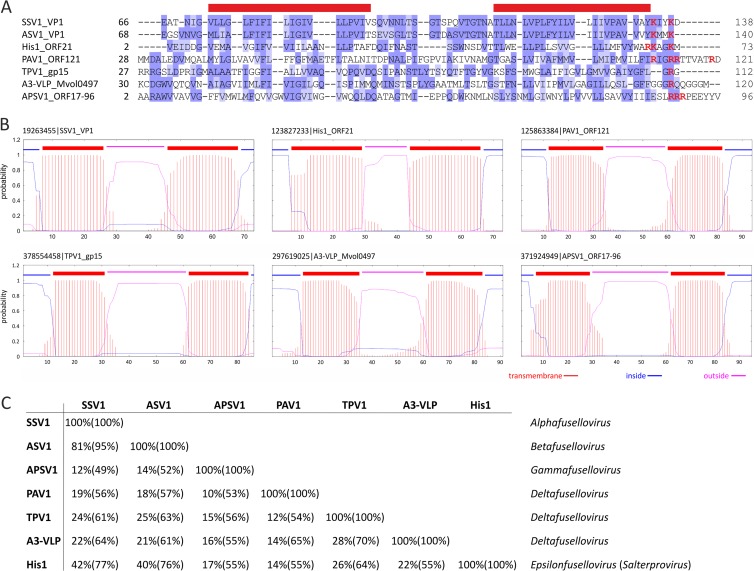
Fuselloviruses and salterprovirus His1 typically display regular spindle-shaped morphology and build their virions using utterly different structural proteins than bicaudaviruses. Whereas MCPs of bicaudaviruses display a unique helix bundle topology (Fig. 2C) (37, 38), those of fuselloviruses and His1 are characterized by two hydrophobic domains (22, 32). Thus, it has been suggested that hyperthermophilic fuselloviruses and halophilic salterprovirus His1 might be evolutionarily related (5, 22), despite infecting hosts residing in different archaeal phyla—Crenarchaeota and Euryarchaeota, respectively. Notably, fuselloviruses encode two paralogous MCPs (VP1 and VP3), while His1 suffices with the product of a single gene (open reading frame 21 [ORF21]). We have investigated the genomes of unclassified spindle-shaped viruses for the presence of ORFs that would (i) display sequence similarity to the MCPs of fuselloviruses and His1 and (ii) share similar hydrophobicity profiles with these proteins. (Homologs were searched for using BLASTP [39], while hydrophobicity profiles were calculated with TMHMM v2 [40]). In all of the viral genomes studied, we could identify ORFs encoding proteins matching our search criteria (Fig. 3A and B). Notably, ORF121 of PAV1, which was identified as a homologue of fuselloviral MCPs (22), has been identified as the major structural component of the PAV1 virions (23), confirming the validity of our approach. Likewise, we identified homologous MCPs in TPV1, A3-VLP, and APSV1 (Fig. 3), which previously eluded functional annotation. The identification of a fusellovirus-like MCP in APSV1 is perhaps most unexpected; based on virion morphology, APSV1 was originally considered to be related to bicaudaviruses (26). However, our analysis shows that it is related to fuselloviruses instead. Although sequence identities between the MCPs of different viruses were generally low, the overall pairwise sequence similarities were typically above 50% (Fig. 3C). (Sequence identities and similarities were calculated using SIAS [http://imed.med.ucm.es/Tools/sias.html], considering the physicochemical properties of aligned amino acids.) Notably, all of the predicted MCPs contain positively charged amino acid residues in the short hydrophilic tail following the C-terminal hydrophobic domain (Fig. 3A). However, the functional significance of these residues remains to be tackled experimentally.
FIG 3.
Evolutionary relationship between tailless spindle-shaped viruses. (A) Multiple alignment of major capsid protein sequences. Red bars above the alignment denote the positions of the two hydrophobic α-helixes. The positively charged residues (R and K) found at the hydrophilic C-terminal tail following the hydrophobic domain are highlighted in red. GenBank identification (GI) numbers: SSV1 VP1, 19263455; ASV1 VP1, 270281782; His1 ORF21, 123827233; PAV1 ORF121, 125863384; TPV1 gp15, 378554458; A3-VLP Mvol0497, 297619025; APSV1 orf17-96, 371924949. (B) Hydrophobicity profiles of the capsid proteins aligned in panel A. (C) Pairwise identity and similarity (in parentheses) values calculated from the alignment shown in panel A using SIAS (http://imed.med.ucm.es/Tools/sias.html). Sequence similarity was calculated by taking into consideration the following physicochemical properties of aligned amino acids: aromatic (F, Y, W), hydrophobic (V, I, L, M, C, A, F, Y, W), aliphatic (V, I, L), positively charged (R, K, H), negatively charged (D, E), polar (N, Q, H, K, R, D, E, T, S), or small (A, T, S, G). The proposed taxonomic classification of the tailless spindle-shaped viruses is shown on the right.
Horizontal gene transfer plays a profound role in shaping the genomic landscape of viruses: any given gene in a viral genome, including those responsible for essential functions, such as genome replication and virion formation, can be replaced by nonhomologous counterparts (41–44). Consequently, virus classification based on a small number of shared characters does not always faithfully represent the evolutionary history of a given viral group. With this caveat in mind, we sought to further validate the grouping of tailless spindle-shaped viruses by performing an exhaustive comparative genomic analysis of spindle-shaped viruses. We found that besides the MCP genes, these viruses share an overlapping set of genes encoding various proteins involved in viral genome replication and integration (Fig. 4). For example, APSV1 encodes four other proteins with homologues in tailless spindle-shaped viruses, including the DnaA-like AAA+ ATPase believed to be involved in genome replication (45). Similarly, in addition to the MCP, PAV1 shares a three-gene cassette with A3-VLP (23, 46), while with TPV1, it shares two other genes for putative minor structural proteins (3). Notably, our analysis has shown that all spindle-shaped viruses encode AAA+ ATPases. Interestingly, however, the DnaA-like ATPases typical of SSV1-like fuselloviruses apparently have been replaced in some of the lineages with nonorthologous AAA+ ATPases from plasmids. Such an exchange is most explicit in the case of PAV1 and a group of Thermococcales plasmids (46), emphasizing the network-like process of evolution in this viral group. Based on the evidence of related capsid proteins (Fig. 3) and the shared overlapping gene content (Fig. 4), we propose to classify all tailless spindle-shaped viruses into different genera within the family Fuselloviridae (Fig. 3C).
FIG 4.
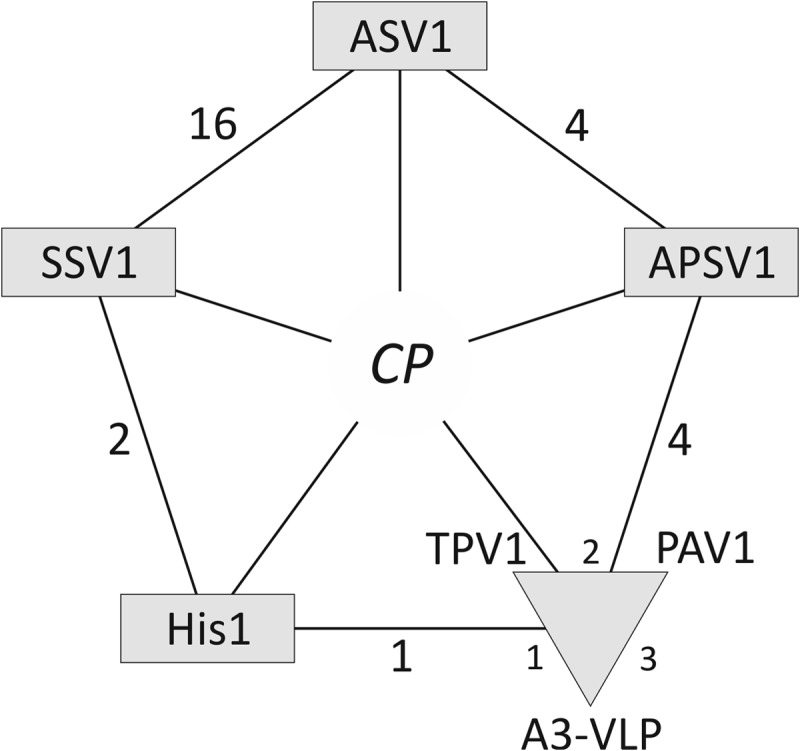
An overlapping gene set shared by tailless spindle-shaped viruses. The diagram shows that in addition to the capsid protein (CP) gene, the viruses share an overlapping set of genes. The numbers next to the lines connecting the viruses denote the number of shared genes. PAV1, TPV1, and A3-VLP are proposed to be grouped into a new genus “Deltafusellovirus,” and are indicated with a triangle.
Here we have addressed a long-standing, unsettled question regarding the evolutionary relationships among spindle-shaped archaeal viruses. Previous efforts failed to reveal links between spindle-shaped viruses infecting phylogenetically distant hosts. Our analysis shows that all known spindle-shaped viruses can be segregated into two distinct groups, corresponding to the families Fuselloviridae and Bicaudaviridae. Peculiarly, similarity in the overall virion morphology for the two viral groups appears to be a result of convergence, rather than divergence; notably, unlike MCPs of fuselloviruses, which are highly hydrophobic (Fig. 3B), the MCPs of bicaudaviruses are predicted to be soluble, consistent with experimental evidence (37). It is rather surprising that spindle-shaped viruses are abundant in archaea but have not been discovered in bacteria or eukaryotes. Presumably, this morphotype is well suited for interaction with archaeal cells, which often dwell in harsh habitats. Clearly, further studies on the biology and structure of spindle-shaped viruses are necessary to explain this specific association. The observation that evolutionarily related spindle-shaped viruses infect hosts thriving in extremely diverse environments and belonging to distinct phylogenetic, metabolic, and physiological groups (acidophiles, hyperthermophiles, methanogens, and halophiles) suggests that the origin of this viral lineage is likely to antedate the radiation of major archaeal groups. More generally, our results demonstrate the utility of the structure-based virus classification (29, 30) and bring additional order to the viral universe.
ACKNOWLEDGMENTS
This work was supported by the Agence Nationale de la Recherche (ANR) program BLANC, project EXAVIR (D.P.) and by the Academy Professor (Academy of Finland) funding grants 255342 and 256518 (D.H.B.). E.R.J.Q. was supported by a fellowship from the Ministère de l'Enseignement Supérieur et de la Recherche of France and the Université Pierre et Marie Curie, Paris, France.
Footnotes
Published ahead of print 11 December 2013
REFERENCES
- 1.Geslin C, Le Romancer M, Gaillard M, Erauso G, Prieur D. 2003. Observation of virus-like particles in high temperature enrichment cultures from deep-sea hydrothermal vents. Res. Microbiol. 154:303–307. 10.1016/S0923-2508(03)00075-5 [DOI] [PubMed] [Google Scholar]
- 2.Geslin C, Le Romancer M, Erauso G, Gaillard M, Perrot G, Prieur D. 2003. PAV1, the first virus-like particle isolated from a hyperthermophilic euryarchaeote, “Pyrococcus abyssi.” J. Bacteriol. 185:3888–3894. 10.1128/JB.185.13.3888-3894.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorlas A, Koonin EV, Bienvenu N, Prieur D, Geslin C. 2012. TPV1, the first virus isolated from the hyperthermophilic genus Thermococcus. Environ. Microbiol. 14:503–516. 10.1111/j.1462-2920.2011.02662.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sime-Ngando T, Lucas S, Robin A, Tucker KP, Colombet J, Bettarel Y, Desmond E, Gribaldo S, Forterre P, Breitbart M, Prangishvili D. 2011. Diversity of virus-host systems in hypersaline Lake Retba, Senegal. Environ. Microbiol. 13:1956–1972. 10.1111/j.1462-2920.2010.02323.x [DOI] [PubMed] [Google Scholar]
- 5.Bath C, Dyall-Smith ML. 1998. His1, an archaeal virus of the Fuselloviridae family that infects Haloarcula hispanica. J. Virol. 72:9392–9395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter K, Russ BE, Dyall-Smith ML. 2007. Virus-host interactions in salt lakes. Curr. Opin. Microbiol. 10:418–424. 10.1016/j.mib.2007.05.017 [DOI] [PubMed] [Google Scholar]
- 7.Oren A, Bratbak G, Heldal M. 1997. Occurrence of virus-like particles in the Dead Sea. Extremophiles 1:143–149. 10.1007/s007920050027 [DOI] [PubMed] [Google Scholar]
- 8.Borrel G, Colombet J, Robin A, Lehours AC, Prangishvili D, Sime-Ngando T. 2012. Unexpected and novel putative viruses in the sediments of a deep-dark permanently anoxic freshwater habitat. ISME J. 6:2119–2127. 10.1038/ismej.2012.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López-Bueno A, Tamames J, Velazquez D, Moya A, Quesada A, Alcami A. 2009. High diversity of the viral community from an Antarctic lake. Science 326:858–861. 10.1126/science.1179287 [DOI] [PubMed] [Google Scholar]
- 10.Rice G, Stedman K, Snyder J, Wiedenheft B, Willits D, Brumfield S, McDermott T, Young MJ. 2001. Viruses from extreme thermal environments. Proc. Natl. Acad. Sci. U. S. A. 98:13341–13345. 10.1073/pnas.231170198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bize A, Peng X, Prokofeva M, MacLellan K, Lucas S, Forterre P, Garrett RA, Bonch-Osmolovskaya EA, Prangishvili D. 2008. Viruses in acidic geothermal environments of the Kamchatka Peninsula. Res. Microbiol. 159:358–366. 10.1016/j.resmic.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 12.Rachel R, Bettstetter M, Hedlund BP, Haring M, Kessler A, Stetter KO, Prangishvili D. 2002. Remarkable morphological diversity of viruses and virus-like particles in hot terrestrial environments. Arch. Virol. 147:2419–2429. 10.1007/s00705-002-0895-2 [DOI] [PubMed] [Google Scholar]
- 13.Wiedenheft B, Stedman K, Roberto F, Willits D, Gleske AK, Zoeller L, Snyder J, Douglas T, Young M. 2004. Comparative genomic analysis of hyperthermophilic archaeal Fuselloviridae viruses. J. Virol. 78:1954–1961. 10.1128/JVI.78.4.1954-1961.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stedman KM, She Q, Phan H, Arnold HP, Holz I, Garrett RA, Zillig W. 2003. Relationships between fuselloviruses infecting the extremely thermophilic archaeon Sulfolobus: SSV1 and SSV2. Res. Microbiol. 154:295–302. 10.1016/S0923-2508(03)00074-3 [DOI] [PubMed] [Google Scholar]
- 15.Zillig W, Arnold HP, Holz I, Prangishvili D, Schweier A, Stedman K, She Q, Phan H, Garrett R, Kristjansson JK. 1998. Genetic elements in the extremely thermophilic archaeon Sulfolobus. Extremophiles 2:131–140. 10.1007/s007920050052 [DOI] [PubMed] [Google Scholar]
- 16.Baker BJ, Comolli LR, Dick GJ, Hauser LJ, Hyatt D, Dill BD, Land ML, VerBerkmoes NC, Hettich RL, Banfield JF. 2010. Enigmatic, ultrasmall, uncultivated Archaea. Proc. Natl. Acad. Sci. U. S. A. 107:8806–8811. 10.1073/pnas.0914470107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comolli LR, Baker BJ, Downing KH, Siegerist CE, Banfield JF. 2009. Three-dimensional analysis of the structure and ecology of a novel, ultra-small archaeon. ISME J. 3:159–167. 10.1038/ismej.2008.99 [DOI] [PubMed] [Google Scholar]
- 18.Prangishvili D. 2013. The wonderful world of archaeal viruses. Annu. Rev. Microbiol. 67:565–585. 10.1146/annurev-micro-092412-155633 [DOI] [PubMed] [Google Scholar]
- 19.Redder P, Peng X, Brugger K, Shah SA, Roesch F, Greve B, She QX, Schleper C, Forterre P, Garrett RA, Prangishvili D. 2009. Four newly isolated fuselloviruses from extreme geothermal environments reveal unusual morphologies and a possible interviral recombination mechanism. Environ. Microbiol. 11:2849–2862. 10.1111/j.1462-2920.2009.02009.x [DOI] [PubMed] [Google Scholar]
- 20.Bath C, Cukalac T, Porter K, Dyall-Smith ML. 2006. His1 and His2 are distantly related, spindle-shaped haloviruses belonging to the novel virus group, Salterprovirus. Virology 350:228–239. 10.1016/j.virol.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 21.Hanhijärvi KJ, Ziedaite G, Pietilä MK, Haeggström E, Bamford DH. 2013. DNA ejection from an archaeal virus—a single-molecule approach. Biophys. J. 104:2264–2272. 10.1016/j.bpj.2013.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietilä MK, Atanasova NS, Oksanen HM, Bamford DH. 2013. Modified coat protein forms the flexible spindle-shaped virion of haloarchaeal virus His1. Environ. Microbiol. 15:1674–1686. 10.1111/1462-2920.12030 [DOI] [PubMed] [Google Scholar]
- 23.Geslin C, Gaillard M, Flament D, Rouault K, Le Romancer M, Prieur D, Erauso G. 2007. Analysis of the first genome of a hyperthermophilic marine virus-like particle, PAV1, isolated from Pyrococcus abyssi. J. Bacteriol. 189:4510–4519. 10.1128/JB.01896-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krupovic M, Bamford DH. 2008. Archaeal proviruses TKV4 and MVV extend the PRD1-adenovirus lineage to the phylum Euryarchaeota. Virology 375:292–300. 10.1016/j.virol.2008.01.043 [DOI] [PubMed] [Google Scholar]
- 25.Wood AG, Whitman WB, Konisky J. 1989. Isolation and characterization of an archaebacterial viruslike particle from Methanococcus voltae A3. J. Bacteriol. 171:93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mochizuki T, Sako Y, Prangishvili D. 2011. Provirus induction in hyperthermophilic archaea: characterization of Aeropyrum pernix spindle-shaped virus 1 and Aeropyrum pernix ovoid virus 1. J. Bacteriol. 193:5412–5419. 10.1128/JB.05101-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiang X, Chen L, Huang X, Luo Y, She Q, Huang L. 2005. Sulfolobus tengchongensis spindle-shaped virus STSV1: virus-host interactions and genomic features. J. Virol. 79:8677–8686. 10.1128/JVI.79.14.8677-8686.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erdmann S, Chen B, Huang X, Deng L, Liu C, Shah SA, Le Moine Bauer S, Sobrino CL, Wang H, Wei Y, She Q, Garrett RA, Huang L, Lin L. 2013. A novel single-tailed fusiform Sulfolobus virus STSV2 infecting model Sulfolobus species. Extremophiles 18:51–60. 10.1007/s00792-013-0591-z [DOI] [PubMed] [Google Scholar]
- 29.Krupovic M, Bamford DH. 2010. Order to the viral universe. J. Virol. 84:12476–12479. 10.1128/JVI.01489-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krupovic M, Bamford DH. 2011. Double-stranded DNA viruses: 20 families and only five different architectural principles for virion assembly. Curr. Opin. Virol. 1:118–124. 10.1016/j.coviro.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 31.Prangishvili D, Krupovic M. 2012. A new proposed taxon for double-stranded DNA viruses, the order “Ligamenvirales.” Arch. Virol. 157:791–795. 10.1007/s00705-012-1229-7 [DOI] [PubMed] [Google Scholar]
- 32.Reiter WD, Palm P, Henschen A, Lottspeich F, Zillig W, Grampp B. 1987. Identification and characterization of the genes encoding 3 structural proteins of the Sulfolobus virus-like particle SSV1. Mol. Gen. Genet. 206:144–153. 10.1007/BF00326550 [DOI] [Google Scholar]
- 33.Schleper C, Kubo K, Zillig W. 1992. The particle SSV1 from the extremely thermophilic archaeon Sulfolobus is a virus—demonstration of infectivity and of transfection with viral-DNA. Proc. Natl. Acad. Sci. U. S. A. 89:7645–7649. 10.1073/pnas.89.16.7645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prangishvili D, Vestergaard G, Häring M, Aramayo R, Basta T, Rachel R, Garrett RA. 2006. Structural and genomic properties of the hyperthermophilic archaeal virus ATV with an extracellular stage of the reproductive cycle. J. Mol. Biol. 359:1203–1216. 10.1016/j.jmb.2006.04.027 [DOI] [PubMed] [Google Scholar]
- 35.Häring M, Vestergaard G, Rachel R, Chen L, Garrett RA, Prangishvili D. 2005. Virology: independent virus development outside a host. Nature 436:1101–1102. 10.1038/4361101a [DOI] [PubMed] [Google Scholar]
- 36.Felisberto-Rodrigues C, Blangy S, Goulet A, Vestergaard G, Cambillau C, Garrett RA, Ortiz-Lombardia M. 2012. Crystal structure of ATV(ORF273), a new fold for a thermo- and acido-stable protein from the Acidianus two-tailed virus. PLoS One 7:e45847. 10.1371/journal.pone.0045847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goulet A, Vestergaard G, Felisberto-Rodrigues C, Campanacci V, Garrett RA, Cambillau C, Ortiz-Lombardia M. 2010. Getting the best out of long-wavelength X-rays: de novo chlorine/sulfur SAD phasing of a structural protein from ATV. Acta Crystallogr. D Biol. Crystallogr. 66:304–308. 10.1107/S0907444909051798 [DOI] [PubMed] [Google Scholar]
- 38.Krupovic M, White MF, Forterre P, Prangishvili D. 2012. Postcards from the edge: structural genomics of archaeal viruses. Adv. Virus Res. 82:33–62. 10.1016/B978-0-12-394621-8.00012-1 [DOI] [PubMed] [Google Scholar]
- 39.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567–580. 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 41.Koonin EV, Dolja VV. 2013. A virocentric perspective on the evolution of life. Curr. Opin. Virol. 3:546–557. 10.1016/j.coviro.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koonin EV, Wolf YI, Nagasaki K, Dolja VV. 2009. The complexity of the virus world. Nat. Rev. Microbiol. 7:250. 10.1038/nrmicro2030-c2 [DOI] [Google Scholar]
- 43.Krupovic M. 2013. Networks of evolutionary interactions underlying the polyphyletic origin of ssDNA viruses. Curr. Opin. Virol. 3:578–586. 10.1016/j.coviro.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 44.Roux S, Enault F, Bronner G, Vaulot D, Forterre P, Krupovic M. 2013. Chimeric viruses blur the borders between the major groups of eukaryotic single-stranded DNA viruses. Nat. Commun. 4:2700. 10.1038/ncomms3700 [DOI] [PubMed] [Google Scholar]
- 45.Koonin EV. 1992. Archaebacterial virus SSV1 encodes a putative DnaA-like protein. Nucleic Acids Res. 20:1143. 10.1093/nar/20.5.1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krupovic M, Gonnet M, Hania WB, Forterre P, Erauso G. 2013. Insights into dynamics of mobile genetic elements in hyperthermophilic environments from five new Thermococcus plasmids. PLoS One 8:e49044. 10.1371/journal.pone.0049044 [DOI] [PMC free article] [PubMed] [Google Scholar]