Abstract
The orf-I gene of human T-cell leukemia type 1 (HTLV-1) encodes p8 and p12 and has a conserved cysteine at position 39. p8 and p12 form disulfide-linked dimers, and only the monomeric forms of p8 and p12 are palmitoylated. Mutation of cysteine 39 to alanine (C39A) abrogated dimerization and palmitoylation of both proteins. However, the ability of p8 to localize to the cell surface and to increase cell adhesion and viral transmission was not affected by the C39A mutation.
TEXT
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia (ATL) and tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM) (1–5). The HTLV-1 genome encodes structural and enzymatic proteins and, at the 3′ end, contains four open reading frames (ORFs) (6). Singly spliced RNA from orf-I encodes a 12-kDa endoplasmic reticulum (ER) resident protein, p12 (7–10). The membrane-associated p12 protein is proteolytically cleaved at the amino terminus to remove a noncanonical ER retention/retrieval signal that results in the generation of p8, which traffics to the cell surface (9).
HTLV-1 p12 and p8 have seemingly opposite effects on T-cell activation (11). The p12 protein promotes T-cell activation by binding to calcineurin to increase ER calcium influx and nuclear factor of activated T cell (NFAT) activity (12–14). HTLV-1 p12 also increases T-cell proliferation by binding to the β and γc chains of the interleukin 2 (IL-2) receptor, resulting in STAT5 phosphorylation, which activates IL-2 production (15, 16). In contrast, the p8 protein induces anergy in T-cell receptor-stimulated cells by downmodulating signaling at the immunological synapse through decreased phosphorylation of linker of activation for T cells (LAT), phospholipase Cγ-1 (PLCγ-1), and Vav (11). Additionally, p8 decreases cell spreading and actin polymerization upon T-cell receptor (TCR) engagement (17), and the orf-I gene protein products increase the motility and migration of T cells toward chemokines (18). In stimulated T cells, p8 increases the virological synapse formation, the length of cellular conduits, and viral infectivity (17).
However, the mechanism by which p8 translocates from the ER to the cell surface remains unclear.
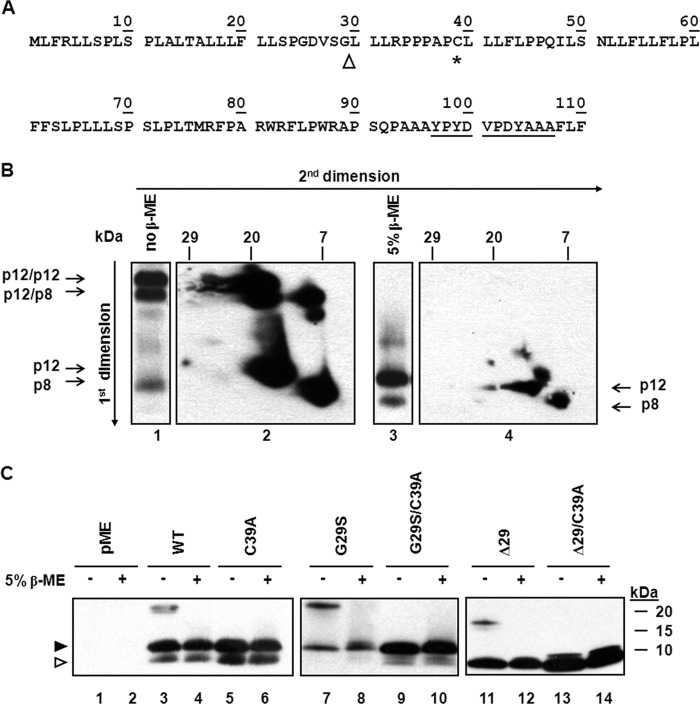
We investigated whether (Fig. 1A) a highly conserved single cysteine residue at position 39 (C39) could form intermolecular disulfide bonds (9), contribute to dimer formation, and regulate the diverse functions of p8 and p12. At first, we immunoprecipitated protein extract from 293T cells transfected with the orf-I cDNA. The immune complexes were treated or not with the reducing agent β-mercaptoethanol (β-ME) and resolved in the first dimension by SDS-PAGE. High-molecular-weight bands observed in the nonreducing conditions were resolved to p12 and p8 in the presence of β-ME (Fig. 1B, lanes 1and 3, respectively). Parallel lanes were excised and placed horizontally at the top of another gel to perform a secondary electrophoresis in reducing or nonreducing conditions. In nonreducing conditions, the predominate high-molecular-weight protein bands were found to contain only p12 or p12 and p8, while the lower faint band was constituted of dimeric p8 (Fig. 1B, lane 1 and panel 2). The reduced immune complexes (lane 3) migrated with a size compatible to that of monomeric p12 and p8 (panel 4). These data are consistent with the ability of these proteins to form homo- and heterodimers. In order to minimize p12 and p8 dimer formation after cell lysis, immunoprecipitates were treated with iodoacetamide (IAN), which covalently modifies the reactive sulfhydryl group on cysteine residues. Immunoblot analysis showed that dimer formation occurred similarly in the presence or absence of IAN (data not shown).
FIG 1.
The p12 and p8 proteins form disulfide-linked dimers. (A) Protein amino acid sequence (single-letter amino acid code) HTLV-1 orf-I gene protein product. The arrowhead indicates a possible site of protein cleavage (9), the asterisk denotes the position of cysteine 39, and underlined residues show the sequence of the HA tag. (B) Extract from 293T cells transfected with the HA-tagged wild-type orf-I gene protein product expression plasmid was treated in the absence (lane 1) or the presence (lane 3) of β-mercaptoethanol (β-ME) and resolved by SDS-PAGE in the first dimension. Gel lanes were excised and resolved in a second dimension in nonreducing (panel 2) and reducing conditions (panel 4). The position of heterodimers is indicated by the horizontal arrows. Long arrows at the left and top of the figure represent the electrophoresis's direction. (C) Extracts from 293T cells transfected with HA-tagged orf-I gene WT and mutants were incubated in the presence or absence of the reducing agent β-ME. Proteins were resolved by SDS-PAGE and immunoblotted with antibodies to HA. The arrowheads at left indicate the positions of p12 (filled) and p8 (hollow).
We mutated C39 to alanine (C39A). pME and HA-tagged WT, G29S, and Δ29 orf-I expression plasmids and the pACH and pAB molecular clones were previously described (1, 11, 19). The orf-I expression plasmids were modified with the addition of a Kozak sequence (underlined). Products were generated by PCR using the oligonucleotides WT-Fwd (5′ ATTACTCGAGGCCACCATGCTGTTTCGCCTTC), Δ29-Fwd (5′ ATTACTCGAGGCCACCATGCTTCTTCTCCGCC), and WT and Δ29-Rev (5′ TCGGTCTAGAAACAACAACAATTGCATT). The PCR products were cleaved with XhoI and XbaI and ligated into the pME backbone plasmid. The C39A mutant was generated by PCR from the QuikChange II site-directed mutagenesis kit (Agilent, Santa Clara, CA) using site-specific mutagenic oligonucleotides. The oligonucleotides C39A-Fwd (5′ CCTCCTGCGCCGGCCCTTCTCCTCTTCCTTC) and C39A-Rev (5′ GAAAAGGAAGGAAGAGGAGAAG) were used. The sequences of all plasmid clones were analyzed to confirm the underlined changes.
Protein extracts from the transfected 293T cells were treated or not with β-ME and resolved on tricine gels, as previously described (9, 11) (Fig. 1C). Immunoblotting of untreated protein extracts from cells expressing the orf-I gene detected the p12 and p8 monomers, as well as slower-migrating bands (Fig. 1C, lane 3) that were not observed following β-ME treatment (Fig. 1C, lane 4) or when cysteine 39 was mutated to alanine (Fig. 1C, lanes 5 and 6), demonstrating the contribution of C39A to dimer formation. In HTLV-1-infected individuals, polymorphisms in the proximity of the cleavage site, within p12, resulted in different ratios of p8 and p12 expression. The natural mutation of glycine 29 to serine (Fig. 1A) resulted in the G29S mutant that produces mainly p12 (9). Expression of G29S demonstrated the p12 monomer and a single slower-migrating complex that was reduced to p12 by β-ME (Fig. 1C, lanes 7 and 8). Indeed, the replacement of cysteine 39 with alanine into G29S (G29S/C39A mutant) resulted in the disappearance of the slow-migrating protein band in nonreducing conditions (Fig. 1C, lane 9). As expected, expression of the Δ29 plasmid encoding only p8 (9) resulted in the detection of the p8 monomer and a single slower-migrating complex that was reduced to the monomeric form by treatment with β-ME (Fig. 1C, lanes 11 and 12). Accordingly, mutation of cysteine 39 to alanine in the Δ29/C39A mutant resulted in the disappearance of the slow-migrating form observed in the Δ29 mutant (Fig. 1C, compare lanes 11, 13, and 14). The difference in the slower-migrating bands in lanes 7 and 11 is compatible with a difference in size of the p12 and p8 dimers. Together, the above-described findings demonstrate that the dimers formed by p12 and p8 are disulfide linked at cysteine 39.
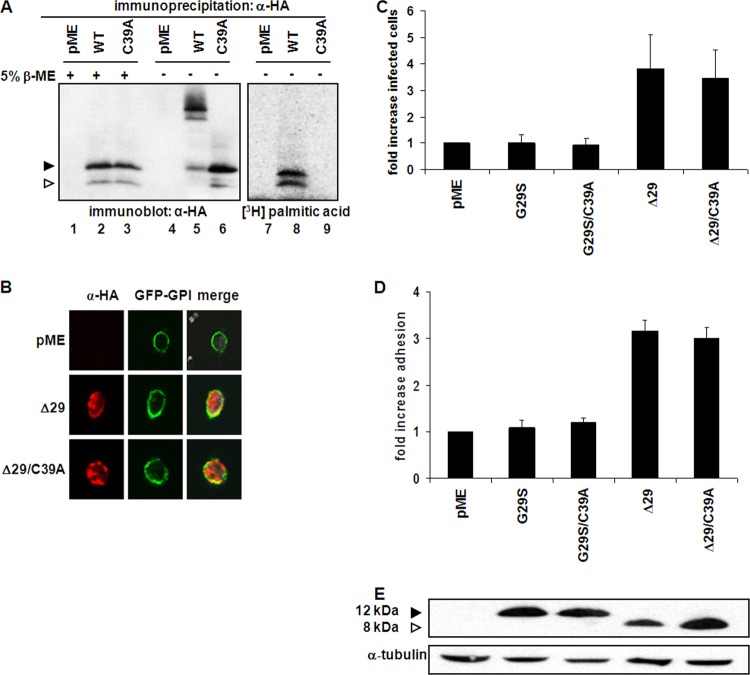
Palmitoylation of cysteine residues appears to be essential for the localization of several transmembrane signaling proteins to the cell surface (19, 20). We therefore hypothesized that p8 and p12 monomers may also be palmitoylated and that this modification may affect p8 localization to the cell surface. 293T cells were transfected with the WT orf-I gene or the C39A mutants and metabolically labeled (21) with [3H]palmitic acid (Fig. 2A). Immunoprecipitated protein extracts from these cells were either treated or not with β-ME and resolved by SDS-PAGE. As expected, immunoblot analysis demonstrated that dimer formation was inhibited by treatment with reducing agent or by mutation at C39 (Fig. 2A, lanes 1 to 6). Fluorography, however, revealed that both the monomeric form of p12 and p8 are palmitoylated and that the palmitoylated proteins did not form dimers in the absence of β-ME (Fig. 2A, lanes 7 and 8). The C39 mutant was not palmitoylated, demonstrating that palmitoylation of both the p12 and p8 proteins occurs on C39 (Fig. 2A, lane 9). These results indicate that the p8 and p12 proteins can either dimerize or undergo palmitoylation and remain monomeric, suggesting a possible mechanism of functional regulation of p8 and p12 functions.
FIG 2.
Functional consequence of palmitoylation at C39 of p12 and p8. (A) 293T cells transfected with empty vector or HA-tagged orf-I gene protein product expression plasmids and parallel plates were metabolically labeled with [3H]palmitic acid. Extract from unlabeled transfected cells was immunoprecipitated with an antibody to HA and treated in the presence or absence of β-mercaptoethanol. Immunoprecipitated proteins were resolved by SDS-PAGE and analyzed by immunoblotting with an antibody to HA (lanes 1 to 6). The [3H]palmitic acid-labeled extract was analyzed by fluorography (lanes 7 to 9) following immunoprecipitation. The arrowheads at the left indicate the positions of p12 (filled) and p8 (hollow). (B) MT-2 cells were transfected with GFP-GPI, together with pME, Δ29, or Δ29/C39A expression plasmids. Cells were then visualized by confocal microscopy. GFP-GPI is shown in green and p8 in red. (C)Transfected MT-2 cells were cocultivated for 48 h with BHK1E6 cells containing a lacZ reporter gene bound to a Tax-responsive promoter. Monolayers were washed, fixed, and stained with X-Gal solution, and β-galactosidase-expressing cells were counted by bright-field microscopy. Control pME-transfected cells were set to 1, and infectivity of cells transfected with orf-I gene protein product expression plasmids was determined relative to this value. Error bars represent the standard errors of the means from three independent experiments. (D) Transfected MT-2 cells were incubated on ICAM-1-coated coverslips and their nuclei stained with crystal violet. Cells were washed and lysed, and released stain was measured at 570 μm. Absorbance for control pME-transfected cells was set to 1, and adhesion of cells transfected with the orf-I gene protein product expression plasmids was determined relative to this value. The error bars represent the standard error of the means from three independent experiments. (E) Protein extract from MT-2 cells transfected with HA-tagged orf-I gene protein product expression plasmids was resolved by SDS-PAGE and immunoblotted with antibodies to HA. The arrowheads at the left indicate the positions of p12 (filled) and p8 (hollow).
We next tested whether the C39A mutant localizes to the cell surface using MT-2 cells cotransfected with a membrane marker, GFP-GPI, together with pME, Δ29, or Δ29/C39A expression plasmids. Forty-eight hours posttransfection, cells were incubated with poly-lysine-coated coverslips to favor cell spreading and visualization. Cells were then fixed, permeabilized, and immunostained with anti-HA, as previously described (9, 11). As shown in Fig. 2B, the membrane marker GFP-GPI (green) localized exclusively to the plasma membrane of MT-2 cells. The Δ29 protein (p8) (red) accumulated in both the cytoplasm, as reported previously (9, 11), and at the cell surface similar to GFP-GPI (yellow) (Fig. 2B, middle). The colocalization of the Δ29 and Δ29/C39A proteins with GFP-GPI was analyzed by line scan measurements in Metamorph (data not shown). We observed no difference in localization of the Δ29/C39A protein compared to the Δ29 protein, suggesting that C39 is dispensable for cell surface localization of p8 (Fig. 2B).
We have previously shown that p8 increases conduit formation, cell contact, and virus transmission in naturally infected T cells (17). Upon direct cell contact with a neighboring cell, p8 polarizes at the cell-to-cell junction and mediates conduit formation (17). Enhanced viral infectivity may therefore be achieved both by virus transmissions along these cellular conduits and from increased cell-to-cell contact (17). To determine the role of C39A mutation in the ability of p8 to increase viral infectivity and cell-to-cell adhesion, the HTLV-1 producer MT-2 cell line was transfected with orf-I gene expression plasmids expressing mainly p12 or p8 carrying a cysteine or an alanine at position 39. HTLV-1-infected MT-2 cells, following transfection with the pME vector or G29S (expresses mainly p12), G29S/C39A, Δ29 (produces mainly p8), or Δ29/C39A cDNA, were cocultivated for 48 h with BHK1E6 cells containing a lacZ reporter gene whose expression is driven by Tax. Monolayers were washed, fixed, and stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) solution, and β-galactosidase-expressing cells were counted by bright-field microscopy, as previously described (17). Cells transfected with G29S or the G29S/C39A cDNAs transmitted similar levels of HTLV-1 to BHK1E6 cells. In contrast, there was a 3-fold increase in the infectivity in cells expressing Δ29 protein, as expected (17), and mutation of cysteine 39 did not affect virus transmission (Fig. 2C). To assess the underlying mechanism, the same batch of transfected MT-2 cells was incubated in parallel, on ICAM-1/Fc-coated coverslips, and then fixed, and their nuclei were stained with crystal violet. The cells were washed and incubated in a solution that releases the stain, and absorbance was measured. As shown in Fig. 2D, expression of p8 increased MT-2 cell adhesion to ICAM-1-coated coverslips 3-fold over that of cells transfected with empty vector, and mutation of cysteine 39 to alanine did not affect the ability to increase cell adhesion of p8. The increase in viral transmission or cell adhesion mediated by p8 or by the Δ29/C39A protein was not due to differences in the expression of the proteins (Fig. 2E). Overall, our results demonstrate that dimerization and palmitoylation are dispensable in p8-augmented cell-to-cell adhesion and viral infectivity.
It seems unlikely for palmitoylation of orf-I-encoded proteins to have been conserved without affecting protein function. Whether palmitoylation or dimerization may be required for the p12/p8-mediated downregulation of MHC class I or STAT5 activation will require further studies.
ACKNOWLEDGMENTS
We thank Teresa Habina for editorial assistance, Lawrence E. Samelson for helpful discussion, and Tatyana Karpova and Tatsuya Morisaki for help with the confocal microscopy and analysis.
Footnotes
Published ahead of print 27 November 2013
REFERENCES
- 1.Gessain A, Barin F, Vernant J-C, Gout O, Maurs L, Calendar A, de The G. 1985. Antibodies to human T-lymphotropic virus type I in patients with tropical spastic paraparesis. Lancet ii:407–410 [DOI] [PubMed] [Google Scholar]
- 2.Nakamura F, Kajihara H, Nakamura M, Sasaki H, Kumamoto T, Okada K. 1989. HTLV-1 associated myelopathy in an HTLV-1 and HBV double carrier family: report of a case and the mode of vertical transmission of both viruses. J. Gastroenterol. Hepatol. 4:387–390. 10.1111/j.1440-1746.1989.tb00850.x [DOI] [PubMed] [Google Scholar]
- 3.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i:1031–1032 [DOI] [PubMed] [Google Scholar]
- 4.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. U. S. A. 77:7415–7419. 10.1073/pnas.77.12.7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida M, Miyoshi I, Hinuma Y. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. U. S. A. 79:2031–2035. 10.1073/pnas.79.6.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seiki M, Hattori S, Kirayama Y, Yoshida M. 1983. Human T-cell leukemia/lymphotropic virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc. Natl. Acad. Sci. U. S. A. 80:3618–3622. 10.1073/pnas.80.12.3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berneman ZN, Gartenhaus RB, Reitz MS, Jr, Blattner WA, Manns A, Hanchard B, Ikehara O, Gallo RC, Klotman MK. 1992. Expression of alternatively spliced human T-lymphotropic virus type I (HTLV-I) pX mRNA in infected cell lines and in primary uncultured cells from patients with adult T-cell leukemia/lymphoma and healthy carriers. Proc. Natl. Acad. Sci. U. S. A. 89:3005–3009. 10.1073/pnas.89.7.3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciminale V, Pavlakis GN, Derse D, Cunningham CP, Felber BK. 1992. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV-I. J. Virol. 66:1737–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukumoto R, Andresen V, Bialuk I, Cecchinato V, Walser JC, Valeri VW, Nauroth JM, Gessain A, Nicot C, Franchini G. 2009. In vivo genetic mutations define predominant functions of the human T-cell leukemia/lymphoma virus p12I protein. Blood 113:3726–3734. 10.1182/blood-2008-04-146928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koralnik I, Gessain A, Klotman ME, Lo Monico A, Berneman ZN, Franchini G. 1992. Protein isoforms encoded by the pX region of the human T-cell leukemia/lymphotropic virus type I. Proc. Natl. Acad. Sci. U. S. A. 89:8813–8817. 10.1073/pnas.89.18.8813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukumoto R, Dundr M, Nicot C, Adams A, Valeri VW, Samelson LE, Franchini G. 2007. Inhibition of T-cell receptor signal transduction and viral expression by the linker for activation of T cells-interacting p12I protein of human T-cell leukemia/lymphoma virus type 1. J. Virol. 81:9088–9099. 10.1128/JVI.02703-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albrecht B, D'Souza CD, Ding W, Tridandapani S, Coggeshall KM, Lairmore MD. 2002. Activation of nuclear factor of activated T cells by human T-lymphotropic virus type 1 accessory protein p12(I). J. Virol. 76:3493–3501. 10.1128/JVI.76.7.3493-3501.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding W, Albrecht B, Kelley RE, Muthusamy N, Kim SJ, Altschuld RA, Lairmore MD. 2002. Human T-cell lymphotropic virus type 1 p12(I) expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells. J. Virol. 76:10374–10382. 10.1128/JVI.76.20.10374-10382.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SJ, Ding W, Albrecht B, Green PL, Lairmore MD. 2003. A conserved calcineurin-binding motif in human T lymphotropic virus type 1 p12I functions to modulate nuclear factor of activated T cell activation. J. Biol. Chem. 278:15550–15557. 10.1074/jbc.M210210200 [DOI] [PubMed] [Google Scholar]
- 15.Mulloy JC, Crowley RW, Fullen J, Leonard WJ, Franchini G. 1996. The human T-cell leukemia/lymphotropic virus type I p12I protein binds the interleukin-2 receptor β and γc chains and affects their expression on the cell surface. J. Virol. 70:3599–3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicot C, Mulloy JC, Ferrari MG, Johnson JM, Fu K, Fukumoto R, Trovato R, Fullen J, Leonard WJ, Franchini G. 2001. HTLV-1 p12(I) protein enhances STAT5 activation and decreases the interleukin-2 requirement for proliferation of primary human peripheral blood mononuclear cells. Blood 98:823–829. 10.1182/blood.V98.3.823 [DOI] [PubMed] [Google Scholar]
- 17.Van Prooyen N, Gold H, Andresen V, Schwartz O, Jones K, Ruscetti F, Lockett S, Gudla P, Venzon D, Franchini G. 2010. Human T-cell leukemia virus type 1 p8 protein increases cellular conduits and virus transmission. Proc. Natl. Acad. Sci. U. S. A. 107:20738–20743. 10.1073/pnas.1009635107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor JM, Brown M, Nejmeddine M, Kim KJ, Ratner L, Lairmore M, Nicot C. 2009. Novel role for interleukin-2 receptor-Jak signaling in retrovirus transmission. J. Virol. 83:11467–11476. 10.1128/JVI.00952-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linder ME, Deschenes RJ. 2007. Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 8:74–84. 10.1038/nrm2084 [DOI] [PubMed] [Google Scholar]
- 20.Veit M, Serebryakova MV, Kordyukova LV. 2013. Palmitoylation of influenza virus proteins. Biochem. Soc. Trans. 41:50–55. 10.1042/BST20120210 [DOI] [PubMed] [Google Scholar]
- 21.Rouquette-Jazdanian AK, Pelassy C, Breittmayer JP, Cousin JL, Aussel C. 2002. Metabolic labelling of membrane microdomains/rafts in Jurkat cells indicates the presence of glycerophospholipids implicated in signal transduction by the CD3 T-cell receptor. Biochem. J. 363:645–655. 10.1042/0264-6021:3630645 [DOI] [PMC free article] [PubMed] [Google Scholar]