ABSTRACT
Dengue virus (DENV) is the cause of a potentially life-threatening disease that affects millions of people worldwide. The lack of a small animal model that mimics the symptoms of DENV infection in humans has slowed the understanding of viral pathogenesis and the development of therapies and vaccines. Here, we investigated the use of humanized “bone marrow liver thymus” (BLT) mice as a model for immunological studies and assayed their applicability for preclinical testing of antiviral compounds. Human immune system (HIS) BLT-NOD/SCID mice were inoculated intravenously with a low-passage, clinical isolate of DENV-2, and this resulted in sustained viremia and infection of leukocytes in lymphoid and nonlymphoid organs. In addition, DENV infection increased serum cytokine levels and elicited DENV-2-neutralizing human IgM antibodies. Following restimulation with DENV-infected dendritic cells, in vivo-primed T cells became activated and acquired effector function. An adenosine nucleoside inhibitor of DENV decreased the circulating viral RNA when administered simultaneously or 2 days postinfection, simulating a potential treatment protocol for DENV infection in humans. In summary, we demonstrate that BLT mice are susceptible to infection with clinical DENV isolates, mount virus-specific adaptive immune responses, and respond to antiviral drug treatment. Although additional refinements to the model are required, BLT mice are a suitable platform to study aspects of DENV infection and pathogenesis and for preclinical testing of drug and vaccine candidates.
IMPORTANCE
INTRODUCTION
Dengue is one of the most significant arthropod-borne viral diseases in the world with respect to morbidity, mortality, and economic cost (1). Approximately 2.5 billion people, two-fifths of the world's population, are now at risk of dengue, and it has been estimated that there may be 390 million dengue virus (DENV) infections every year, of which 96 million manifest an apparent level of disease severity (2). The disease dengue fever (DF) is now endemic in more than 100 countries. The causative agent is DENV, a positive-sense, single-stranded RNA virus belonging to the family Flaviviridae. Four genetically and antigenically distinct serotypes, DENV-1 to DENV-4, have been described.
Humans are infected by the bite of the mosquitoes Aedes aegypti or Aedes albopictus. Initial dengue virus infection may be asymptomatic or may result in disease ranging from acute, self-limiting febrile illness (DF) to life-threatening dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS) (3), which are more pronounced and more frequent following secondary infection with a heterologous DENV serotype. The precise mechanism of how viral and host factors contribute to disease severity remains incompletely understood (4).
The natural host tropism of DENV is limited to humans and some nonhuman primates. Experimental inoculation of rhesus (5) or cynomolgus macaques (6, 7) with clinical DENV isolates or tissue culture-adapted strains results in low-level viremia and is largely asymptomatic except for low to moderate thrombocytopenia. To complement nonhuman primate models and overcome some of their limitations (cost, availability, genetic heterogeneity), several rodent dengue models have been explored (reviewed in reference 8).
Humanized mice, i.e., animals engrafted with human tissue and/or that express human genes, have emerged as versatile tools to study human-tropic pathogens (9). They can be generated by injecting human hematopoietic stem cells (HSC) into conditioned immunodeficient recipients, which can result in considerable human hematopoietic chimerism (reviewed in reference 10). To foster T cell development and to improve T cell functionality, small pieces of autologous fetal liver and thymus are implanted under the kidney capsule of severely immunocompromised NOD/SCID mice. Xenorecipients are then conditioned by sublethal irradiation and injected with HSC, resulting in so-called “bone-marrow/liver/thymus” (BLT) mice (11, 12).
Such human immune system (HIS) mice have become versatile challenge models for numerous human pathogens with limited host ranges, including HIV (reviewed in reference 13), Epstein Barr virus (EBV) (14, 15), Kaposi's sarcoma-associated herpesvirus (16), human T cell leukemia virus (17), human cytomegalovirus (18), and also bacterial pathogens such as Salmonella enterica serovar Typhi (19) and Borrelia hermsii (20). HSC-transplanted mice were shown to be susceptible to DENV infection and their responses mimicked many of the associated clinical features, including fever and rash (21–25, 32, 41–44).
In this study, we aimed to assess the utility of the BLT mouse model for DENV infection and preclinical testing of antiviral drugs. We found that, following inoculation with a previously uncharacterized clinical DENV-2 isolate, humanized BLT mice became viremic and exhibited slight increases in body temperature and decreased platelet counts, symptoms reminiscent of DENV infection in humans. NS1 is detectable in the circulation, and DENV antigens are detectable primarily in human cells. DENV infection elicits humoral immune responses. In vivo-primed T cells become activated and acquire effector functions when restimulated ex vivo with DENV-infected dendritic cells (DCs). Antigen recognition is HLA specific, as anti-major histocompatibility complex (MHC) class I and II antibodies significantly decrease the release of effector cytokines. Furthermore, administration of a previously described inhibitor of the DENV NS5 polymerase that had only been tested in immunodeficient AG129 mice (26) significantly reduced viral load in HIS BLT mice. These data establish proof-of-concept for the utility of HIS BLT mice for preclinical assessment of the efficacy of directly acting antivirals against primary DENV isolates replicating in human cells.
MATERIALS AND METHODS
Generation of BLT-NOD/SCID mice.
NOD.Cg-Prkdcscid (NOD/SCID) mice were purchased from the Jackson Laboratory. Mice were maintained under pathogen-free conditions, with irradiated food supplemented with antibiotics and acidified water, at the Comparative Bioscience Center (CBC) of the Rockefeller University according to guidelines established by the Institutional Animal Committee.
Six- to 8-week-old NOD/SCID mice were anesthetized and surgically implanted with human fetal thymus and liver under the kidney capsule. Fetal organs, 16 to 22 weeks of gestation, were obtained from Advanced Bioscience Resources, Inc. (Alameda, CA) and the Human Fetal Tissue Depository at Albert Einstein College of Medicine (Bronx, NY). Three days after implantation, the mice were sublethally irradiated with 325 cGy and transplanted intravenously with 0.2 × 106 to 1 × 106 human CD34+ HSC. Human CD34+ cells from autologous fetal liver tissue were isolated with a CD34+ HSC isolation kit (StemCell Technologies) according to the manufacturer's protocol and cryopreserved until transplantation in mice. Twelve to 16 weeks after HSC transplantation, mice were bled through the retro-orbital route and analyzed for human immune system reconstitution. Approximately 120 male and female mice transplanted with CD34+ cells derived from various human donors were used in this study. All experiments in mice were performed at the CBC under protocols approved by the Institutional Review Board and the Institutional Animal Care and Use Committee at Rockefeller University.
Dengue virus.
The low-passage dengue virus serotype 2 Colombia 362981 TVP-3521 (DENV-2 Col) used in this study was generously provided by Robert Tesh at the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA). The virus was originally isolated in 1993 from serum of an infected patient from Colombia and has been passaged three times in C6/36 (Aedes Albopictus) mosquito cells. Viral stocks were prepared as follows: tissue culture flasks (175 cm2) were seeded with 1.5 × 106 C6/36 cells (grown in alpha minimal essential medium [MEM; Gibco], 10% fetal bovine serum, 0.1 mM nonessential amino acids) and 24 h later were infected with dengue virus at a multiplicity of infection (MOI) of 0.01. After 1 h of incubation, virus was removed and fresh C6/36 medium was added to reach 15 ml. Cell supernatants were harvested 7 days postinfection, centrifuged at 600 × g for 10 min to remove cells, and concentrated 10-fold using a stirred ultrafiltration cell unit (Millipore) with a 100-kDa-cutoff cellulose membrane (Millipore), aliquoted, frozen in liquid nitrogen, and stored at −80°C. The virus stock titer was determined by means of an endpoint dilution (50% tissue culture infective dose [TCID50]) assay in C6/36 cells. For some experiments, virus was inactivated by exposure to short-wave UV (200 mJ for 10 min) in a UV light chamber (GS Gene Linker; Bio-Rad). Inactivation of virus infectivity was verified by endpoint dilution assay in C6/36 cells and in infection experiments in HIS BLT mice.
In vitro dengue virus infections.
RAJI and RAJI-DC cells (kindly provided by Ana Fernandez-Sesma, Mt. Sinai School of Medicine) and THP-1 cells (ATCC) were cultured in RPM1 1640 medium supplemented with 10% fetal bovine serum (Gibco). K562 cells (ATCC) were cultured in Iscove's modified Dulbecco's medium (IMDM) supplemented with 10% fetal bovine serum (Gibco). Virus infections were performed at an MOI of 0.5, for 1 h at 37°C, washed three times with 1× phosphate-buffered saline (PBS), and incubated at 37°C in appropriate cell culture medium.
Dengue virus infections of humanized mice and clinical scoring.
Mice with engraftment levels of 50 to 90% in peripheral blood were infected intravenously with 1 × 106 TCID50 of DENV-2 Col. Control groups were infected with same volume of UV-inactivated DENV-2 or C6/36 cell culture medium, prepared and concentrated in the same way as the virus stock. Mouse temperatures and body weights were monitored daily. Temperature was measured using a RET-3 rectal probe coupled to a Microtherma 2 type T thermometer (Braintree Scientific). Mice were anesthetized with isoflurane and bled prior to DENV-2 Col infection, at day 1 postinfection and every 2 days thereafter, by the retro-orbital route using plain calibrated capillary tubes (Chase; Scientific Glass). Blood was transferred to EDTA-K-covered microvette tubes (Sarstedt) for further processing. Plasma was separated by centrifugation at 1,000 × g for 5 min and used for RNA extraction, enzyme-linked immunosorbent assays (ELISAs), and cytokine analyses. For thrombocytopenia determination, 20 μl of blood was collected as described above, diluted 10-fold in PBS (GIBCO/Invitrogen) containing 5% bovine serum albumin, and human platelets were measured within 1 h after blood collection using an automated dual-angle light-scattering instrument (ADVIA120; Bayer Diagnostics). Mouse infections were performed in a biosafety level 2 (BSL-2) containment facility at the CBC of the Rockefeller University.
Leukocyte isolation.
EDTA was used as the anticoagulant in blood collection. Peripheral blood mononuclear cells were isolated by lymphocyte separation medium (Cellgro) gradient centrifugation at 930 × g for 20 min. Leukocytes were collected from the interphase and washed twice with cold PBS. For isolation of splenocytes, spleens were homogenized through a cell strainer (100 μm; BD Biosciences) and digested for 20 min at 37°C in collagenase-containing digestion medium (Hanks balanced salt solution with 0.1% collagenase IV [Sigma-Aldrich], 40 mM HEPES, 2 M CaCl2, and 2 U/ml DNase I [Roche]). After digestion, leukocytes were isolated using lymphocyte separation medium gradient centrifugation, as described for peripheral blood mononuclear cell isolation. To isolate bone marrow cells, tibias and femurs were flushed with PBS. The bone marrow was pressed through a cell strainer to obtain a single-cell suspension, and leukocytes were isolated by gradient centrifugation as described above. For isolation of intrahepatic leukocytes, the liver was perfused with 20 ml of cold PBS, minced, and pressed through a cell strainer (100 μm; BD Biosciences). The homogenized liver was resuspended in cold RPMI 1640 (Gibco) and centrifuged for 10 min at 330 × g. The pellet was resuspended in collagenase-containing digestion medium and incubated at 37°C for 30 min. After digestion, the liver suspension was centrifuged at 330 × g for 10 min. The pellet was resuspended in RPMI 1640 and gently overlaid onto a lymphocyte separation medium gradient as described above.
Flow cytometry and antibodies.
The protocol for surface marker staining consisted of blocking the cells for 10 min at room temperature with anti-mouse CD16/CD32 Fc block (BD Biosciences), washing with staining buffer (PBS containing 1% fetal bovine serum), staining with the appropriate antibodies for 15 min at room temperature, washing again twice with staining buffer, fixation with 4% paraformaldehyde for 15 min at 4°C, and resuspension of the cells in staining buffer. For intracellular staining cells, were fixed and permeabilized (Cytofix/Cytoperm; BD Biosciences) prior to staining with the appropriate antibody.
The following antihuman antibodies were used: CD45-Pacific Orange, CD14-AlexaFluor 700, CD16-Pacific Blue, CD3-phycoerythrin (PE)-Texas Red (Invitrogen Corp.); CD8-fluorescein isothiocyanate (FITC), CD19-allophycocyanin (APC), CD4-PE, CD33-peridinin chlorophyll protein (PerCP)-Cy5.5, HLA-DR–APC, HLA-A2–FITC (BD Biosciences); CD3-APC-eFluor780, CD8-APC-eFluor780, CD45RO-PerCP-eFluor710, CD27-PeCy7 (eBioscience); CD4-Pacific Blue, CD56-FITC (Biolegend). The following anti-mouse secondary antibodies were used: CD45-PeCy7 (eBioscience) and CD45-PerCP (Biolegend). For intracellular staining of infected cells, we utilized a mouse anti-DENV envelope monoclonal antibody (MAb; 4G2; ATCC) coupled to Alexa 647. Appropriate isotype controls were also purchased from each company.
Fluorescence-activated cell sorter (FACS) analysis was performed with a BD LSRII flow cytometer (BD Biosciences), and data were analyzed with FlowJo software 9.3.1 (Tree Star).
Human cytokine analysis.
Human cytokines and chemokines were measured in the plasma of infected and control BLT-NOD/SCID mice by Milliplex MAP human cytokine/chemokine technology (MPXHCYTO-60K-17; Millipore). Plasma samples were obtained at days 1, 3, and 10 postinfection, as described above, and 30-μl aliquots of the samples were used for testing expression of 17 human cytokines (granulocyte colony-stimulating factor [G-CSF], granulocyte-macrophage CSF [GM-CSF], alpha interferon 2 [IFN-α2], IFN-γ-induced protein 10 [IP-10], tumor necrosis factor beta [TNF-β], interleukin-1α [IL-1α], TNF-α, IFN-γ, IL-2, soluble IL-2 receptor [sIL-2R], IL-6, IL-8, IL-10, monocyte chemoattractant protein 1 [MCP-1], IL-2RA, IL-1RA, and vascular endothelial growth factor [VEGF]), according to the manufacturer's instructions. Quantification was performed using the Luminex xMAP platform and Xponent 3.1 software (Luminex). Results are expressed in picograms per milliliter.
Viral RNA extraction and real-time quantitative RT-PCR.
To determine viremia, viral RNA was extracted from 30 μl of mouse plasma by using TRIzol LS reagent (Invitrogen) following the manufacturer's instructions. Eluted RNA was finally resuspended in 30 μl of DEPC water, and 5 μl of RNA was used for quantification by the real-time quantitative reverse transcription-PCR (qRT-PCR) TaqMan system. PCR primers specific for DENV-2 strain 16681 along with the corresponding TaqMan probe were designed using the Biosearch Real-Time design software (Biosearch Technologies). PCR primers and TaqMan probes were obtained from Biosearch Technologies. The following forward and reverse primers were used: S07100620_FP (5′-CCGTCACACTGGGTTCCAA-3′), S07100620_RT (5′-CCGTCGTCATCCATTCATGCT-3′), and the 9920 probe (5′–6-carboxyfluorescein–CGAACAACCTGGTCCATACACGCT–black hole quencher 1–3′). The primers amplified a region of the NS5 gene of DENV-2. For the qRT-PCR assay, the LightCycler 480 master mix (Roche Diagnostics) was used. The final concentration of each PCR primer was 0.5 μM, and the concentration of the probe was 0.25 μM in a 20-μl total reaction mixture volume. For reverse transcription and amplification on the LightCycler 480, the cycling conditions were 63°C for 3 min, followed by 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 1 s. Cooling was done at 40°C for 10 s. The results were quantified by interpolation via a standard curve consisting of 10-fold serial dilutions of in vitro-transcribed DENV-2 RNA (by use of the Mega script kit [Ambion]). Data were analyzed with the LightCycler 480 software (Roche).
ELISAs.
Total human IgG and IgM antibodies were detected and quantified by using a human IgG or IgM ELISA quantitation set (Bethyl Laboratories), according to the manufacturer's protocol. For qualitative detection of DENV-specific IgG and IgM antibodies in plasma, we utilized a dengue virus IgG indirect ELISA kit (Panbio) or a dengue IgM capture ELISA kit (Panbio), which detected circulating DENV IgG and IgM antibodies to the four serotypes. Mouse plasma samples were diluted 1:10 and 1:100 fold in the kit's sample diluent and analyzed according to the manufacturer's instructions. Dengue virus NS1 antigen circulating in plasma of humanized mice was detected qualitatively from 30 μl of plasma by using a dengue virus early ELISA kit (Panbio), according to the company's protocol.
FACS-based neutralization assay.
Mouse serum samples collected at days 9, 12, and 25 were tested for neutralization activity in a fluorescence-activated cell sorting (FACS) neutralization test (FNT) in C6/36 cells (27). Briefly, 96-well plates were seeded at a density of 1.3 × 105 cell/well and incubated overnight at 28°C. Mouse sera were serially diluted and incubated with DENV-2 Col in a total volume of 100 μl for 1 h at 37°C. The virus-serum mixture was then used to inoculate C6/36 cells at a multiplicity of infection of 0.2 (determined relative to the number of seeded cells) for 1 h at room temperature. Wells were then washed with 1× PBS and incubated in culture medium for 24 h. Virus infectivity was determined by FACS analysis after staining with 4G2 antibody conjugated to Alexa 647, as previously described. Sera from mock-infected humanized mice were used as negative controls for neutralization. The FNT80 was defined as the highest 2-fold dilution of serum that produced a >80% reduction in DENV infection in C6/36 cells.
Analysis of ex vivo DENV-specific T cell responses.
Human monocytes were isolated from buffy coats of healthy donors (New York Blood Center) by using a magnetically activated cell sorting CD14 isolation kit (Milteny Biotec), according to the manufacturer's protocol. CD14+ cells were then differentiated from naive monocyte-derived dendritic cells (mDCs) via a 5-day incubation in DC medium (RPMI 1640 supplemented with 10% fetal bovine serum, 1.5% HEPES, and 1% penicillin-streptomycin) and 500 U/ml human GM-CSF (PeproTech) and 1,000 U/ml human IL-4 (PeproTech).
After 5 days in culture, mDCs were infected for 1 h at an MOI of 1 of DENV-2 Col or conditioned C6/36 cell culture medium (mock control), prepared and concentrated in the same way as the virus stock. Infected cells were then incubated for 48 h before coculture with T cells. The frequency of mDC infection was monitored at 24 and 48 h by flow cytometry using an antibody against the DENV E protein, as described above.
Human T cells were isolated from BLT-NOD/SCID mouse peripheral blood and spleen leukocytes by using a human CD3 cell isolation kit (Miltenyi Biotec). Purified CD3+ T cells were cocultured with mDCs in a 48-well plate at a mDC/T cell ratio of 1:3. In HLA-blocking experiments, target cells were incubated with 10 μg/ml anti-HLA-A/B/C (clone W6/32; eBioscience) and 10 μg/ml anti-HLA-DR/DP/DQ (clone Tü39; BD Pharmingen). After 4 days of coculture, the levels of secreted human IFN-γ and TNF-α in the supernatants were determined by cytometric bead array (CBA; BD Biosciences) on an LSR II flow cytometer (BD Biosciences).
Antiviral efficacy in DENV-infected BLT mice.
Antiviral NITD008 (kindly provided by Pei Yong Shi) was administered following two different experimental regimens. The first regimen consisted of intraperitoneal (i.p.) administration of the NITD008 compound to 10 mice at a dose of 15 mg/kg followed by intravenous (i.v.) injection of 1 × 106 TCID50 of DENV-2 Col. Antiviral dosing was continued twice a day for 7 days. The control group (6 mice) received an equal volume of dimethyl sulfoxide (DMSO) instead of NITD008 and followed identical infection and treatment protocols as the experimental group. The second antiviral protocol consisted of first infecting mice i.v. with 1 × 106 TCID50 of DENV-2 Col. Two days after virus inoculation, the infected mice were divided into 3 groups of 5 animals each and subjected to different treatment doses (administered in equal volumes). One group received 2.5 mg/kg of NITD008 (26), a second group received 15 mg/kg of NITD008, and a third group (control) received an equal volume of DMSO. For both protocols, the drug was dissolved in DMSO and diluted in PBS to achieve a final volume of 200 μl, which was administered i.p. twice a day, with an interval of 12 h between injections. Mice were bled through the retro-orbital route and monitored daily. Viral RNA was isolated from plasma and quantified by qRT-PCR.
Statistical analyses.
Statistical analyses were performed using unpaired Student's t test within GraphPad Prism version 5.0c. Plotted data represented means ± standard deviations. P values below 0.05 were considered statistically significant. The correlation between DENV titer and each cytokine was determined using nonparametric Spearman rank correlations (GraphPad Prism version 5.0c).
Ethics statement.
Use of all human tissue was reviewed and approved by the Rockefeller University's Institutional Review Board and ethics committee. All human tissues (fetal thymi and livers and buffy coats of healthy donors) were anonymized. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Research Council (62). All animal experiments were executed in adherence with RU CBC protocol number 12536, which was reviewed and approved by the Rockefeller University's Animal Care and Use Committee.
RESULTS
Human immune cell reconstitution in BLT-NOD/SCID mice.
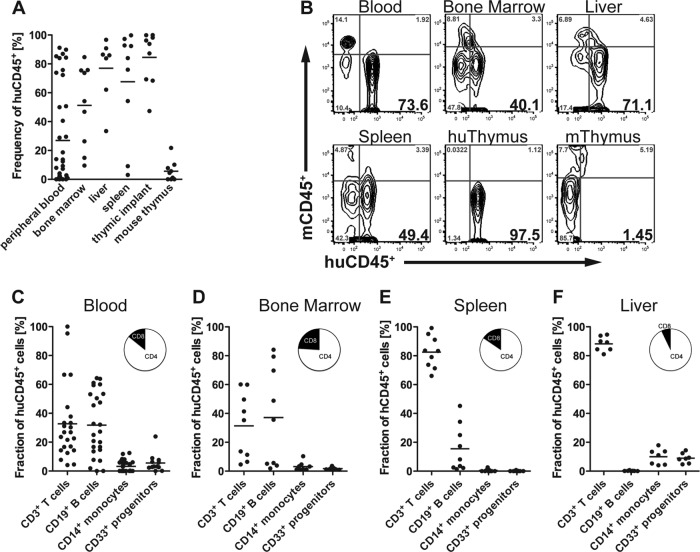
We generated humanized mice by transplanting nonobese NOD/SCID xenorecipients with an organoid consisting of small pieces of human fetal liver and thymus under the kidney capsule and subsequent intravenous injection of autologous HSC. BLT-NOD/SCID mice were analyzed for human immune cell reconstitution 16 weeks posttransplantation by flow cytometry of peripheral blood, spleen, bone marrow, liver, thymus, and human thymus organoid cells (Fig. 1A and B). In accordance with previous reports, we routinely achieved high levels of human hematopoietic stem cell reconstitution in lymphoid and nonlymphoid compartments (11, 28). Overall repopulation of lymphoid as well as nonlymphoid organs with human leukocytes exceeded 50% in most mice, even though the frequency of human cells in circulation was highly variable. As expected, almost all human thymocytes were found in the human thymic implant and not the murine thymus. Lineage analysis of CD45+-expressing human leukocytes in blood, bone marrow, spleen, and liver revealed levels of human T cells and B cells as well as myeloid cells comparable to levels described for humanized mice in general (Fig. 1C to F) (12, 15, 28, 29).
FIG 1.
Reconstitution of humanized BLT-NOD/SCID mice with human hematopoietic stem cells. (A and B) Frequencies (percentages) (A) and representative flow cytometry plots (B) of human CD45+ lymphocytes (huCD45+) in peripheral blood, bone marrow, liver, spleen, human thymic implant (huThymus), and mouse thymus (mThymus) at 16 weeks posttransplantation (n = 6 to 20 mice per group). Numbers in bold in the bottom right quadrants represent the percentage of mCD45− huCD45+ cells. (C to G) Human lymphocyte reconstitution in peripheral blood (C), bone marrow (D), spleen (E), and liver (F) with CD3+ T cells, CD19+ B cells, CD14+ monocytes, and CD33+ myeloid progenitor cells. The pie chart insets represent percentages of CD3+ CD4+ T helper cells (white) and CD3+ CD8+ cytotoxic T cells (black).
Replication and clinical signs of disease of a DENV clinical isolate in BLT-NOD/SCID mice.
For infection and pathogenesis studies in BLT-NOD/SCID mice, we utilized a serotype 2 DENV that was isolated from a human patient from Colombia in 1993 (Colombia 362981 TVP-3521, here referred to as DENV-2 Col). This virus was passaged 3 times in C6/36 mosquito cells, yielding a titer of 5 × 105 TCID50/ml. Supernatants were further concentrated 10-fold to increase virus titers for subsequent mouse inoculations. We assume that this DENV isolate did not adapt (or only minimally adapted) to growth in cultured human cells, given that virus was passaged only in mosquito cells. Nevertheless, DENV-2 Col was able to infect in vitro primary and immortalized human cells (data not shown), suggesting a natural tropism for human cells.
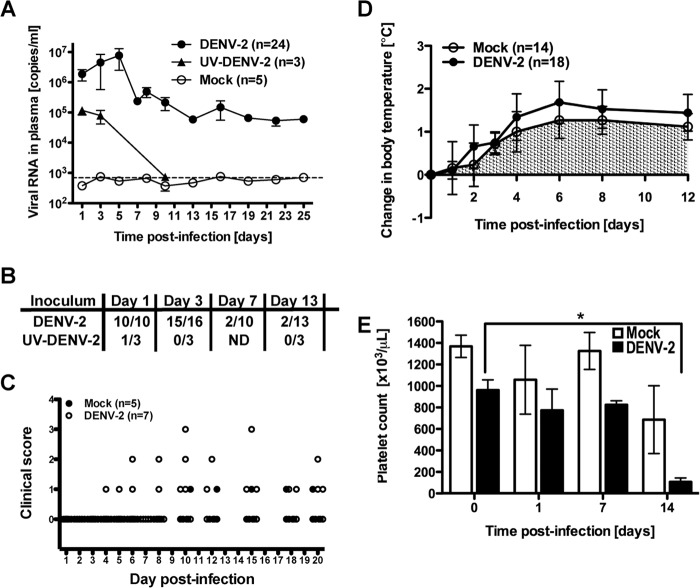
In order to minimize interexperimental variability, we used animals with hematopoietic human chimerism in blood greater than 50% at 16 weeks after tissue transplantation. BLT-NOD/SCID mice were infected i.v. with 1 × 106 TCID50 DENV-2 Col, or an equal volume of mock-infected C6/36 cell culture supernatants that was processed in the same manner as virus stock. In one experiment, we included three animals that were infected with UV-inactivated DENV-2 Col. UV treatment, as was shown previously for other viruses (30), rendered the DENV-2 Col noninfectious in vitro and also abrogated viremia in vivo (Fig. 2A). Animals were monitored for signs of morbidity, mortality, and viremia. Body weight, body temperature, posture, and motility, all of which have been observed to fluctuate in humans infected with DENV, were monitored daily. To determine the DENV-2 Col load, mice were bled every other day; viral RNA was extracted from the plasma, and the copy number was assessed by real-time qRT-PCR. Viral RNA was detected as early as 24 h postinfection and peaked between days 4 and 6 (Fig. 2A). We detected low levels of circulating infectious DENV in a fraction (20 to 40%) of infected animals until day 10 postinfection, but not thereafter (data not shown). Secreted DENV nonstructural protein 1 (NS1), another marker of productive virus replication, was also monitored. NS1 antigen dropped below the limit of detection by day 7 postinfection, confirming the kinetics of virus replication observed with qRT-PCR (Fig. 2B). RNA from UV-inactivated DENV-2 Col-infected mice was not detected in plasma after day 3 postinfection, again providing evidence for productive DENV-2 replication in the BLT-NOD/SCID mice (Fig. 2A). A total of four control nonhumanized NOD/SCID mice were infected in parallel. Of these, three animals had detectable levels of circulating viral RNA but showed consistently lower viremia levels than the humanized mice, with a maximum peak of 1 × 104 RNA copies/ml. By day 6 postinfection, RNA levels in control animals were undetectable (data not shown). When we compared reconstituted mice with a human chimerism exceeding 50%, no correlation was observed between the level of human engraftment and virus replication; the mice reconstituted to the highest level in this group did not show increased viremia compared to mice with a lower level of human chimerism.
FIG 2.
DENV infection and disease in BLT-NOD/SCID mice. (A) Indicated numbers of mice were inoculated i.v. with 1 × 106 TCID50 of DENV-2 Col, the same volume of UV-inactivated DENV-2 Col, or C6/36 cell culture medium. Mice were bled at the indicated times postinfection, and viral RNA was extracted from plasma and quantified by qRT-PCR. Results are presented as copies of viral RNA per ml of plasma. (B) DENV-2 NS1 antigen was detected by ELISA in plasma of infected animals at the indicated time points. Numbers in the nominators indicate positive signals for NS1 antigen, and denominators correspond to numbers of mice tested. NS1 antigen was not detected in mock-infected animals. ND, not detected. (C) Clinical scores measured and recorded daily for mock-infected (filled circles) and DENV-infected (open circles) mice. A clinical score was assigned as follows: 0, posture normal, appearance with smooth, shiny fur; 1, posture hunched, appearance with ruffled fur, loss of tone, loss of weight; 2, posture hunched, trembling, shaky, appearance with ruffled fur, loss of weight, rash; 3, posture severely hunched, appearance disheveled, significant (30%) body weight loss; 4, death. (D) Slight increase in body temperature in DENV-2 Col-infected mice measured at the indicated time points postinfection. (E) Thrombocytopenia in DENV-2-infected mice was observed at 14 days postinfection. Four mice were used in each experimental group. Error bars indicate standard errors of the means, and the asterisk indicates a statistically significant difference (P < 0.05) between the indicated columns. For panels A to E, data were pooled from 3 independent experiments involving mice reconstituted with cells or tissues from at least 6 different human donors.
Body weight and body temperature were measured, and a clinical scoring system that provided a quantification of the degree of illness observed in the infected mice was applied daily. Overall, neither mortality nor changes in body weight were consistently observed; however, several DENV-2 Col-infected mice exhibited significant weight loss and showed signs of illness, including ruffled fur and hunching (Fig. 2C). Fever is a hallmark of DENV infection in humans, and DENV-infected mice showed only a marginal increase in body temperature of no statistical significance compared to the control group (Fig. 2D). Higher viremia did not correlate with increased body temperature in infected mice. An additional characteristic of severe dengue disease in humans is decreased levels of circulating platelets. Platelet counts were determined longitudinally throughout the infection period. Up to day 4 postinfection, no significant changes in platelet numbers were detected in infected animals. At day 7 postinfection, we started to detect a drop in the total platelet count that only became significant (P < 0.05) at day 14 postinfection (Fig. 2E), which is consistent with previous published work. In summary, these data confirm that BLT-NOD/SCID mice can be infected with a low-passage, clinical isolate serotype 2 dengue virus, and productive infection is supported by the presence of human cells. This virus can replicate in vitro and in vivo and is capable of causing measurable signs of disease in these mice, such as thrombocytopenia and a trend toward elevated body temperature that did not reach statistical significance.
Cell tropism of DENV-2 Col infection in different organs of BLT-NOD/SCID mice.
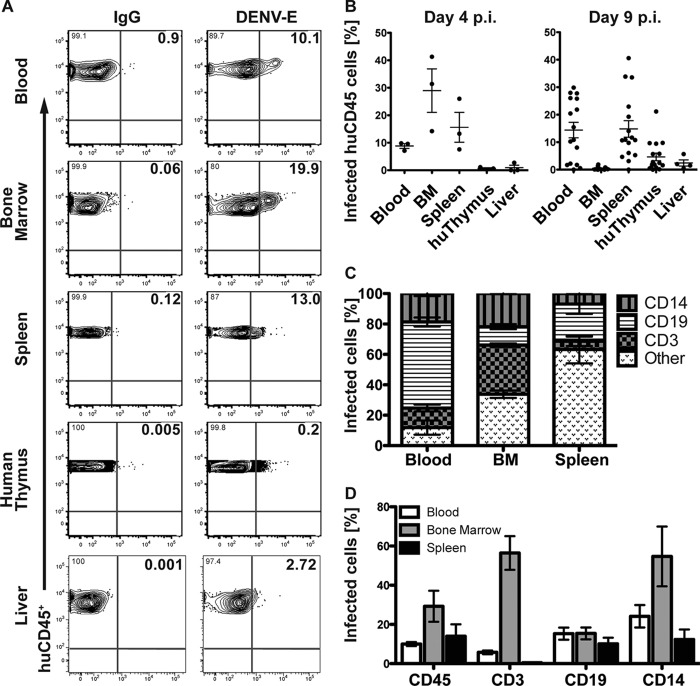
To identify the human lymphoid cells from peripheral blood and other immune-relevant organs that become infected upon DENV-2 Col inoculation, infected and control BLT-NOD/SCID mice were bled and sacrificed at day 4 (n = 3) and 9 (n = 16) postinfection. Human CD45+ leukocytes, B cells (CD19+), T cells (CD3+), and monocytes (CD14+) isolated from spleen, bone marrow, liver, and human thymus were analyzed for the presence of DENV E protein by flow cytometry. Human CD45+ leukocytes from the peripheral blood infected with DENV but not with the UV-inactivated DENV control (data not shown) were detected as early as 1 day postinfection in the peripheral blood. At day 4 postinfection, CD45+ cells derived from blood, bone marrow, and spleen also showed infection with DENV (Fig. 3A). The percentage of infected human CD45+ cells from blood and spleen remained constant until day 9; however, CD45+ cells from bone marrow only showed marginal signs of infection at this time point. In contrast, at day 9 an increased level of DENV E antigen was detected in CD45+ cells derived from the human thymus implant, compared to day 4 postinfection (Fig. 3B). Overall, we observed a very low frequency of infection in human leukocytes isolated from the liver. No more than 2% of total CD45+ cells isolated from the liver stained positive for DENV E antigen, and only a modest increase in the percentage of infected CD45+ cells (up to 5%) was observed at 9 days postinfection.
FIG 3.
Cellular tropism of DENV in different organs in infected BLT-NOD/SCID mice. (A) Representative flow cytometry plots of human CD45+ leukocytes infected with DENV-2 Col. Single, live cells were doubly gated for human CD45+ cells from the indicated body compartments and DENV envelope protein (DENV-E) or IgG (control) at day 4 postinfection. Bold numbers in top right corners of the graphs represent percentages of double-positive stained cells. (B) Frequency of DENV-E antigen-expressing cells in human CD45+ leukocytes from the indicated organs at days 4 and 9 following DENV-2 Col inoculation. Plots represent the means and standard errors of the means (SEM) for 3 mice (day 4) or 16 mice (day 9). For liver samples, data corresponding to 3 mice (day 4) or 6 mice (day 9) are presented. (C) Frequency of distinct CD45+ leukocyte populations infected by DENV in blood, bone marrow, and spleen at day 4 postinfection. Plots represent mean with SEM for 3 mice. (D) Percentage of infected human CD45+ leukocytes, CD3+ T cells, CD19+ B cells, and CD14+ monocytes from the indicated body compartments at day 4 postinfection. Plots represent mean with SEM for 3 mice. BM, bone marrow.
To provide a more detailed picture of the cellular tropism of DENV, we quantified the number of DENV antigen-positive cells in different immune cell subsets in the blood, spleen, and bone marrow (Fig. 3C and D). Given the small numbers of human leukocytes recovered from the liver, we were not able to discern reliably the different lineages of the infected cells in this organ. In the peripheral blood at 4 days postinfection, CD19+ B cells constituted the major immune cell population carrying DENV-E antigen, followed by CD14+ monocytes and CD3+ T cells. Conversely, of all the leukocytes infected with DENV in the bone marrow, more CD3+ cells harbored DENV E. Only a small fraction of infected leukocytes in the bone marrow corresponded to B cells. In the spleen, most of the infected CD45+ cells corresponded to myeloid and other cell lineages, followed by CD19+ B cells. Only a few infected T cells were detected in the spleen at this time point (Fig. 3C).
An alternative presentation of these data highlights the frequency of DENV E-positive cells within the indicated cell lineages in the corresponding immune compartments (Fig. 3D). In the peripheral blood at day 4 postinfection, approximately 30% of circulating CD14+ monocytes and 20% of CD19+ B cells expressed DENV-E antigen. The frequency of infected CD3+ T cells circulating in the blood was less than 5%. In the bone marrow, more than 30% of resident CD45+, CD3+, and CD14+ cells were infected with DENV. On the other hand, in the spleen, none of the cell lineages exceeded a 20% infection frequency. DENV-infected CD3+ T cells were almost absent in the spleen at this time point (Fig. 3D).
Overall, these results confirmed that human leukocytes are early targets for DENV infection (31, 32). In the peripheral blood of BLT-NOD/SCID mice, monocytes, myeloid cells, and B cells are the primary early targets of DENV infection. Infected CD3+ T cells may be recruited to the bone marrow, possibly in response to the infection of local cells, such as CD19+ B cells and CD14+ monocytes.
Induction of human cytokines and chemokines in DENV-infected BLT-NOD/SCID mice.
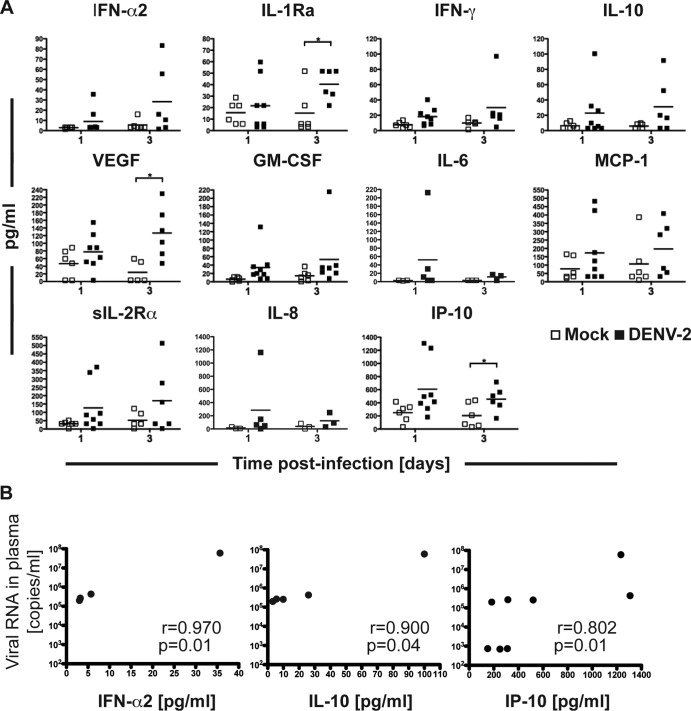
We then characterized the profiles of human cytokines and chemokines produced by human cells in the BLT-NOD/SCID mice at early time points (days 1 and 3) after DENV infection. We chose a panel of human cytokines that have been reported to be elevated in patients with severe dengue disease (reviewed in reference 33). Out of 17 cytokines assayed, 11 were detected in mouse plasma, but only 3 were significantly induced compared to the control group. Results are presented as the mean values from 3 to 8 mice (Fig. 4A). Of two soluble human receptor antagonists that have been associated with severe dengue disease, sIL-2Ra and IL-1 receptor 1 antagonist (IL-R1A), only IL-R1A was significantly elevated at day 3 postinfection (34, 35). The mast cell-derived mediator VEGF, first identified and characterized as a potent stimulator of endothelial permeability (36) and shown to be elevated in dengue patients with hemorrhagic syndrome (37, 38), was also significantly elevated in infected versus control mice at day 3 postinfection. Likewise, IP-10, which was previously shown to be a strong proinflammatory marker in dengue virus-infected patients at the febrile phase (39, 40), was present at elevated levels at 3 days postinfection. Increased circulating IL-1Rα may exert antipyretic actions in an effort to counteract the already-increased concentrations of proinflammtory cytokines, such as IL-1β, which was shown to be elevated in dengue virus-infected patients (40) but was not part of our cytokine panel. Although differences in the remaining cytokines/chemokines did not reach statistical significance, trends were observed. IFN-α2, IL-10, IL-6, and IL-8 showed slight increases at day 1 postinfection; levels of IFN-γ and GM-CSF were elevated in infected mice at day 3. For most of the cytokines/chemokines, increased levels correlated with circulating virus levels. IFN-α2, IL-10, and IP-10 are the three cytokines that showed statistically significant correlations between viral titer and cytokine secretion (Fig. 4B). The exception was monocyte chemoattractant protein 1 (MCP-1), for which we did not observed such a correlation. Furthermore, the levels of secreted cytokines in mice inoculated with UV-inactivated DENV-2 (data not shown) at day 3 postinfection corresponded to the levels observed in mock-infected animals. At an earlier time point, five cytokines (IL-1Ra, IL-10, MCP-1, sIL-2Rα, and IP-10) were slightly elevated in one of the UV-DENV-2 infected mice but not in the other, suggesting that these specific cytokines are mostly but not exclusively secreted in response to virus replication.
FIG 4.
Cytokine and chemokine secretion in dengue virus-infected mice. The tested panel of cytokines and chemokines included some that were detected in plasma of infected humanized mice at days 1 and 3 postinfection. Plots represent the individual mice in each group (ranging from 3 to 8 mice per group at each time point). Results are expressed in picograms per ml. *, statistically significant difference (P < 0.05) between the indicated experimental groups. (B) Spearman correlation coefficients (r) between viral titers and cytokine secretion levels in infected animals. IFN-α2, IL-10, and IP-10 were the only cytokines that showed statistically significant correlations between the viral titer and circulating cytokine level.
DENV infection in BLT-NOD/SCID mice elicits an increase in human IgM but not IgG.
To evaluate humoral responses in infected humanized mice, we measured the concentrations of circulating human IgG and IgM. Significant levels of human IgM antibodies were detected starting at day 13 postinfection. At this time, the majority of infected animals (71.4%) had an IgM titer at least 3-fold higher than the control group titer, with considerable variation between individual mice. At day 25 postinfection, high IgM levels persisted in 50% of the animals, but by day 32, human IgM titers returned to background levels (Fig. 5A). IgM titers peaked at 25 days after infection in all mice tested. We assayed for the presence of DENV-specific IgM antibodies in infected and control mice serum by utilizing a qualitative dengue IgM capture ELISA. We detected DENV-specific IgM antibodies in 30% of infected animals as early as 13 days postinfection. We then tested the neutralizing activity of these DENV-specific antibodies by performing a FACS-based neutralization test (27) in C6/36 cells (Fig. 5B). Four of the analyzed sera (mice 1122, 1123, 1125, and 9720) showed neutralizing activity, whereas the serum from a mock-infected mouse (mouse 9716) was unable to reduce DENV infection in this assay. We did not detect human IgG antibodies in plasma of humanized mice even at later time points (25 to 32 days postinfection). These results confirmed that antibody responses toward DENV are predominantly of the IgM isotype in this humanized mouse model. This is a reflection of the general inability of B cells in humanized mice to produce IgG due to lack of class switching.
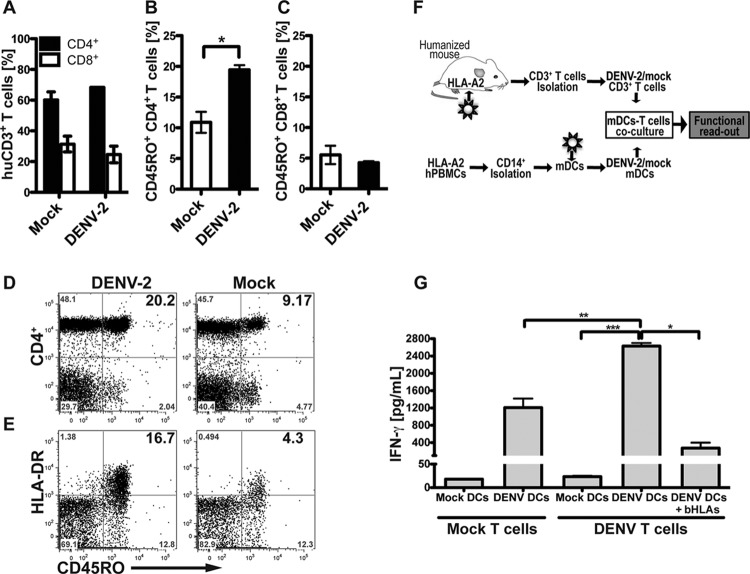
FIG 5.
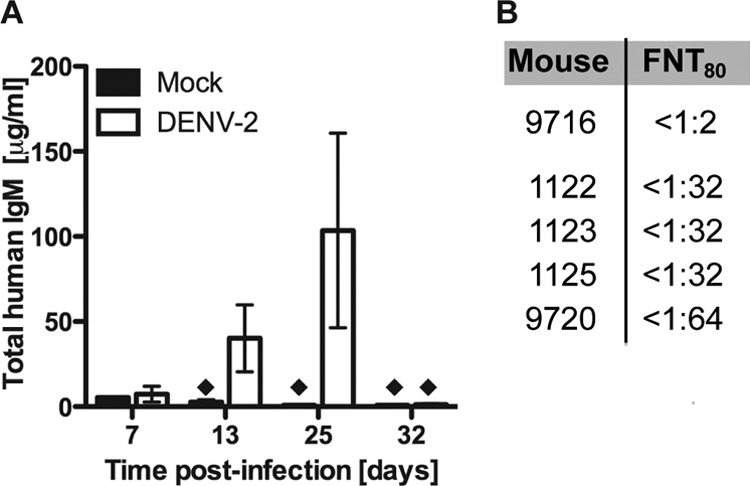
Human humoral immune responses in BLT-NOD/SCID mice infected with DENV-2. (A) Total human IgM antibodies circulating in the bloodstream of DENV-2 Col-infected and control animals were measured by ELISA at days 7, 13, 25, and 32 postinfection. Black diamonds represent less than 5 μg/ml of IgM antibody in the sample. (B) Antibody neutralization titers determined in the FACS-based neutralization test.
DENV-specific cellular immune responses in BLT-NOD/SCID mice.
To investigate whether humanized BLT mice can mount a functional T cell response to virus infection, we monitored phenotypic changes of circulating T cell populations in DENV-2 Col-infected humanized mice by using surface stains for markers of maturation. We did not observe an increase in the frequency of all CD3+ T cells in the blood of infected animals, and the ratio of CD4+ and CD8+ cells were equivalent to the control mice at 10 days postinfection (Fig. 6A). When the CD4+ T helper cell population from peripheral blood was analyzed, we found that the infected animals had a more mature/late-differentiated CD27− CD28− phenotype (data not shown) with relatively higher expression of the memory/effector surface marker CD45RO+ (Fig. 6B and D). These phenotypic changes in memory/effector markers were not observed in the circulating CD8+ T cell population (Fig. 6C) or in CD4+ T cells from mice inoculated with UV-inactivated DENV-2 (data not shown). Overall, these results suggest that DENV infection induces T cell-mediated immune responses in these mice and elicits phenotypic changes in CD4+ helper cells from the peripheral blood toward an activated and functional state.
FIG 6.
DENV infection enhances the effector/memory phenotype and specific response to DENV antigens by T cells. (A) Frequency of human CD3+ T cell subsets CD4+ and CD8+ from peripheral blood of DENV- or mock-infected mice, analyzed by flow cytometry. (B and C) Frequency of effector/memory (CD45RO+) phenotype of CD4+ helper T cells (B) and CD8+ T cells (C). (D and E) Representative flow cytometry plots showing CD45RO+ expression on CD4+ T cells (D) and HLA-DR expression on CD45RO+ CD4+ effector helper T cells (E). For all panels, frequencies and activation levels of CD3+ T cells were analyzed at day 10 postinfection. Bold numbers in top right quadrants of each graph represent corresponding double-positive stained cells. (F) Schematic representation of the experimental layout. CD14+ monocytes were obtained from HLA-A2 blood donors and differentiated to mDC with a conditional medium containing IL-4 and GM-CSF for 5 days. mDC were then mock infected or DENV infected for 48 h and then mixed with T cells obtained from splenocytes from mock-infected or DENV-infected BLT-NOD/SCID mice. After 4 days in culture, human IFN-γ release in supernatants was measured as the functional readout. (G) Human IFN-γ secreted into supernatants by activated T cells, after 4 days of mDC-T cell coculture. Data were pooled from two independent experiments. Results are expressed in picograms per ml. Based on Student's t test, ***, P ≤ 0.0005; **, P ≤ 0.005; *, P ≤ v0.05.
We next examined whether T cells from infected BLT-NOD/SCID mice also acquired effector functions. Previous studies (21, 32, 41–44) using humanized mice as a model for dengue disease utilized CD34+ HSC-transplanted BALB/c Rag2−/− IL2Rγ null, NOD/SCID, or NOD/SCID IL2Rγ null (NSG) mice, which are by and large less suitable for monitoring virus-specific T cell immunity compared to BLT HIS mice. Currently, there is only one study that has characterized dengue virus infection in humanized BLT mice (24), and it showed some evidence of T cell activation in response to peptide pools and HLA-A2-restricted peptides. However, few DENV T cell epitopes identified in humans have been confirmed in humanized mice, and the hierarchy of T cell epitopes can actually differ drastically between humans and humanized mice (15). Therefore, we decided to investigate the ability of the infected BLT mice to generate the DENV-specific effector T cells by priming with DENV-infected dendritic cells. For this, we restimulated T cells isolated from mock- or DENV-infected mice with DENV-infected DCs, which are capable of presenting DENV-2 Col epitopes in the context of HLA (Fig. 6F). While it would have been desirable to use autologous DC for these restimulation assays, the number of animals required to produce enough stimulator cells exceeded the maximum cohort sizes that we were able to generate from a given fetal tissue donor. As an alternative, we generated monocyte-derived DC from HLA-A2-matched human donors and infected them with DENV-2 Col for 2 days. Virus- and mock-infected mDC were then coincubated for 4 days with CD3+ T cells derived from infected and control BLT-NOD/SCID mice that had been reconstituted with CD34+ HSC from an HLA-A2 donor. IFN-γ release was monitored as the signature for specific T cell activation and acquisition of effector functions. T cells from infected animals developed a vigorous specific response by secreting significantly higher cytokine levels than controls upon incubation with infected mDC (Fig. 6G). Addition of blocking antibodies against HLA-A/B/C and HLA-DR/DP/DQ significantly reduced cytokine production, providing evidence that DENV antigens were being recognized in the context of human MHC antigens. Coculture of human T cells with uninfected mDC resulted in very low IFN-γ production, confirming that the cytokine release was not due to recognition of allogeneic antigens as a consequence of mismatches in minor alleles between stimulator and effector cells. In summary, our data demonstrate that HLA-restricted, DENV-specific human CD3+ T cells were primed in BLT-NOD/SCID mice upon infection with DENV-2 Col.
Antiviral efficacy in DENV-infected BLT-NOD/SCID mice.
In order to validate the use of humanized BLT mice as a platform for preclinical antiviral testing, we tested the efficacy of the adenosine nucleoside inhibitor NITD008 (26) by following two different drug administration regimens. In the first protocol, we injected NITD008 intraperitoneally at 15 mg/kg of body weight and intravenously infected mice with DENV-2 immediately thereafter. This drug dosage was continued for 7 days, and mice were bled through the retro-orbital route and monitored daily. We observed a significant decrease in circulating virus RNA as measured by qRT-PCR, and virus RNA remained lower than the vehicle control mice for the duration of the treatment (Fig. 7A). For the second protocol, we aimed to recreate a scenario of DENV infection that would be more relevant for postexposure treatment in humans. Therefore, we first infected mice i.v. with 1 × 106 TCID50 of DENV-2 Col, and 2 days later we treated them with three different regimens. The first group of mice received 2.5 mg/kg of NITD008, the second group received 15 mg/kg of NITD008, and the third group (control) received DMSO in the same volume as the experimental groups. In all cases, the drug was administered i.p. twice a day, with an interval of 12 h between injections. Efficacy of treatment was determined by the decrease in viremia in treated animals. Animals treated with the maximum dose of 15 mg/kg twice a day showed a significant reduction of 15- fold in viremia after 3 days of treatment (Fig. 7B) compared to the control-treated animals.
FIG 7.
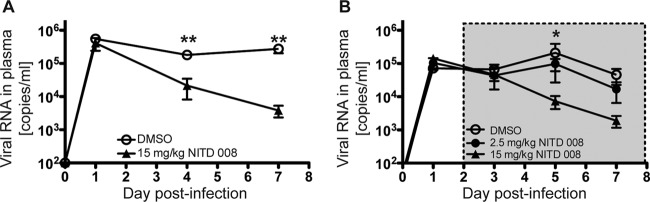
Antiviral efficacy of NITD008 in BLT-NOD/SCID mice. Humanized mice were inoculated i.v. with 1 × 106 TCID50 of DENV-2 Col, and antiviral efficacy was evaluated by using two different protocols. (A) The antiviral compound NITD008 was administered i.p. (to 10 mice) at the indicated concentrations immediately before viral inoculation, and dosing continued every 12 h for 7 days. Equal volumes of the DMSO vehicle were administered to the control group (6 mice). (B) Antiviral treatment was started at day 2 postinfection. Two groups of mice (n = 5 per group) were dosed i.p. twice a day with the indicated amount of the antiviral agent NITD008. The control group (9 mice) was treated with DMSO twice a day. Mice were bled at the indicated times postinfection, and viral RNA was extracted from plasma and quantified by qRT-PCR. Results are presented as copies of viral RNA per ml of plasma. The gray box indicates the duration of antiviral treatment. Based on Student's t test: **, P ≤ 0.005; *, P ≤ 0.05.
DISCUSSION
Previous studies of humanized mice infected with dengue virus reported that the clinical outcome for infected mice depended greatly on the virus strain used as the inoculum (32, 43, 44). In this study, we sought to establish an experimental system that recreated human infections with DENV, and therefore we used a DENV isolate from a human patient; the virus had only been grown in mosquito cells and had been passaged no more than three times in these cells. We demonstrated that humanized BLT mice were susceptible to DENV infection and developed modest signs of dengue disease. Infected animals showed slightly elevated body temperature, which correlated with the peak of viremia (day 4 to 5 postinfection), similar to what has been described in humans. Worth mentioning is that the duration of the peak of viremia observed in our model was shorter than that of other studies in humanized mice. This could be attributed to the route of infection chosen by our group (i.v.), which differed from the subcutaneous, intradermal, or mosquito bite route used in studies that have shown prolonged high viral loads (32, 42). An alternative but not mutually exclusive explanation for the difference in the kinetics of viremia could be the nature of the viral strain used by our group, an uncharacterized isolate of DENV-2 that has not been previously adapted to human or mouse cells. Consistent with previous studies (21, 32), we also observed a significant decrease in circulating total platelets, i.e., thrombocytopenia, another hallmark of DENV infection in humans. Thrombocytopenia is frequently observed among dengue fever patients, and two mechanisms have been proposed to explain it: antibody-mediated depletion of platelets from peripheral blood (45–49) and deficient platelet production by megakaryocytes in the bone marrow (25, 50). The latter hypothesis is supported by a study that demonstrated that human megakaryocytes from the bone marrow were highly permissive for DENV infection in vitro, suggesting that megakaryocytes may also be targeted in vivo, thus providing a plausible mechanism for thrombocytopenia during acute infection in humans (50). Furthermore, a recent study on DENV in humanized mice showed that DENV infection inhibited the production of human megakaryocytes and their progenitor cells in the bone marrow of NSG mice, resulting in depletion of circulating human platelets (25). Intriguingly though, in our infection model we did not observe a correlation between peak viremia and decreased platelet levels. Instead, we found a delay in the loss of platelets compared to the levels of circulating viral RNA, which peaked between days 5 and 7 postinfection. Although the modest antibody response in humanized mice does not support the antibody-mediated mechanism as an explanation for the loss of circulating platelets, the kinetics of thrombocytopenia in our mouse model does not discard this hypothesis. More work still has to be done to develop a general model for platelet loss during DENV infection in the BLT-NOD/SCID mouse model.
It has been largely established that mononuclear phagocytes, such as dendritic cells, monocytes, and macrophages, are the primary cellular targets of DENV infection in humans. More recent human studies have shown that B cells can also be infected and may play an important role in dengue virus pathogenesis. In our mouse model, we found that, as expected, monocytes were infected. Unfortunately, however, due to the low frequency of human dendritic cells in this mouse model, we could not make a reliable analysis of infection in this lineage. Interestingly, most of the DENV-infected cells in the peripheral blood of the BLT mice corresponded to CD19+ B cells, and these infected cells were detected as early as 3 days postinfection. This observation was in agreement with reports suggesting that B cells are important sites for infection and dissemination of DENV in humans (51–53). Infected B cells were also detected in the spleen and, to a lesser extent, in the bone marrow at this time point.
Whether T lymphocytes become infected with DENV in the course of human infection is still contradictory (54, 55). We detected infected CD3+ T cells in blood, spleen, and bone marrow, the latter organ being the one with a higher frequency of infected T cells (20% of all DENV-positive leukocytes). This result is in agreement with another published study on DENV in humanized mice, which demonstrated that approximately half of the T cells from the bone marrow carried DENV antigen (32).
T lymphocytes from infected BLT-NOD/SCID mice also acquired effector functions. In contrast to other study groups, who primarily relied on detection of antigen-specific T cell responses, which they measured by using recombinant peptides (24, 41), we demonstrated that in vivo-primed T cells become activated and acquire effector functions when restimulated ex vivo with DENV-infected DC. Antigen recognition is HLA specific, as anti-MHC class I and II antibodies significantly decreased the release of effector cytokines.
DENV infection induced a broad spectrum of proinflammatory cytokines and chemokines, some of which were previously shown to be increased in patients with dengue (56, 57). In accordance with previous reports, BLT humanized mice also mounted specific and neutralizing IgM but not IgG responses to DENV infection (21, 24, 32, 41, 44). This was likely a reflection of the general inability of current versions of humanized mice to switch classes efficiently, possibly due to the aberrant architecture of secondary lymphoid organs.
While these data and data from other groups who have studied DENV infection in humanized mice (21, 24, 41, 44) are encouraging, it is clear that the humanized mouse model will require additional refinements to recapitulate faithfully human immune responses to DENV infection and immune-enhanced pathogenesis (reviewed in reference 58). Arguably, more-adequate human T cell selection can be achieved in BLT mice by transplantation of small pieces of human thymus under the kidney capsule prior to injection of autologous HSC (11). While we provided evidence for DENV-specific T cell activation, overall T cell immunity did not seem to be dramatically improved, which is in accordance with previous reports characterizing EBV (11) or HIV infection in humanized BLT mice (28). This may be explained in part by the lack expression of HLA in nonhematopoietic tissues in the periphery, where a continued interaction of naive T cells with self peptide has been shown to be important for maintenance of the immune response (59, 60) and viral epitopes are presented by mouse MHC molecules and are consequently less efficiently recognized. Recently, it was reported that infection of BLT-NSG mice expressing HLA-A2 with tissue culture-derived DENV resulted in enhanced humoral and cellular immune responses (24). While these data are intriguing, additional refinements of the immune responses are undoubtedly needed.
In future studies, a major focus should be on detailed characterization of secondary immune responses to DENV. Currently, there is little evidence for the induction of memory B and T cells in humanized mice with any pathogen tested thus far. Both B and T cells become activated, which is reflected in phenotypic changes and the acquisition of effector functions. However, inadequately organized lymphoid organs may result in aberrant priming of memory T cells, and lack of cross-reactivity between human and mouse cytokines, such as interleukins 7 and 15, may efficiently maintain memory pools. Consequently, current versions of humanized mice may not (yet) be suitable to recapitulate the immune enhancement of dengue virus pathogenesis, which is frequently observed in preimmune individuals who become exposed to heterologous DENV serotypes.
Despite these shortcomings, mice harboring components of a human hemato-lymphoid system can be of use for testing preclinical efficacies of antiviral agents. Currently, no specific directly acting antiviral drug (DAA) regimens have been approved for DENV treatment. A broad range of DENV proteins and host factors are being considered as antiviral targets (reviewed in reference 61). Several compounds that exhibited anti-DENV activity in preclinical assays have been identified, but few have advanced into clinical trials. In order to prioritize more effectively lead compounds and to accelerate their development toward human trials, predictive preclinical models are desired. An adenosine nucleoside inhibitor, NITD008, was previously shown to inhibit the RNA-dependent RNA polymerase of DENV, presumably through its action as a chain terminator during viral RNA replication. NITD was shown to reduce viremia in AG129 mice (lacking IFN-αβ and IFN-γ receptors) infected with DENV-2 strain TSV01 (26). Here, we chose NITD008 as a prototype DAA to establish proof of concept for the utility of humanized mice to evaluate the efficacy of pharmacological inhibitors of DENV. Our data show that NITD008 significantly reduces viremia in xenorecipients harboring components of a human immune system infected with a DENV-2 patient isolate and are thus consistent with previous observations in inbred mouse models of DENV disease. Thus, we have provided, for the first time, proof of concept for use of humanized mice for preclinical testing of antivirals. Humanized mice allow the monitoring of drug efficacy against clinical DENV isolates in the context of human cells. This may also open up opportunities to simultaneously assess human-specific idiosyncratic drug toxicities, which are frequently immune mediated. These are important differences from the AG129 model, which relies on a highly adapted, murine-tropic DENV strain that only replicates in severely immunocompromised mice.
In conclusion, humanized BLT mice may serve as a useful model to study DENV infection, but additional improvements of the xenorecipients and humanization protocols are needed to recapitulate faithfully DENV infection. However, humanized mice can already be deployed for preclinical evaluation of antiviral treatment regimens.
ACKNOWLEDGMENTS
We thank William C. Budell, Mary Ellen Castillo, Brenna Flatley, Tamar Friling, Joshua A. Horwitz, Kathy Mu, and Julia Sable for laboratory support; Juana Gonzalez from the Translational Technology Core Laboratory at The Rockefeller University for help with the xMAP Luminex assay; Robert Tesh from the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA) for providing DENV clinical isolates; Pei-Yong Shi (Novartis Institute for Tropical Diseases) for the NITD008 compound; Ana Fernandez-Sesma and Dabeiba Bernal-Rubio (Mount Sinai School of Medicine, NY) for providing reagents and technical assistance with handling DENVs; Naglaa Shoukry for insightful discussions; and Andrew Tager for advice and training on the BLT surgical procedures.
This study was supported in part by the Greenberg Medical Institute, the Starr Foundation, Center for Translational Science Award (CTSA) pilot grant CCL3001018 (to A.P.), CTSA grant UL1 RR024143 (to The Rockefeller University) from the National Center for Research Resources (NCRR), a component of NIH, and a U19 AI057266 subcontract with Emory University (to C.M.R. and A.P.). Natalia Frias-Staheli is a recipient of a Rockefeller University Women in Science Fellowship. Eva Billerbeck and Marcus Dorner are recipients of postdoctoral fellowships from the Deutsche Forschungsgemeinschaft (DFG). Alexander Ploss is a recipient of a Liver Scholar Award from the American Liver Foundation.
Footnotes
Published ahead of print 11 December 2013
REFERENCES
- 1.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. 2010. Dengue: a continuing global threat. Nat. Rev. Microbiol. 8:S7–S16. 10.1038/nrmicro2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitehorn J, Simmons CP. 2011. The pathogenesis of dengue. Vaccine 29:7221–7228. 10.1016/j.vaccine.2011.07.022 [DOI] [PubMed] [Google Scholar]
- 4.Rothman AL. 2011. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat. Rev. Immunol. 11:532–543. 10.1038/nri3014 [DOI] [PubMed] [Google Scholar]
- 5.Goncalvez AP, Engle RE, St. Claire M, Purcell RH, Lai CJ. 2007. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc. Natl. Acad. Sci. U. S. A. 104:9422–9427. 10.1073/pnas.0703498104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koraka P, Benton S, van Amerongen G, Stittelaar KJ, Osterhaus AD. 2007. Characterization of humoral and cellular immune responses in cynomolgus macaques upon primary and subsequent heterologous infections with dengue viruses. Microbes Infect. 9:940–946. 10.1016/j.micinf.2007.03.012 [DOI] [PubMed] [Google Scholar]
- 7.Guirakhoo F, Pugachev K, Zhang Z, Myers G, Levenbook I, Draper K, Lang J, Ocran S, Mitchell F, Parsons M, Brown N, Brandler S, Fournier C, Barrere B, Rizvi F, Travassos A, Nichols R, Trent D, Monath T. 2004. Safety and efficacy of chimeric yellow fever-dengue virus tetravalent vaccine formulations in nonhuman primates. J. Virol. 78:4761–4775. 10.1128/JVI.78.9.4761-4775.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yauch LE, Shresta S. 2008. Mouse models of dengue virus infection and disease. Antiviral Res. 80:87–93. 10.1016/j.antiviral.2008.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Legrand N, Ploss A, Balling R, Becker PD, Borsotti C, Brezillon N, Debarry J, de Jong Y, Deng H, Di Santo JP, Eisenbarth S, Eynon E, Flavell RA, Guzman CA, Huntington ND, Kremsdorf D, Manns MP, Manz MG, Mention JJ, Ott M, Rathinam C, Rice CM, Rongvaux A, Stevens S, Spits H, Strick-Marchand H, Takizawa H, van Lent AU, Wang C, Weijer K, Willinger T, Ziegler P. 2009. Humanized mice for modeling human infectious disease: challenges, progress, and outlook. Cell Host Microbe 6:5–9. 10.1016/j.chom.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shultz LD, Ishikawa F, Greiner DL. 2007. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7:118–130. 10.1038/nri2017 [DOI] [PubMed] [Google Scholar]
- 11.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, Garcia JV. 2006. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 12:1316–1322. 10.1038/nm1431 [DOI] [PubMed] [Google Scholar]
- 12.Wege AK, Melkus MW, Denton PW, Estes JD, Garcia JV. 2008. Functional and phenotypic characterization of the humanized BLT mouse model. Curr. Top. Microbiol. Immunol. 324:149–165. 10.1007/978-3-540-75647-7_10 [DOI] [PubMed] [Google Scholar]
- 13.Leung C, Chijioke O, Gujer C, Chatterjee B, Antsiferova O, Landtwing V, McHugh D, Raykova A, Munz C. 2013. Infectious diseases in humanized mice. Eur. J. Immunol. 43:2246–2254. 10.1002/eji.201343815 [DOI] [PubMed] [Google Scholar]
- 14.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. 2004. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 304:104–107. 10.1126/science.1093933 [DOI] [PubMed] [Google Scholar]
- 15.Strowig T, Gurer C, Ploss A, Liu YF, Arrey F, Sashihara J, Koo G, Rice CM, Young JW, Chadburn A, Cohen JI, Munz C. 2009. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J. Exp. Med. 206:1423–1434. 10.1084/jem.20081720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dittmer D, Stoddart C, Renne R, Linquist-Stepps V, Moreno ME, Bare C, McCune JM, Ganem D. 1999. Experimental transmission of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) to SCID-hu Thy/Liv mice. J. Exp. Med. 190:1857–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerjee P, Tripp A, Lairmore MD, Crawford L, Sieburg M, Ramos JC, Harrington W, Jr, Beilke MA, Feuer G. 2010. Adult T-cell leukemia/lymphoma development in HTLV-1-infected humanized SCID mice. Blood 115:2640–2648. 10.1182/blood-2009-10-246959 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Smith MS, Goldman DC, Bailey AS, Pfaffle DL, Kreklywich CN, Spencer DB, Othieno FA, Streblow DN, Garcia JV, Fleming WH, Nelson JA. 2010. Granulocyte-colony stimulating factor reactivates human cytomegalovirus in a latently infected humanized mouse model. Cell Host Microbe 8:284–291. 10.1016/j.chom.2010.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song J, Willinger T, Rongvaux A, Eynon EE, Stevens S, Manz MG, Flavell RA, Galan JE. 2010. A mouse model for the human pathogen Salmonella typhi. Cell Host Microbe 8:369–376. 10.1016/j.chom.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vuyyuru R, Liu H, Manser T, Alugupalli KR. 2011. Characteristics of Borrelia hermsii infection in human hematopoietic stem cell-engrafted mice mirror those of human relapsing fever. Proc. Natl. Acad. Sci. U. S. A. 108:20707–20712. 10.1073/pnas.1108776109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bente DA, Melkus MW, Garcia JV, Rico-Hesse R. 2005. Dengue fever in humanized NOD/SCID mice. J. Virol. 79:13797–13799. 10.1128/JVI.79.21.13797-13799.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beaumier CM, Jaiswal S, West KY, Friberg H, Mathew A, Rothman AL. 2010. Differential in vivo clearance and response to secondary heterologous infections by H2(b)-restricted dengue virus-specific CD8+ T cells. Viral Immunol. 23:477–485. 10.1089/vim.2010.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanya S, Kim SS, Abraham S, Yao J, Kumar M, Kumar P, Haridas V, Lee SK, Shultz LD, Greiner D, Manjunath N, Shankar P. 2010. Targeted delivery of small interfering RNA to human dendritic cells to suppress dengue virus infection and associated proinflammatory cytokine production. J. Virol. 84:2490–2501. 10.1128/JVI.02105-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaiswal S, Pazoles P, Woda M, Shultz LD, Greiner DL, Brehm MA, Mathew A. 2012. Enhanced humoral and HLA-A2-restricted dengue virus-specific T-cell responses in humanized BLT NSG mice. Immunology 136:334–343. 10.1111/j.1365-2567.2012.03585.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sridharan A, Chen Q, Tang KF, Ooi EE, Hibberd ML, Chen J. 2013. Inhibition of megakaryocyte development in the bone marrow underlies dengue virus-induced thrombocytopenia in humanized mice. J. Virol. 87:11648–11658. 10.1128/JVI.01156-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin Z, Chen YL, Schul W, Wang QY, Gu F, Duraiswamy J, Kondreddi RR, Niyomrattanakit P, Lakshminarayana SB, Goh A, Xu HY, Liu W, Liu B, Lim JY, Ng CY, Qing M, Lim CC, Yip A, Wang G, Chan WL, Tan HP, Lin K, Zhang B, Zou G, Bernard KA, Garrett C, Beltz K, Dong M, Weaver M, He H, Pichota A, Dartois V, Keller TH, Shi PY. 2009. An adenosine nucleoside inhibitor of dengue virus. Proc. Natl. Acad. Sci. U. S. A. 106:20435–20439. 10.1073/pnas.0907010106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambeth CR, White LJ, Johnston RE, de Silva AM. 2005. Flow cytometry-based assay for titrating dengue virus. J. Clin. Microbiol. 43:3267–3272. 10.1128/JCM.43.7.3267-3272.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brainard DM, Seung E, Frahm N, Cariappa A, Bailey CC, Hart WK, Shin HS, Brooks SF, Knight HL, Eichbaum Q, Yang YG, Sykes M, Walker BD, Freeman GJ, Pillai S, Westmoreland SV, Brander C, Luster AD, Tager AM. 2009. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J. Virol. 83:7305–7321. 10.1128/JVI.02207-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Billerbeck E, Barry WT, Mu K, Dorner M, Rice CM, Ploss A. 2011. Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor, GM-CSF and interleukin 3 expressing NOD SCID IL2Rγ null humanized mice. Blood 117:3076–3086. 10.1182/blood-2010-08-301507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walters KA, Syder AJ, Lederer SL, Diamond DL, Paeper B, Rice CM, Katze MG. 2009. Genomic analysis reveals a potential role for cell cycle perturbation in HCV-mediated apoptosis of cultured hepatocytes. PLoS Pathog. 5(1):e1000269. 10.1371/journal.ppat.1000269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durbin AP, Vargas MJ, Wanionek K, Hammond SN, Gordon A, Rocha C, Balmaseda A, Harris E. 2008. Phenotyping of peripheral blood mononuclear cells during acute dengue illness demonstrates infection and increased activation of monocytes in severe cases compared to classic dengue fever. Virology 376:429–435. 10.1016/j.virol.2008.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mota J, Rico-Hesse R. 2011. Dengue virus tropism in humanized mice recapitulates human dengue fever. PLoS One 6(6):e20762. 10.1371/journal.pone.0020762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srikiatkhachorn A, Green S. 2010. Markers of dengue disease severity. Curr. Top. Microbiol. Immunol. 338:67–82. 10.1007/978-3-642-022159-9_6 [DOI] [PubMed] [Google Scholar]
- 34.Suharti C, van Gorp EC, Dolmans WM, Setiati TE, Hack CE, Djokomoeljanto R, van der Meer JW. 2003. Cytokine patterns during dengue shock syndrome. Eur. Cytokine Netw. 14:172–177 [PubMed] [Google Scholar]
- 35.Valero N, Larreal Y, Espina LM, Reyes I, Maldonado M, Mosquera J. 2008. Elevated levels of interleukin-2 receptor and intercellular adhesion molecule 1 in sera from a venezuelan cohort of patients with dengue. Arch. Virol. 153:199–203. 10.1007/s00705-007-1080-4 [DOI] [PubMed] [Google Scholar]
- 36.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. 1983. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219:983–985. 10.1126/science.6823562 [DOI] [PubMed] [Google Scholar]
- 37.Tseng CS, Lo HW, Teng HC, Lo WC, Ker CG. 2005. Elevated levels of plasma VEGF in patients with dengue hemorrhagic fever. FEMS Immunol. Med. Microbiol. 43:99–102. 10.1016/j.femsim.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 38.Furuta T, Murao LA, Lan NT, Huy NT, Huong VT, Thuy TT, Tham VD, Nga CT, Ha TT, Ohmoto Y, Kikuchi M, Morita K, Yasunami M, Hirayama K, Watanabe N. 2012. Association of mast cell-derived VEGF and proteases in dengue shock syndrome. PLoS Negl. Trop. Dis. 6(2):e1505. 10.1371/journal.pntd.0001505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rathakrishnan A, Wang SM, Hu Y, Khan AM, Ponnampalavanar S, Lum LC, Manikam R, Sekaran SD. 2012. Cytokine expression profile of dengue patients at different phases of illness. PLoS One 7(12):e52215. 10.1371/journal.pone.0052215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de-Oliveira-Pinto LM, Gandini M, Freitas LP, Siqueira MM, Marinho CF, Setubal S, Kubelka CF, Cruz OG, Oliveira SA. 2012. Profile of circulating levels of IL-1Ra, CXCL10/IP-10, CCL4/MIP-1β and CCL2/MCP-1 in dengue fever and parvovirosis. Mem. Inst. Oswaldo Cruz 107:48–56. 10.1590/S0074-02762012000100007 [DOI] [PubMed] [Google Scholar]
- 41.Jaiswal S, Pearson T, Friberg H, Shultz LD, Greiner DL, Rothman AL, Mathew A. 2009. Dengue virus infection and virus-specific HLA-A2 restricted immune responses in humanized NOD-scid IL2Rγ null mice. PLoS One 4(10):e7251. 10.1371/journal.pone.0007251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox J, Mota J, Sukupolvi-Petty S, Diamond MS, Rico-Hesse R. 2012. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J. Virol. 86:7637–7649. 10.1128/JVI.00534-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mota J, Rico-Hesse R. 2009. Humanized mice show clinical signs of dengue fever according to infecting virus genotype. J. Virol. 83:8638–8645. 10.1128/JVI.00581-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuruvilla JG, Troyer RM, Devi S, Akkina R. 2007. Dengue virus infection and immune response in humanized RAG2−/− γc−/− (RAG-hu) mice. Virology 369:143–152. 10.1016/j.virol.2007.06.005 [DOI] [PubMed] [Google Scholar]
- 45.Sun DS, King CC, Huang HS, Shih YL, Lee CC, Tsai WJ, Yu CC, Chang HH. 2007. Antiplatelet autoantibodies elicited by dengue virus non-structural protein 1 cause thrombocytopenia and mortality in mice. J. Thromb. Haemost. 5:2291–2299. 10.1111/j.1538-7836.2007.02754.x [DOI] [PubMed] [Google Scholar]
- 46.Saito M, Oishi K, Inoue S, Dimaano EM, Alera MT, Robles AM, Estrella BD, Jr, Kumatori A, Moji K, Alonzo MT, Buerano CC, Matias RR, Morita K, Natividad FF, Nagatake T. 2004. Association of increased platelet-associated immunoglobulins with thrombocytopenia and the severity of disease in secondary dengue virus infections. Clin. Exp. Immunol. 138:299–303. 10.1111/j.1365-2249.2004.02626.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang S, He R, Patarapotikul J, Innis BL, Anderson R. 1995. Antibody-enhanced binding of dengue-2 virus to human platelets. Virology 213:254–257. 10.1006/viro.1995.1567 [DOI] [PubMed] [Google Scholar]
- 48.Lin CF, Lei HY, Liu CC, Liu HS, Yeh TM, Wang ST, Yang TI, Sheu FC, Kuo CF, Lin YS. 2001. Generation of IgM anti-platelet autoantibody in dengue patients. J. Med. Virol. 63:143–149. [DOI] [PubMed] [Google Scholar]
- 49.Chen MC, Lin CF, Lei HY, Lin SC, Liu HS, Yeh TM, Anderson R, Lin YS. 2009. Deletion of the C-terminal region of dengue virus nonstructural protein 1 (NS1) abolishes anti-NS1-mediated platelet dysfunction and bleeding tendency. J. Immunol. 183:1797–1803. 10.4049/jimmunol.0800672 [DOI] [PubMed] [Google Scholar]
- 50.Clark KB, Noisakran S, Onlamoon N, Hsiao HM, Roback J, Villinger F, Ansari AA, Perng GC. 2012. Multiploid CD61+ cells are the pre-dominant cell lineage infected during acute dengue virus infection in bone marrow. PLoS One 7(12):e52902. 10.1371/journal.pone.0052902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srikiatkhachorn A, Wichit S, Gibbons RV, Green S, Libraty DH, Endy TP, Ennis FA, Kalayanarooj S, Rothman AL. 2012. Dengue viral RNA levels in peripheral blood mononuclear cells are associated with disease severity and preexisting dengue immune status. PLoS One 7(12):e51335. 10.1371/journal.pone.0051335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baclig MO, Gervacio LT, Suarez LA, Buerano CC, Matias RR, Kumatori A, Inoue S, Morita K, Natividad FF, Hasebe F. 2010. Flow cytometric analysis of dengue virus-infected cells in peripheral blood. Southeast Asian J. Trop. Med. Public Health 41:1352–1358 [PubMed] [Google Scholar]
- 53.King AD, Nisalak A, Kalayanrooj S, Myint KS, Pattanapanyasat K, Nimmannitya S, Innis BL. 1999. B cells are the principal circulating mononuclear cells infected by dengue virus. Southeast Asian J. Trop. Med. Public Health 30:718–728 [PubMed] [Google Scholar]
- 54.Blackley S, Kou Z, Chen H, Quinn M, Rose RC, Schlesinger JJ, Coppage M, Jin X. 2007. Primary human splenic macrophages, but not T or B cells, are the principal target cells for dengue virus infection in vitro. J. Virol. 81:13325–13334. 10.1128/JVI.01568-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kou Z, Quinn M, Chen H, Rodrigo WW, Rose RC, Schlesinger JJ, Jin X. 2008. Monocytes, but not T or B cells, are the principal target cells for dengue virus (DV) infection among human peripheral blood mononuclear cells. J. Med. Virol. 80:134–146. 10.1002/jmv.21501 [DOI] [PubMed] [Google Scholar]
- 56.Priyadarshini D, Gadia RR, Tripathy A, Gurukumar KR, Bhagat A, Patwardhan S, Mokashi N, Vaidya D, Shah PS, Cecilia D. 2010. Clinical findings and pro-inflammatory cytokines in dengue patients in western India: a facility-based study. PLoS One 5(1):e8709. 10.1371/journal.pone.0008709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Restrepo BN, Ramirez RE, Arboleda M, Alvarez G, Ospina M, Diaz FJ. 2008. Serum levels of cytokines in two ethnic groups with dengue virus infection. Am. J. Trop. Med. Hyg. 79:673–677 [PubMed] [Google Scholar]
- 58.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. 2012. Humanized mice for immune system investigation: progress, promise and challenges. Nat. Rev. Immunol. 12:786–798. 10.1038/nri3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takeda S, Rodewald HR, Arakawa H, Bluethmann H, Shimizu T. 1996. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span. Immunity 5:217–228. 10.1016/S1074-7613(00)80317-9 [DOI] [PubMed] [Google Scholar]
- 60.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. 1997. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science 276:2057–2062. 10.1126/science.276.5321.2057 [DOI] [PubMed] [Google Scholar]
- 61.Noble CG, Chen YL, Dong H, Gu F, Lim SP, Schul W, Wang QY, Shi PY. 2010. Strategies for development of Dengue virus inhibitors. Antiviral Res. 85:450–462. 10.1016/j.antiviral.2009.12.011 [DOI] [PubMed] [Google Scholar]
- 62.National Research Council 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC [Google Scholar]