Abstract
The recent outbreak of H7N9 influenza virus infections in humans in China has raised concerns about the pandemic potential of this strain. Here, we test the efficacy of H3 stalk-based chimeric hemagglutinin universal influenza virus vaccine constructs to protect against H7N9 challenge in mice. Chimeric hemagglutinin constructs protected from viral challenge in the context of different administration routes as well as with a generic oil-in-water adjuvant similar to formulations licensed for use in humans.
TEXT
In early spring 2013, the Chinese authorities reported the first human cases of avian H7N9 influenza virus infections (1), and a total of 137 cases and 45 fatalities have been officially confirmed by the World Health Organization by 25 October 2013 (including 2 cases in October 2013) (2). Characterization of viral isolates revealed that H7N9 binds to α2.6-linked sialic acids and that it can replicate in the mammalian upper respiratory tract, indicating that it could be able to acquire sustained human-to-human transmissibility (3–5). These findings, together with the fact that neuraminidase (NA) inhibitor-resistant mutants have arisen quickly in treated patients, without apparent fitness loss (6), have generated concerns for an H7N9 pandemic.
The H7 hemagglutinin (HA) falls phylogenetically within the group 2 influenza A HAs and shares conserved epitopes in the membrane proximal stalk domain with other members of this group (H3, H4, H10, H14, and H15 HAs). This region of the HA is, however, considered to be immunologically subdominant. Antibodies against this subdominant region of the HA are generally not induced by regular, H3-containing, seasonal trivalent inactivated vaccines (TIV) and only to low titers by natural H3N2 infection (7–9). However, monoclonal antibodies recognizing epitopes in the stalk domain have been isolated from mice and humans and exhibit broadly neutralizing activity against divergent group 2 influenza virus subtypes (10–13).
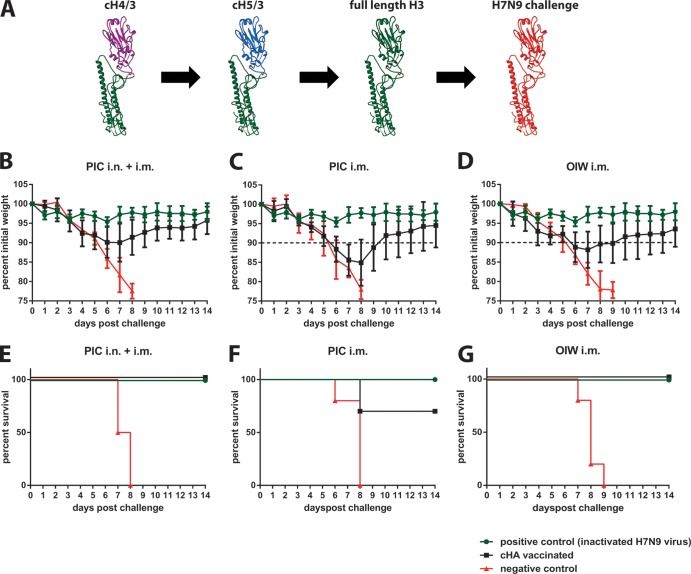
We have recently reported that a vaccination regimen based on chimeric HAs (cHA) can elevate the immunogenicity of this domain and thus focus the antibody response toward conserved epitopes in the HA. Importantly, elicited anti-HA stalk antibody titers were sufficient to protect against influenza virus challenges in both mice and ferrets (13–16) (F. Krammer, R. Hai, M. Yondola, G. S. Tan, V. Leyva-Grado, A. Ryder, J. K. Rose, P. Palese, A. Garcia-Sastre, R. A. Albrecht, submitted for publication). Importantly, protection is limited to viruses that express an HA belonging to the same HA group used for vaccination, and cross-reactivity among HAs belonging to different groups was also not observed (14, 16). Here, we aim to evaluate whether the titers of cross-reactive stalk antibodies elicited by a group 2 (H3) HA stalk-based vaccination regimen are sufficient to confer protection against the novel H7N9 virus. Furthermore, to study the importance of mucosal responses for protection, we applied an experimental setup that induces both mucosal and systemic immunity and compared it to one that would induce only systemic immunity. We also compared two adjuvants, poly(I·C) (PIC), which we successfully used in combination with cHAs in animals before, and a generic oil-in-water (OIW) adjuvant (17). The latter is similar to adjuvants that have been licensed for use in humans (17). Animals (n = 10 per group, female 6- to 8-week-old BALB/c mice) were primed with a DNA plasmid expressing a cH4/3 protein (H4 head derived from A/duck/Czech/56 on top of an H3 stalk domain derived from A/Perth/16/09) (16) via intramuscular (i.m.) electroporation with a TriGrid electroporation device (Ichor Medical Systems) (Fig. 1A). It is of note that DNA vaccination is not essential for induction of broad protection by cHA vaccination regimens. Vaccination with just proteins (no DNA) yielded results comparable to vaccination with DNA priming in earlier studies (18). Three weeks postprime, animals received a recombinant cH5/3 protein (H5 head derived from A/Vietnam/1203/04 on top of an H3 stalk derived from A/Perth/16/09) (16). Animals in one group received 5 μg of cH5/3 protein intranasally (i.n.) and 5 μg i.m. adjuvanted with 5 μg of PIC (high molecular weight; Invivogen) (PIC i.n. and i.m.). A second group received only the i.m. dose with PIC (5 μg HA in total) (PIC i.m.), while a third group received 5 μg of cH5/3 protein with a generic OIW-based adjuvant (OIW i.m.) (Fig. 1A). All mice were boosted a second time 3 weeks later with full-length H3 protein using the same vaccination routes, adjuvants, and immunogen amounts (Fig. 1A). All recombinant proteins used for vaccination were expressed in the baculovirus expression system with a C-terminal T4 foldon trimerization domain and a hexahistidine tag to facilitate purification (19). The OIW adjuvant (20 mM citrate, 0.5% polysorbate 80 [pH 6.5], 0.5% span-85 [sorbitan trioleate], 4.3% squalene) was prepared as described before (17) (Fig. 2). Positive controls (n = 5) received one i.m. vaccination of formalin-inactivated A/Shanghai/1/13 H7N9 (SH1; 6:2 reassortant with HA and NA derived from A/Shanghai/1/13 and the internal genes from A/Puerto Rico/8/34) whole virus preparation. Negative controls (n = 4 or 5) received a mock DNA vaccination followed by two boosts with bovine serum albumin (BSA) administered in the same amounts and route as the respective cHA vaccination regimens they were compared to. Four weeks after the last immunization, animals were bled and then challenged with 10 murine 50% lethal doses (mLD50) of SH1 virus. Weight loss was monitored over a period of 14 days, and mice that lost more than 20% of their initial body weight were euthanized. Animals in the PIC i.n. and i.m. group lost an average of 10% of their initial body weight (Fig. 1B) compared to 15% in the PIC i.m. group (Fig. 1C), suggesting that the mucosal immunity at the site of infection has a significant role in protection. Furthermore, the mice in the OIW i.m. group lost only 12% of their initial body weight on average (Fig. 1D), which is also reflected in the observed survival. All PIC i.m. and i.n. vaccinated animals survived the challenge (Fig. 1E), compared to only 70% of the PIC i.m. animals (Fig. 1F). However, survival in the OIW i.m. group was 100% (Fig. 1F), indicating a better protective effect with the OIW adjuvant. To quantitatively assess the titers of H7 HA antibodies elicited by the three different vaccination regimens, we performed enzyme-linked immunosorbent assays (ELISA) using a recombinant SH1 H7 HA as the substrate. Since the cHA-vaccinated animals were not exposed to an H7 head domain, we concluded that any observed reactivity to H7 would be predominantly derived from cross-reactive antistalk antibodies. To exclude binding to the hexahistidine tag or the trimerization domain, we used a recombinant SH1 H7 HA expressed with a GCN pII leucine zipper trimerization domain and a Strep-tag II (14, 20). The highest endpoint titers were detected in serum collected from PIC i.n. and i.m. vaccinated animals, followed by the OIW i.m. animals (Fig. 3A). Mice that received the PIC i.m. vaccine had statistically significant lower titers (P = 0.03) than the OIW i.m. animals. This difference in endpoint titers correlates well with the differences in weight loss and survival. The antibody isotype distribution can be strongly influenced by adjuvants and can have a high impact on the protective efficiency of antibodies. However, we did not detect significant differences in the isotype profile when we compared the three vaccination regimens (Fig. 3B). This suggest that, in the case of i.m. only vaccination, the antibody titer was the main correlate of protection. Sera from the vaccine and control groups were also tested in a microneutralization assay, but results for the cHA-vaccinated groups were negative, probably due to the limit of detection (1:20) of this assay (data not shown). Since we observed that animals immunized via both routes showed lower morbidity than animals that were vaccinated only intramuscularly, we can speculate that mucosal IgA antibodies induced by the i.n. vaccination had a substantial contribution to protection. Differences in weight loss between the PIC i.n. and i.m. group and the PIC i.m. group were statistically significant on day 7 (P = 0.0383) and day 8 (P = 0.0136) (unpaired t test). However, it should be noted that these animals also received twice as much antigen as the i.m.-only animals. In conclusion, we show that mice can be protected against the novel H7N9 virus using a stalk-based immunization regimen with cHA constructs that do not possess an H7 head domain. Furthermore, an oil-in-water-based adjuvant similar to those licensed for use in humans (17, 21) performed well with the cHA vaccine candidates, suggesting that this combination could also be considered for testing in humans. An HA stalk-based vaccine providing universal influenza virus protection could replace strain-specific seasonal vaccines and further enhance our preparedness against potential pandemic influenza virus strains like H7N9.
FIG 1.
Vaccination with H3 stalk-based cHA protects mice from challenge with H7N9 virus. (A) Schematic representation of the vaccination regimen. Structures are based on protein database structure number 1RU7. Mice in panels B to G received a DNA prime coding for cH4/3 protein (H4 head in combination with an H3 stalk) and were then boosted with cH5/3 (H5 head on top of an H3 stalk) and full-length H3 protein. The animals were challenged 4 weeks after the last immunization with H7N9 virus. Weight loss curves are shown in panels B to D, and survival curves are shown in panels E to G. The dashed lines in panels C and D indicate the highest average weight loss (day 7) of the PIC i.n. and i.m. group shown in panel B. Mice shown in panels B and E (PIC i.n. and i.m.) were boosted sequentially with cH4/3 and cH5/3 protein intranasally and intramuscularly in the presence of poly(I·C) as the adjuvant. Animals in panels C and F received the same proteins as boosts but received the immunizations only intramuscularly (PIC i.m.). Mice in panels D and G were immunized intramuscularly only with an oil-in-water adjuvant (OIW i.m.). Animals in the control groups received BSA with the respective adjuvant and via the respective routes. Positive control animals (same group was compared to all three experimental conditions) were vaccinated intramuscularly with inactivated matched challenge virus.
FIG 2.
Oil-in-water (OIW) adjuvant preparation and characteristics. (A) Scheme of the OIW adjuvant preparation. The OIW emulsion is prepared at a 20- to 40-ml scale. Span-85 is dissolved in squalene, and polysorbate 80 is dissolved in deionized water and then mixed with citrate-citric acid buffer. Oil and aqueous phase fractions are combined and mixed into a coarse emulsion by vortexing and is then passed through an LV1 Microfluidizer (Microfluidics, Westwood, MA) at 12,000 lb/in2. The eluent is collected and repassaged for a total of 5 passes through the Microfluidizer to yield a stable and homogeneous emulsion. The final pass is filtered through a 0.22-μm-pore-size filter and filled into sterile glass vials. (B) Particle size of the OIW adjuvant prepared by microfluidization was determined by dynamic light scattering on a Malvern Nano 3ZS. The sizes shown are the Z-average means from 2 independently prepared batches. These preparations are considered monomodal, as polydispersity after the final passage through the Microfluidizer and sterile filtration ranged from 8 to 11%.
FIG 3.
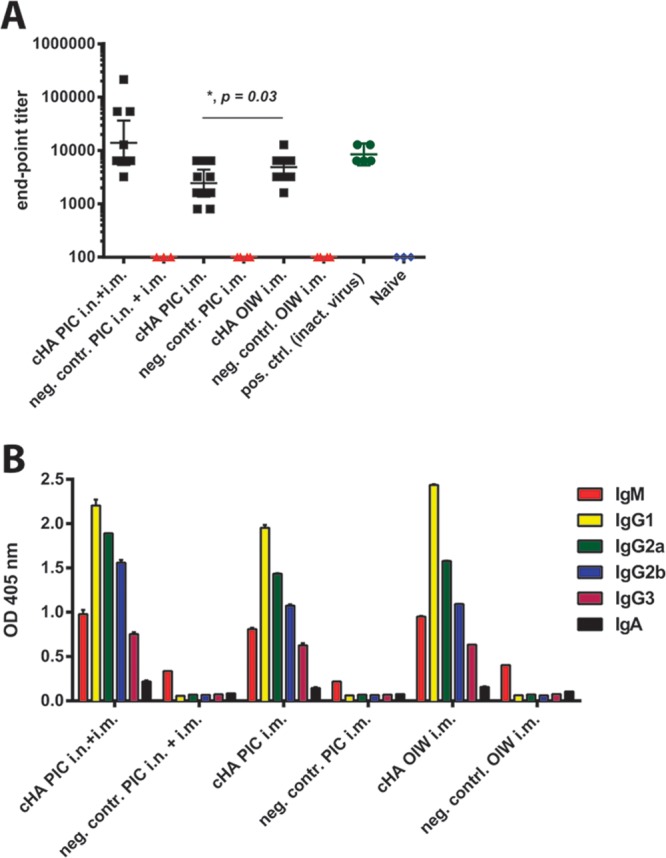
Vaccination with H3 stalk-based cHAs induces high antibody titers against H7 hemagglutinin in mice. (A) IgG endpoint titers of cHA-vaccinated mice against H7 (A/Shanghai/1/13) HA. The one-tailed P value was calculated using an unpaired t test. (B) Immunoglobulin isotype distribution directed to H7 HA in sera from mice vaccinated via different routes and with different adjuvants. The analysis was performed at a 1:200 dilution.
ACKNOWLEDGMENTS
We thank Chen Wang for excellent technical support.
Florian Krammer was supported by an Erwin Schrödinger fellowship (J3232) from the Austrian Science Fund (FWF). This work was partially supported by a Centers for Excellence for Influenza Research and Surveillance (CEIRS) grant (HHSN26620070010C to P.P.), the NIH program project grant 1P01AI097092-01A1 (to P.P.), and HHSN272200900032C (Innate Immune Receptors and Adjuvant Discovery; to P.P.).
Footnotes
Published ahead of print 4 December 2013
REFERENCES
- 1.Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y. 2013. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 368:1888–1897. 10.1056/NEJMoa1304459 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization 2013. Number of confirmed human cases of avian influenza A(H7N9) reported to WHO. WHO, Geneva, Switzerland: http://www.who.int/influenza/human_animal_interface/influenza_h7n9/Data_Reports/en/ [Google Scholar]
- 3.Ramos I, Krammer F, Hai R, Aguilera D, Bernal-Rubio D, Steel J, García-Sastre A, Fernandez-Sesma A. 2013. H7N9 influenza viruses interact preferentially with α2,3-linked sialic acids and bind weakly to α2,6-linked sialic acids. J. Gen. Virol. 94:2417–2423. 10.1099/vir.0.056184-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe T, Kiso M, Fukuyama S, Nakajima N, Imai M, Yamada S, Murakami S, Yamayoshi S, Iwatsuki-Horimoto K, Sakoda Y, Takashita E, McBride R, Noda T, Hatta M, Imai H, Zhao D, Kishida N, Shirakura M, de Vries RP, Shichinohe S, Okamatsu M, Tamura T, Tomita Y, Fujimoto N, Goto K, Katsura H, Kawakami E, Ishikawa I, Watanabe S, Ito M, Sakai-Tagawa Y, Sugita Y, Uraki R, Yamaji R, Eisfeld AJ, Zhong G, Fan S, Ping J, Maher EA, Hanson A, Uchida Y, Saito T, Ozawa M, Neumann G, Kida H, Odagiri T, Paulson JC, Hasegawa H, Tashiro M, Kawaoka Y. 2013. Characterization of H7N9 influenza A viruses isolated from humans. Nature 501:551–555. 10.1038/nature12392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiong X, Martin SR, Haire LF, Wharton SA, Daniels RS, Bennett MS, McCauley JW, Collins PJ, Walker PA, Skehel JJ, Gamblin SJ. 2013. Receptor binding by an H7N9 influenza virus from humans. Nature 499:496–499. 10.1038/nature12372 [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Lu S, Song Z, Wang W, Hao P, Li J, Zhang X, Yen HL, Shi B, Li T, Guan W, Xu L, Liu Y, Wang S, Tian D, Zhu Z, He J, Huang K, Chen H, Zheng L, Li X, Ping J, Kang B, Xi X, Zha L, Li Y, Zhang Z, Peiris M, Yuan Z. 2013. Association between adverse clinical outcome in human disease caused by novel influenza A H7N9 virus and sustained viral shedding and emergence of antiviral resistance. Lancet 381:2273–2279. 10.1016/S0140-6736(13)61125-3 [DOI] [PubMed] [Google Scholar]
- 7.Moody MA, Zhang R, Walter EB, Woods CW, Ginsburg GS, McClain MT, Denny TN, Chen X, Munshaw S, Marshall DJ, Whitesides JF, Drinker MS, Amos JD, Gurley TC, Eudailey JA, Foulger A, DeRosa KR, Parks R, Meyerhoff RR, Yu JS, Kozink DM, Barefoot BE, Ramsburg EA, Khurana S, Golding H, Vandergrift NA, Alam SM, Tomaras GD, Kepler TB, Kelsoe G, Liao HX, Haynes BF. 2011. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS One 6:e25797. 10.1371/journal.pone.0025797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671. 10.1038/nature06890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margine I, Hai R, Albrecht RA, Obermoser G, Harrod AC, Banchereau J, Palucka K, García-Sastre A, Palese P, Treanor JJ, Krammer F. 2013. H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J. Virol. 87:4728–4737. 10.1128/JVI.03509-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang TT, Tan GS, Hai R, Pica N, Petersen E, Moran TM, Palese P. 2010. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog. 6:e1000796. 10.1371/journal.ppat.1000796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJ, Brandenburg B, Vogels R, Brakenhoff JP, Kompier R, Koldijk MH, Cornelissen LA, Poon LL, Peiris M, Koudstaal W, Wilson IA, Goudsmit J. 2011. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 333:843–850. 10.1126/science.1204839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, Silacci C, Fernandez-Rodriguez BM, Agatic G, Bianchi S, Giacchetto-Sasselli I, Calder L, Sallusto F, Collins P, Haire LF, Temperton N, Langedijk JP, Skehel JJ, Lanzavecchia A. 2011. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333:850–856. 10.1126/science.1205669 [DOI] [PubMed] [Google Scholar]
- 13.Krammer F, Palese P. 2013. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr. Opin. Virol. 3:521–530. 10.1016/j.coviro.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krammer F, Pica N, Hai R, Margine I, Palese P. 2013. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol. 87:6542–6550. 10.1128/JVI.00641-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, Banach D, Wrammert J, Belshe RB, García-Sastre A, Palese P. 2012. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc. Natl. Acad. Sci. U. S. A. 109:2573–2578. 10.1073/pnas.1200039109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margine I, Krammer F, Hai R, Heaton NS, Tan GS, Andrews SA, Runstadler JA, Wilson PC, Albrecht RA, García-Sastre A, Palese P. 2013. Hemagglutinin stalk-based universal vaccine constructs protect against group 2 influenza A viruses. J. Virol. 87:10435–10446. 10.1128/JVI.01715-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ott G, Radhakrishnan R, Fang J-H, Hora M. 2000. The adjuvant MF59: a 10-year perspective, p 211–228 In O'Hagan DT. (ed), Vaccine adjuvants, vol 42 Springer, New York, NY [Google Scholar]
- 18.Goff PH, Eggink D, Seibert CW, Hai R, Martínez-Gil L, Krammer F, Palese P. 2013. Adjuvants and immunization strategies to induce influenza virus hemagglutinin stalk antibodies. PLoS One 8:e79194. 10.1371/journal.pone.0079194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krammer F, Margine I, Tan GS, Pica N, Krause JC, Palese P. 2012. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS One 7:e43603. 10.1371/journal.pone.0043603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weldon WC, Wang BZ, Martin MP, Koutsonanos DG, Skountzou I, Compans RW. 2010. Enhanced immunogenicity of stabilized trimeric soluble influenza hemagglutinin. PLoS One 5:e12466. 10.1371/journal.pone.0012466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Hagan DT, Ott GS, Nest GV, Rappuoli R, Giudice GD. 2013. The history of MF59(®) adjuvant: a phoenix that arose from the ashes. Expert Rev. Vaccines 12:13–30. 10.1586/erv.12.140 [DOI] [PubMed] [Google Scholar]