Abstract
Measles virus (MV) immunosuppression is due to infection of SLAM-positive immune cells, whereas respiratory shedding and virus transmission are due to infection of nectin4-positive airway epithelial cells. The vaccine lineage MV strain Edmonston (MV-Edm) acquired an additional tropism for CD46 which is the basis of its oncolytic specificity. VSVFH is a vesicular stomatitis virus (VSV) encoding the MV-Edm F and H entry proteins in place of G. The virus spreads faster than MV-Edm and is highly fusogenic and a potent oncolytic. To determine whether ablating nectin4 tropism from VSVFH might prevent shedding, increasing its safety profile as an oncolytic, or might have any effect on CD46 binding, we generated VSVFH viruses with H mutations that disrupt attachment to SLAM and/or nectin4. Disruption of nectin4 binding reduced release of VSVFH from the basolateral side of differentiated airway epithelia composed of Calu-3 cells. However, because nectin4 and CD46 have substantially overlapping receptor binding surfaces on H, disruption of nectin4 binding compromised CD46 binding and greatly diminished the oncolytic potency of these viruses on human cancer cells. Thus, our results support continued preclinical development of VSVFH without ablation of nectin4 binding.
INTRODUCTION
The live attenuated Edmonston (Edm) strain of measles virus (MV) induces extensive cytopathic effects (CPE) of intercellular fusion (syncytia) in infected cells and has promising antitumor activity against numerous cancer types (1). MV-NIS has been engineered to express the human sodium iodide symporter (NIS) gene to enable noninvasive, real-time monitoring of the pharmacokinetics of viral replication using I-123 and single photon emission computed tomography/computed tomography (SPECT/CT) imaging, in which two different types of scans are taken and the images or pictures from each are fused or merged together (2, 3). The virus is being evaluated in several phase I clinical trials in patients with ovarian cancer (intraperitoneal administration), multiple myeloma (intravenous), glioblastoma (intracavity and intratumor), squamous cell carcinoma of the head and neck (intratumor), and mesothelioma (intrapleural) (2, 4–6). In a recently completed phase I trial of patients with recurrent ovarian cancer, the best objective response using a closely related MV (MV-CEA) was dose-dependent disease stabilization in 14 of 21 patients with a median duration of 92.5 days (range, 54 to 277 days). Five patients had significant decreases in their ovarian cancer marker CA-125 levels. The median survival time of patients in the study was 12.15 months (range, 1.3 to 38.4 months), comparing favorably to an expected median survival of 6 months in this patient population. However, the level of viral replication and gene expression was modest (4).
To improve the oncolytic potency of MV, we engineered a hybrid MV/vesicular stomatitis virus (VSV) to incorporate the replication machinery of VSV (7). The replication of MV is relatively slow, and the cytopathic effects of syncytium formation are not typically evident in an infected cell culture until 30 to 36 h postinfection at low multiplicities of infection (MOIs). In contrast, VSV has fast replication kinetics and large burst size and yields significantly higher titers in infected cells than MV (7). The glycoprotein (G) of VSV was deleted from the full-length infectious clone of VSV and replaced with two additional transcriptional units encoding MV F and H, yielding VSVFH (7). Transmission electron microscope pictures show that VSVFH has a structure very similar to that of VSV. In addition to rapid replication, the hybrid virus is highly fusogenic and has a restricted tropism compared to that of VSV, using only CD46, SLAM (signaling lymphocyte activation molecule), and nectin4/PVRL4 (poliovirus receptor-related 4) as receptors for infection and spread. When tested in vivo by intratumoral or intravenous administration in immunocompromised mice with local or disseminated human myeloma, the virus was not toxic and exclusively infected human myeloma cells, inducing rapid regression of the local plasmacytomas and extended survival of mice (7).
Measles virus immunosuppression is due to infection of SLAM-positive immune cells, whereas respiratory shedding and virus transmission are due to infection of nectin4-positive airway epithelial cells. To fine-tune VSVFH as an oncolytic agent for cancer therapy, we hypothesized that engineering VSVFH to bind exclusively to CD46 and eliminating SLAM and nectin4 interactions should make it more specific as an anticancer therapeutic. While wild-type MV uses SLAM (found on immune cells) and nectin4 (on epithelial cells) as receptors, the Edm strain that has been attenuated through passage in tissue culture has adapted to also use CD46 as a receptor (8–10). The adaptation involves a mutation at residue 481 from asparagine (N) to tyrosine (Y) in the MV hemagglutinin (H) attachment protein (11). The amino acid residues with which H interacts with SLAM and nectin4 are also identified (10, 12). Importantly, transient immunosuppression induced by MV is attributed to H interaction with SLAM, while H binding to nectin4 is responsible for shedding of wild-type MV into the respiratory tract (13–16). In this study, we performed site-directed mutagenesis on H to specifically ablate SLAM, nectin4, and/or CD46 binding sites. The recombinant VSVFH viruses bearing mutant H proteins were rescued and their receptor usage, oncolytic potency, and shedding in a polarized epithelial layer (Calu cells) were evaluated in vitro.
MATERIALS AND METHODS
Cell lines.
The African green monkey kidney cell line Vero and Vero expressing human SLAM receptor (Vero-hSLAM) were cultured in Dulbecco's modified Eagle's medium (DMEM; Mediatech, Herndon, VA) supplemented with 5% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO). SW579 human thyroid squamous cell carcinoma (ATCC HTB-107), SW480 human colorectal adenocarcinoma (ATCC CCL-228), SKBr3 human mammary carcinoma (ATCC HTB-30), LNCaP human prostate carcinoma (ATCC CLR-1740), COLO 205 human colorectal adenocarcinoma (ATCC CCL-222), Calu-3 human pulmonary adenocarcinoma (ATCC HTB-55), and BHK (baby hamster kidney; ATCC CCL-10) cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). SW579, SW480, SKBr3, Calu-3, and BHK cells were maintained in DMEM supplemented with 10% FBS. LNCaP and COLO 205 cells were cultured in RPMI medium (Mediatech) supplemented with 10% FBS. CHOpgsA754 Chinese hamster ovary (CHO) cells, kindly provided by Chris Richardson of Dalhousie University, were maintained in DMEM–nutrient mixture F-12 (Invitrogen, Carlsbad, CA) supplemented with 10% FBS. CHO cells expressing human CD46 receptor (CHO-CD46) were maintained in DMEM supplemented with 10% FBS and 5 mg/ml of G418 (17). CHO cells expressing human SLAM receptor (CHO-SLAM) were maintained in RPMI medium supplemented with 10% FBS, 0.5% sodium bicarbonate, and 5 mg/ml of G418 (17).
CHO cells expressing human nectin4.
To obtain nectin4 cDNA, total RNA was extracted from SKBr3 cells using the RNeasy Plus Universal minikit (Qiagen, Frederick, MD). Oligo(dT), provided with the SuperScript III First-Strand synthesis system (Invitrogen, Carlsbad, CA), was used to produce the cDNA. Nectin4-specific primers were used to amplify nectin4 double-stranded DNA (dsDNA) from the cDNA (forward, 5′-ATGCCCCTGTCCCTGGGAGCCGAGA-3′, and reverse, 5′-ATTATTGCGGCCGCTTAGACCAGGTG-3′). The nectin4 dsDNA was first ligated into a TOPO plasmid from a ZeroBlunt TOPO PCR cloning kit (Invitrogen). Another set of primers (forward, 5′-AAAAAAGGATCCATGCCCCTGTCCCT-3′, and reverse, 5′-ATTATTGCGGCCGCTTAGACCAGGTG-3′) was used to introduce BamHI and NotI restriction sites at the 5′ and 3′ ends of nectin4 cDNA, respectively. The PCR product was subcloned into a lentiviral vector plasmid, pHR-SIN-CSGW dlNotI, using BamHI and NotI restriction enzymes to create pHR-Nectin4-SIN. Lentiviral vectors encoding nectin4 were produced by transient cotransfection of 293T cells with pHR′CMV-Nectin4-SIN, a human immunodeficiency virus type 1-derived packaging plasmid (p8.91), and a plasmid encoding the VSV glycoprotein (pMD.G), as described previously (18), and were used to transduce CHO cells. Nectin4 expression was confirmed by flow cytometry using mouse anti-human nectin4 antibody conjugated with phycoerythrin (PE) (Fab2659P; R&D, Minneapolis, MN).
VSVFH constructs and virus rescue.
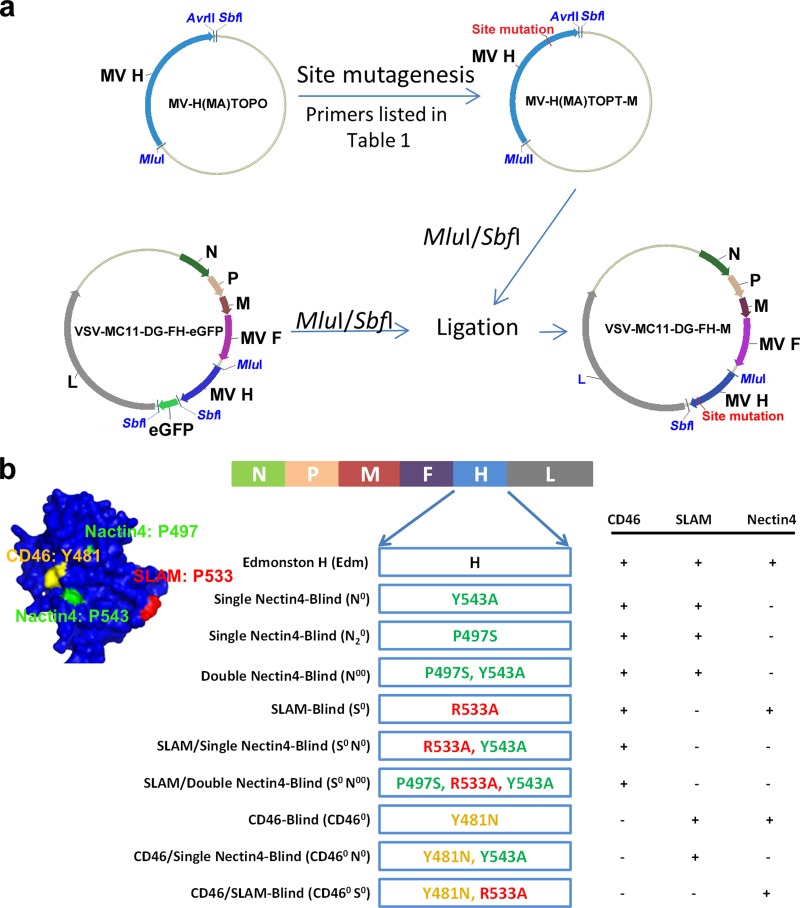
The schema for cloning the full-length infectious clone of VSVFH with specific mutations in the receptor binding sites is shown in Fig. 1a. A plasmid expressing MV H protein (pCGH) (17) and TOPO plasmid from the ZeroBlunt TOPO PCR cloning kit (Invitrogen, Carlsbad, CA) carrying MV H genome [pMV-H(MA)TOPO (K.-H. Peng and C. Ayala-Breton, unpublished data] were used as templates for PCR mutagenesis. PCR mutagenesis was performed using the QuikChange II XL site-directed mutagenesis kit (Agilent Technologies) with 1 μl of the previous template (as outlined in Table 1) and 100 ng of the appropriate primers (Table 1). PCR conditions were as follows: 95°C for 1 min; 95°C for 50 s, 60°C for 50 s, and 68°C for 5.5 min for 18 cycles; and 68°C for 7 min. The PCR product was then digested with DpnI and transformed onto XL10 Gold Ultracompetent Escherichia coli (Agilent). All pCGH and TOPO vector products were sequenced to confirm the mutation. The TOPO vector products were subcloned into the full-length plasmid (pVSV-DG-FH-eGFP [Peng and Ayala-Breton, unpublished]) using MluI and SbfI enzymes, replacing the “H-eGFP” part of the full-length plasmid with different H mutants. To rescue the VSVFH hybrid viruses, the full-length plasmids were transfected along with pMD.G by following the VSV rescue system previously described (19).
FIG 1.
Schematic representation of the cloning strategy and the mutation constructs used in this study. (a) Strategy used for cloning the full-length infectious clone of VSVFH with specific mutations in the receptor binding sites. TOPO plasmid carrying MV H genome [pMV-H(MA)TOPO] was used as a first template for PCR mutagenesis. Primers and templates used for all the mutants are listed in Table 1. The PCR mutagenesis products were subcloned into the full-length plasmid (pVSVDG-FH-GFP) with MluI and SbfI enzymes replacing “H-eGFP” with “H mutants.” (b) A total of nine different VSVFH constructs displaying H proteins ablated for binding to specific receptors were made by mutagenesis of the parental Edm H. The left side shows the structure of H protein and the location of each receptor binding site. The right side represents the predicted receptor binding ability of each construct.
TABLE 1.
Primers and templates used for site-directed mutagenesis
| Mutant | Template | Primera |
|---|---|---|
| MV-H(MA)-N0 | MV-H(MA)TOPO | 5′-GAACATGCTGTGGTTTATTACGTTGCAAGCCCAAGCCGCTC-3′ |
| MV-H(MA)-N20 | MV-H(MA)TOPO | 5′-GGCGAAGACTGCCATGCCAGTACATACCTACCTGCGG-3′ |
| MV-H(MA)-N00 | MV-H(MA)-N0 | 5′-GGCGAAGACTGCCATGCCAGTACATACCTACCTGCGG-3′ |
| MV-H(MA)-S0 | MV-H(MA)TOPO | 5′-GGCAACCTACGATACTTCCGCTGTTGAACATGCTGTGG-3′ |
| MV-H(MA)-S0N0 | MV-H(MA)-N0 | 5′-GGCAACCTACGATACTTCC>GCTGTTGAACATGCTGTGG-3′ |
| MV-H(MA)-S0N00 | MV-H(MA)-S0N0 | 5′-GGCGAAGACTGCCATGCCAGTACATACCTACCTGCGG-3′ |
| MV-H(MA)-CD460 | MV-H(MA)TOPO | 5′-AGATTCAAGGTTAGTCCCAACCTCTTCAATGTCCCAATT-3′ |
| MV-H(MA)-CD460N0 | MV-H(MA)-CD460 | 5′-GAACATGCTGTGGTTTATTACGTTGCAAGCCCAAGCCGCTC-3′ |
| MV-H(MA)-CD460S0 | MV-H(MA)-CD460 | 5′-GGCAACCTACGATACTTCCGCTGTTGAACATGCTGTGG-3′ |
Primers shown here are forward primers. The reverse primers used for site-directed mutagenesis are complemented to the forward primers. The mutation sites are shown in bold.
Immunoblotting for viral proteins.
Protein lysates fractionated by 10% acrylamide SDS-PAGE were transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). Membranes were blocked with 5% nonfat milk in Tris-buffered saline (TBS)–Tween for 1 h at room temperature, followed by incubation with primary antibodies, polyclonal rabbit anti-MV-H, polyclonal rabbit anti-MV-F, rabbit anti-human MX1/2/3 (SC-34128; Santa Cruz, Dallas, TX), or polyclonal rabbit anti-VSV proteins (20, 21). After five washes in TBS-Tween, membranes were incubated with the appropriate peroxidase-conjugated secondary antibodies. The signal was developed using a Pierce ECL Western blotting substrate kit (Thermo Scientific, Waltham, MA) according to the manufacturer's instructions.
CHO cell transfection and infection.
CHO, CHO-CD46, CHO-SLAM, CHO-nectin4, and Vero cells were seeded into 12-well culture plates at a density of 105 cells/well. Twenty-four hours later, cells were either transfected with 1.5 μg of plasmid expressing the fusion protein of MV (pCGF) and 1.5 μg of pCGH or pCGH vector products using the SuperFect transfection reagent (Qiagen, Frederick, MD) or infected with the viruses at a multiplicity of infection (MOI) of 1.0. Twenty-four hours to 48 h after transfection or infection, cells were fixed with 4% paraformaldehyde (Santa Cruz, Dallas, TX), washed with phosphate-buffered saline (PBS), and then stained with 1% crystal violet. Images were captured with Nikon Digital Sight DS-2Mv digital camera with NIS Elements software on a Nikon ECLIPSE TS100 microscope (Nikon, Tokyo, Japan).
Infection assays on human cancer cells.
For the infection assays, cancer cells (7,000 cells/well in a 96-well plate) were exposed to VSVFH at the MOIs indicated below. Antibody blocking assays were performed by adding antibodies against CD46 (mouse monoclonal; Santa Cruz, Dallas, TX) and/or nectin4 (goat monoclonal; R&D, Minneapolis, MN) 30 min before virus. Cell viability was assessed at 48 h postinfection using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell proliferation assay according to the manufacturer's instructions (Promega, Madison, WI).
Infection of human airway epithelial cells.
Calu-3 cells were used at passage number 5 to 19 after receipt from the ATCC (HTB-55). The cells were seeded onto collagen-coated (0.29 μg/ml, 200 μl/insert), semipermeable membranes with a 0.4-μm pore size (Millicell-HA; surface area, 0.6 cm2; Millipore Corporation) in 12-well Transwell filters at 1.5 × 105 cells/filter. Calu-3 cells were grown in a liquid cover culture (LCC) so the culture medium was replaced in both the apical and basolateral chambers every other day. The studies were performed on polarized layers of Calu-3 cells between 14 and 18 days of growth. Polarization of cells was measured by adding sodium fluorescein (0.5 mg/ml) to the apical compartment and nonfluorescent buffer to the basolateral compartment of cells. After 1 h of incubation, the amount of sodium fluorescein that diffused into the basolateral compartment was determined by spectrophotometric analysis and compared to the sodium fluorescein standard curve (22). Viruses (MOI = 1) were diluted in Opti-MEM, and 100 μl of the solution was applied to either the apical or basolateral surface of airway epithelial cells. To infect through the basolateral surface, the Transwell filters containing polarized Calu-3 cells were turned over and the viruses were applied to the basolateral surface. In some assays, antibodies against CD46 (sc-52647; Santa Cruz, Dallas, TX) and/or nectin4 (AF2659; R&D, Minneapolis, MN) were applied onto the surface 30 min before virus incubation. After incubation for 2 h at 37°C, unbound viruses were removed by washes with PBS, and the filters were turned upright and further incubated at 37°C. Supernatant were obtained from both apical and basolateral chambers at the time points indicated below and replaced with fresh growth medium, and the amount of infectious virus was determined by 50% tissue culture infective dose (TCID50) assays on Vero-SLAM cells.
RESULTS
Characterization of nectin4-, SLAM-, or CD46-ablated hemagglutinin protein.
A total of nine different pCGH vector products or VSVFH viruses displaying H proteins ablated for binding to specific receptors were generated by mutagenesis of the parental Edmonston (Edm) H (Fig. 1). Single or dual mutations at residues 543 and/or 497 are reported to ablate nectin4 binding (16). Thus, we generated 3 different nectin4-blind H constructs, where residue 543 was changed from Y to A (N0 [Y543A]), residue 497 was changed from P to S (N20 [P497S]), or both residues were mutated (N00 [Y543A and P497S]). The SLAM-blind construct has a mutation at residue 533 (S0 [R533A]) (16); SLAM and nectin4 dually blind constructs were also generated by incorporating the mutations in the same H (N0S0 [R533A and Y543A] or N00S0 [R533A, Y543A, and P497S]). The following constructs were generated by mutation of residue 481: CD46 blind (CD460 [Y481N]) (12), CD46 and nectin4 blind (CD460N0 [Y481N] and [Y543A]), and CD46 and SLAM blind (CD460S0 [Y481N and R533A]).
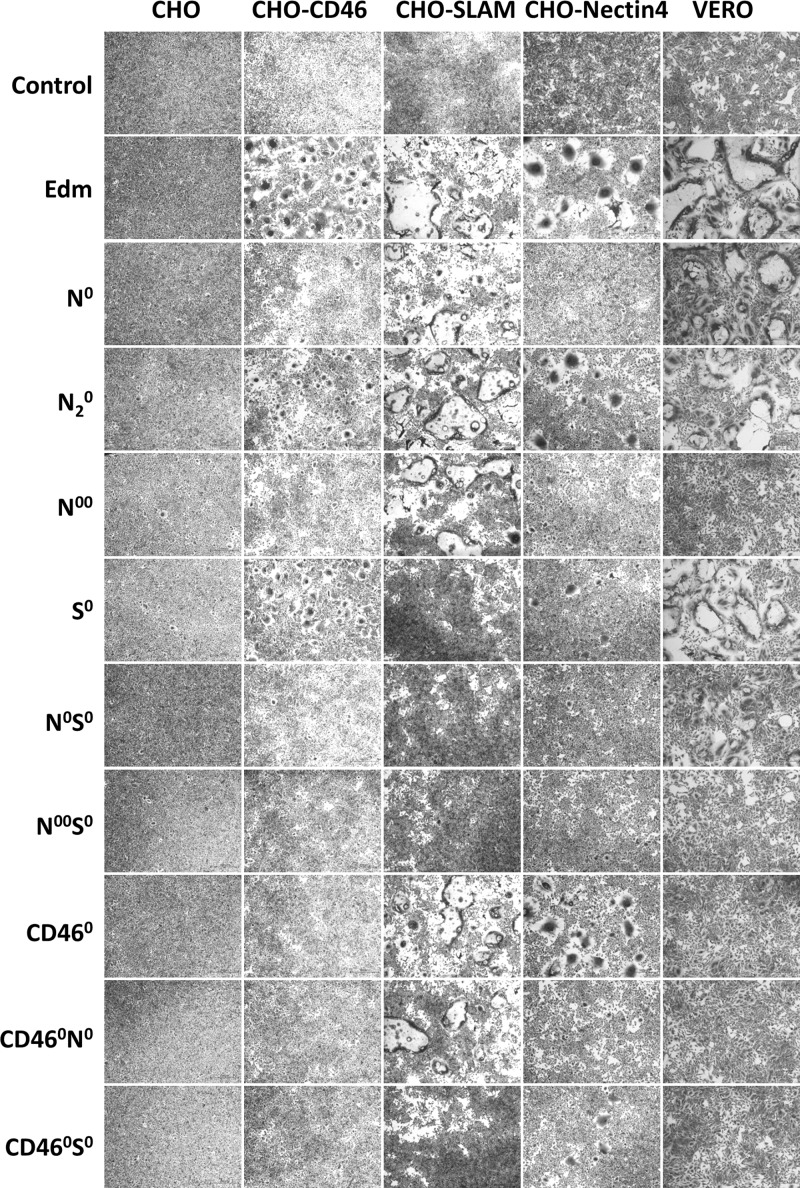
To first evaluate the expression of these H mutants on the cellular surface and their ability to induce intercellular fusion with F, the pCGH vector products were cotransfected with an F-expressing plasmid into Vero cells or CHO cells expressing different MV receptors, human CD46, human SLAM, or human nectin4 (Fig. 2). The fusogenic activities of the mutants were quite consistent with what we predicted (Fig. 1b, right side) in most cases (Fig. 2). The vector products with the CD46 binding site ablated (pCGH-CD460, CD460N0, and CD460S0) lost their fusogenic activity on CHO-CD46 cells. Also, on CHO-SLAM cells, no cell-cell fusion was observed when cells were transfected with vector products with SLAM receptors binding site ablated (pCGH-S0, N0S0, N00S0, and CD460S0). However, surprisingly, not only did the constructs with the nectin4 binding site ablated, specifically Y543A (pCGH-N0 and N0S0), lose their fusogenic activity on CHO-nectin4 cells, but also no cell-cell fusion was observed on CHO-CD46 cells. When both nectin4 binding sites were ablated (Y543A and P497S), no cell-cell fusion was observed, even on Vero cells. These results suggest that mutations at nectin4, SLAM, and CD46 binding sites all effectively knock out receptor binding, but nectin4 ablation also interferes with CD46 binding.
FIG 2.
Fusogenic activity of different pCGH vector products with F protein. Different pCGH vector products were cotransfected with an F-expressing plasmid into Vero cells or CHO cells expressing different MV receptors, human CD46, human SLAM, or human nectin4. Forty-eight hours later, cells were washed, fixed, and stained with crystal violet.
Characterization of nectin4-, SLAM-, or CD46-ablated hybrid viruses.
The TOPO vector products with different mutation constructs were subcloned into the full-length infectious clone of VSVFH-GFP to yield replication-competent viruses incorporating MV F and H proteins, whose cellular infection is dependent on the MV coat proteins. Most of the VSVFH mutants were successfully rescued using either African green monkey kidney Vero cells (expressing CD46) or Vero cells transfected to express human SLAM receptor (Vero-hSLAM). The only viruses that could not be rescued on these cells were N00S0 (SLAM and double nectin4 blind) and CD460S0 (CD46 and SLAM blind). These two mutants are not able to bind CD46 or SLAM, as seen in Fig. 2, and thus cannot be propagated on Vero or Vero-hSLAM cells. These viruses were not pursued further in this study.
Immunoblotting was performed on a supernatant containing released virions from infected Vero-SLAM producer cells (MOI, 0.01, 24 h postinfection). All the viruses incorporated MV H and F proteins in the virions, as well as VSV nucleocapsid (N) protein, phosphoprotein (P), and matrix (M) proteins (Fig. 3a). Viral titers for the mutants were slightly lower than for the parental virus (VSVFH-Edm) but were not significantly different. There was more released virus in the supernatant (about 1 log higher) than in the infected cell lysates (Fig. 3b). We next evaluated receptor usage of the viruses on CHO, CHO-CD46, CHO-SLAM, and CHO-nectin4 cells as described above (Fig. 3c). CHO cells cannot be infected by any of the VSVFH viruses because they do not express receptors for MV-H binding (Fig. 3c). CD46-blind viruses (VSVFH-CD460 and CD460N0) lost their fusogenic activity on CHO-CD46 cells. Similarly, no cell-cell fusion was observed in CHO-SLAM cells after infection by SLAM-blind viruses (VSVFH-S0 and N0S0). Nectin4-blind viruses, specifically the viruses with the Y543A mutation (VSVFH-N0 and N0S0), were not able to interact with nectin4 receptor on CHO-nectin4 cells, and no syncytium formation was observed. Similar to what we showed in the pCGH vector products (Fig. 2), these VSVFH viruses with the Y543A mutation were also compromised in their ability to use and bind CD46 (Fig. 3c). These viruses could not induce syncytium formation in CHO-CD46 cells but were found to induce intercellular fusion in Vero cells, probably due to Vero cells being their propagation cell lines: viruses could replicate better in Vero cells. Cell-cell fusion was observed on CHO-CD46 and CHO-nectin4 cells infected with virus with the P497S mutation (VSVFH-N20), but the size and numbers of the syncytia were significantly reduced. When both Y543A and P497S were ablated (VSVFH-N00), no syncytium formation was seen, even on Vero cells. Overall, these results show that VSVFH incorporating H mutations to ablate nectin4, SLAM, or CD46 binding are functional, having robust propagation and titers that are not significantly lower than those of the parental virus. Importantly, the mutations were able to effectively block virus interaction with the respective receptors. The unexpected result was that ablation of nectin4 binding compromised H usage of CD46 on CHO-CD46 and Vero cells.
FIG 3.
Characterization of the viruses with different mutations. (a) Immunochemical analysis of VSVFH mutants. Viral supernatants were purified, and proteins were fractionated by SDS-PAGE. MV and VSV proteins were detected with polyclonal anti-MV or anti-VSV antibodies. (b) Titers of different VSVFH mutants. Vero-hSLAM cells were infected with different mutants (MOI, 0.02), and the cells and supernatant were harvested 48 h later separately. Titers were determined using a TCID50 assay. Results represent the averages of three independent experiments. (c) Fusogenic activity of different VSVFH viruses, including the parental VSVFH-Edm (Edm). CHO cells expressing different human proteins, CD46, SLAM, or nectin4, and Vero cells were infected with different VSVFH mutants (MOI, 1.0). Forty-eight hours later, cells were washed, fixed, and stained with crystal violet.
Effect of nectin4 ablation on viral shedding.
The effect of ablating nectin4 binding on virus shedding was studied by using a human airway epithelial cell line, Calu-3, that expresses both CD46 and nectin4 and not SLAM (Fig. 5a and data not shown). Receptor usage of VSVFH on monolayers of nonpolarized Calu-3 cells was first tested using antibodies that specifically block CD46 or nectin4 binding. Nectin4-specific antibody alone (at 12.5 μg/ml) was sufficient to block the CD46-blind VSVFH-CD460 killing of Calu-3 cells (Fig. 4a). In contrast, both CD46 and nectin4 antibodies were required to completely block killing of VSVFH-Edm (Fig. 4b). Thus, VSVFH can efficiently kill Calu-3 cells by binding to CD46 and/or nectin4.
FIG 5.
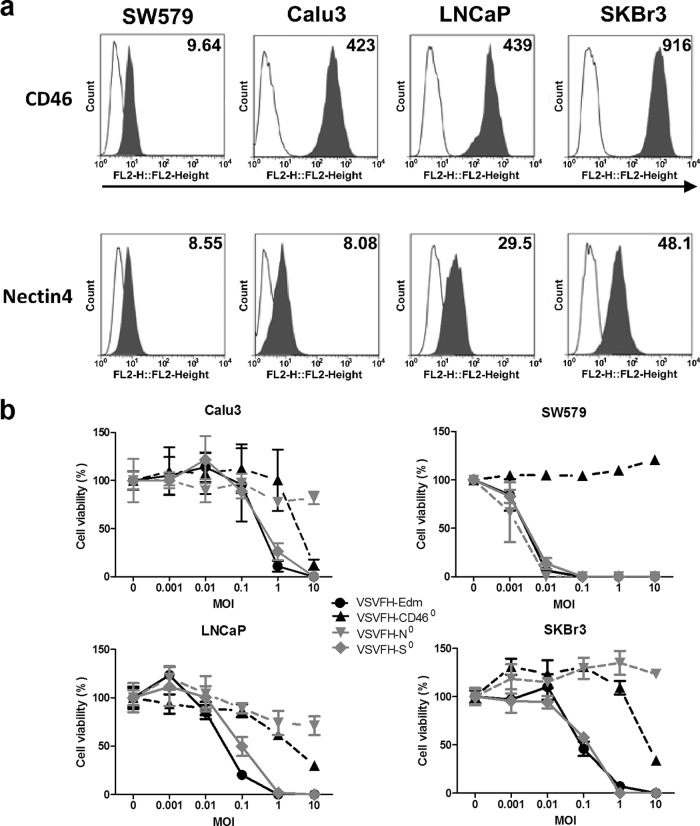
Effect of CD46, SLAM, and nectin4 ablation on oncolytic activity. (a) Flow cytometry was used to evaluate the surface CD46 (top) and nectin4 (bottom) expression by various human cancer cell lines. The presence of CD46 and nectin4 on the cell surface was detected using PE-conjugated anti-human CD46 (624048; BD, Franklin Lakes, NJ) and PE-conjugated anti-human nectin4 (Fab2659P; R&D, Minneapolis, MN) antibodies (solid gray histograms) and was compared to their isotype control (open histograms). Numbers within the panels indicate the mean fluorescence intensities of the populations expressing either CD46 or nectin4 on the surface. (b) Cell viability in various VSV mutant-infected cultures was evaluated by the MTS assay at 48 h after infection at the respective MOIs. Results are expressed as the means ± SDs of three different experiments.
FIG 4.
Effect of nectin4 ablation on viral shedding. Calu-3 cells in the 96-well plate were infected with VSVFH-CD460 (a) or VSVFH-Edm (b) in the presence of monoclonal antibodies specifically against human nectin4 and/or CD46 or an isotype control at the indicated concentrations. Cell viability was assessed at 48 h postinfection using the MTS cell proliferation assay. (c) Evaluation of virus shedding in vitro using the Calu-3 human airway epithelial model. Polarized layers of Calu-3 cells on 12-well Transwell filters were infected with VSVFH-Edm from either the apical or basolateral surface of airway epithelial cells as indicated on the x axis. Supernatants were collected from either basolateral chambers (apical infection) or apical chambers (basolateral infection) at the indicated time points. (d) Calu-3 cells on 12-well Transwell filters were infected with VSVFH-N0, VSVFH-S0, VSVFH-CD460, or VSVFH-Edm basolaterally in the absence or presence (200 μg/ml) of antibodies as indicated below the graph. The amounts of infectious viral particles released to the supernatant obtained from apical chamber at 48 h, 96 h, and 144 h postinfection were determined by TCID50 assays on Vero-SLAM cells. Results are expressed as the means ± SDs of three different experiments. *, P < 0.05 (unpaired student t test). N.D., not detectable.
We next evaluated virus infection and shedding in vitro using the Calu-3 human airway epithelial model, which closely resembles the human airway in which cells are polarized and develop apical adhesion complexes with tight and adherens junctions. Polarization of Calu-3 cells was confirmed by measuring the passive diffusion of sodium fluorescein through the cell monolayer as previously described (22, 23). In our study, the amount of sodium fluorescein that diffused into the basolateral compartment declined from 8.3% of the initial amount of dye added to the apical compartment when cells were nonpolarized (3 days postseeding) to less than 1% (0.3%, 14 days postseeding). Calu-3 cells were considered to be completely polarized once the amount of sodium fluorescein that diffused into the basolateral compartment was less than 1% of the initial amount in the apical compartment, the threshold of polarization (22, 23).
Viruses were diluted in sterile Opti-MEM in the presence or absence of anti-CD46 or nectin4 blocking antibodies to an MOI of 1 (as determined on Vero-SLAM cells), and 100 μl of the solution was applied to either the apical or basolateral surface. Two hours postinfection, viruses were removed, and cells were washed and placed back into the LCC condition. A supernatant from either the apical surface (basolateral infection) or the basolateral surface (apical infection) of Calu-3 cells was collected at various time points postinfection, and titers were determined on Vero-SLAM cells (Fig. 4c and d). As shown in Fig. 4c, greater viral titers were present in the supernatant from the apical compartment following basolateral infection of VSVFH-Edm compared to the supernatant from the basolateral compartment following apical infection at all the time points. With the expression of CD46 receptors equally distributed on both the apical and basolateral surfaces on polarized monolayer Calu-3 cells (data not shown), our result indicated that VSV/MV hybrid virus preferentially transduces the basolateral surface of Calu-3 human airway epithelial cells and is released in an apical direction. This is actually similar to findings for MV, which has been shown to also preferentially transduce the basolateral surface of well-differentiated human airway epithelia, even though the CD46 expression was predominantly on the apical side of primary cultures of human airway epithelial cells (24). Antinectin4 antibody was able to slightly inhibit virus shedding, but virus shedding into the apical surface was significantly inhibited by anti-CD46 antibody. Additions of both CD46 and nectin4 antibodies were potently inhibitory on VSVFH-Edm infection and shedding via the Calu-3 epithelial layer (Fig. 4d). In contrast, shedding of the CD46-blind VSVFH-CD460 into the apical surface was significantly inhibited by antinectin4 antibody alone. Importantly, ablation of nectin4 binding (VSVFH-N0) successfully reduced virus infection and release from the apical side of the Calu-3 epithelial layer (Fig. 4d). The amounts of SLAM-blind VSVFH-S0 and parental VSVFH-Edm released into the supernatant were not significant different, but the amount of VSVFH-N0 released into the supernatant was significantly reduced compared to either one of them.
Oncolysis of human cancer cells.
Ablation of nectin4 severely compromised VSVFH-N0 usage of CD46. The cytopathic effects (CPE) of syncytium formation in Vero and CHO-CD46 cells were negligible or inhibited (Fig. 2 and 3c). This raises the concern that the oncolytic activity of VSVFH-N0 might be compromised. Human cancer cell lines expressing different levels of CD46 and nectin4 were infected with the panel of viruses. Except for SW579, most of the cell lines, SKBr3, LNCaP, and Calu-3, express relatively high levels of CD46 (Fig. 5a). The expression levels of nectin4 on SW579 and Calu-3 cells were relatively low compared to those on SKBr3 and LNCaP cells. While all four viruses induced comparable killing on Vero-hSLAM cells (data not shown), the oncolytic activities of the viruses on human cancer cell lines were more variable (Fig. 5b). In general, VSVFH-Edm and VSVFH-S0 were superior to VSVFH-N0 and VSVFH-CD460 (Fig. 5b). Since VSVFH-N0 is compromised in its usage of CD46, these data suggest that CD46 usage is important for oncolysis of this panel of human cancer cell lines. VSVFH-CD460 oncolysis is severely attenuated in SW579 (low nectin4) and required a high MOI, 10, to reach 80 to 90% killing of SKBr3 cells that express the highest level of nectin4. Similar results were seen with the nectin4-blind VSVFH-N0 (Fig. 5b). At the highest MOI tested (10), VSVFH-N0 killing of human cancer cell lines was significantly lower than that by VSVFH-Edm (P < 0.05). In conjunction with data from Fig. 2 and 3c, these data suggest that ablation of nectin4 severely compromised the oncolytic activity of VSVFH-Edm.
DISCUSSION
The goal of this study was customize the fusogenic VSVFH-Edm hybrid virus for cancer therapy so that the virus uses CD46 exclusively as a receptor for infection and syncytium formation. CD46 is a negative regulator of complement activation, protecting cells from self-lysis by complement proteins (25). CD46 is highly attractive as a cancer target, as it is expressed at low levels on all cells (except erythrocytes) but is overexpressed on numerous cancer cell types (26). Indeed, overexpression of CD46 on cancer cells is a major obstacle in immunotherapy, as CD46 protects cells against antibody and complement-mediated lysis (27–29). For example, malignant plasma (myeloma) cells express 7 to 10 times more CD46 on their surface compared to the rest of the normal hematopoietic cells in the bone marrow (30). Recently, microarray gene expression profiling identified the CD46 gene as a target for activated STAT3 signaling in human breast and prostate cancer cells (31). CD46 mRNA can be induced by activation of STAT3 and interleukin-6 (31). Since STAT3 is constitutively activated with high frequency in many different human tumor cell lines and primary tumors compared with their normal counterparts, the study reveals a potential mechanism whereby oncogenic signaling contributes to enhanced CD46 expression on cancer cells, resulting in tumor cell evasion of antibody-mediated immunity (31).
While wild-type measles virus uses only SLAM and nectin4 as receptors, passage of the wild-type virus results in rapid adaptation of the measles H protein and acquisition of the ability to use CD46 due to a mutation in residue 481 from Asn to Tyr (11). Nectin4 was recently identified as the receptor responsible for measles virus shedding from the airway epithelial layer from the infected host (8, 10). Usage of nectin4 is thus important in pathogenesis and transmission of wild-type measles virus. It remains to be seen if VSVFH-Edm is shed through the epithelial layers in cancer patients who receive the virus. However, since residues that enable H to bind nectin4 are known, ablation of nectin4 binding could easily be accomplished in the early preclinical development stage. The nectin4-blind VSVFH recombinant was compromised in its release from the basolateral side of the Calu-3 LCC airway epithelial layer. However, mutagenesis of residues responsible for nectin4 binding unexpectedly impaired the ability of the virus to interact with CD46, compromising virus infection and syncytium formation. Hence, nectin4 ablation of H in VSVFH is not compatible with the goal of this study. Recent structural studies revealed that all three receptors of MV bind close to a hydrophobic groove located between blades 4 and 5 (β4-β5 groove) of the measles H protein β-propeller head (32–34). Mateo et al. recently reported that there is a strong overlap between the functional footprints of nectin4 and CD46 but not SLAM (35). Based on these studies, it is thus not surprising that ablation of nectin4 binding in VSVFH would result in a significant compromise in H usage of CD46 and impairment of its oncolysis of human cancer cells.
SLAM (CD150) is expressed at low levels on immune cells such as T and B lymphocytes, macrophages, and dendritic cells, and its expression levels are significantly increased in activated cells (36). While CD46 was identified as the receptor for MV in 1994, it was not until 2000 and 2011 that SLAM and nectin4 were, respectively, identified as receptors of measles virus (8, 10, 37, 38). SLAM usage is associated with immunosuppression induced by measles virus, which results in transient leukopenia in individuals infected by wild-type measles virus (39). The mixed-lymphocyte reaction (MLR) assay using lymphocytes from 3 to 5 different donors is a well-established in vitro method to evaluate agents that inhibit lymphocyte proliferation. Mutations in H that ablate SLAM interaction result in reduced lymphocyte proliferation in MLR assays and reduced leukopenia in nonhuman primates (15). Using parental Edmonston strain MV and SLAM-blind MV (MV-S0), we showed that SLAM ablation of H in VSVFH, unlike nectin4 ablation, did not impair the ability of the virus to interact with CD46, compromising virus infection and syncytium formation (Peng and Liu, data unpublished). Further studies with nonhuman primates would be needed to evaluate if VSVFH-Edm induces immunosuppression and if ablation of the SLAM binding site in VSVFH-Edm could reverse this effect.
In summary, we have generated a panel of recombinant VSVFH viruses that bind to one or more of the MV receptors. All the mutations successfully yielded the intended phenotype such that virus usage of intended receptor was abolished. However, ablation of nectin4 binding is not compatible with the intended use of VSVFH as an oncolytic agent, as the killing potential of nectin4-blind virus is severely compromised. Results from this study do not support modification of the H protein to remove nectin4 binding sites but do support continuation of preclinical development of VSVFH displaying the parental H protein.
ACKNOWLEDGMENTS
We are grateful to Chris Richardson for the gift of CHO-nectin4 cells.
This study was supported by grants from the National Institutes of Health (R01CA118488 and R01CA129193) and the Mayo Foundation.
S.J.R. and K.-W.P. have financial conflicts of interest related to this research. They are cofounders of Omnis Pharma, an oncolytic-VSV company.
Footnotes
Published ahead of print 11 December 2013
REFERENCES
- 1.Lech PJ, Russell SJ. 2010. Use of attenuated paramyxoviruses for cancer therapy. Expert Rev. Vaccines 9:1275–1302. 10.1586/erv.10.124 [DOI] [PubMed] [Google Scholar]
- 2.Dingli D, Peng KW, Harvey ME, Greipp PR, O'Connor MK, Cattaneo R, Morris JC, Russell SJ. 2004. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood 103:1641–1646. 10.1182/blood-2003-07-2233 [DOI] [PubMed] [Google Scholar]
- 3.Peng KW, Facteau S, Wegman T, O'Kane D, Russell SJ. 2002. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat. Med. 8:527–531. 10.1038/nm0502-527 [DOI] [PubMed] [Google Scholar]
- 4.Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA, Kaur JS, Haluska PJ, Jr, Aderca I, Zollman PJ, Sloan JA, Keeney G, Atherton PJ, Podratz KC, Dowdy SC, Stanhope CR, Wilson TO, Federspiel MJ, Peng KW, Russell SJ. 2010. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 70:875–882. 10.1158/0008-5472.CAN-09-2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC, Russell SJ. 2002. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 62:4656–4662 [PubMed] [Google Scholar]
- 6.Phuong LK, Allen C, Peng KW, Giannini C, Greiner S, TenEyck CJ, Mishra PK, Macura SI, Russell SJ, Galanis EC. 2003. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 63:2462–2469 [PubMed] [Google Scholar]
- 7.Ayala-Breton C, Suksanpaisan L, Mader EK, Russell SJ, Peng KW. 2013. Amalgamating oncolytic viruses to enhance their safety, consolidate their killing mechanisms, and accelerate their spread. Mol. Ther. 51:1930–1937. 10.1038/mt.2013.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mühlebach MD, Mateo M, Sinn PL, Prufer S, Uhlig KM, Leonard VH, Navaratnarajah CK, Frenzke M, Wong XX, Sawatsky B, Ramachandran S, McCray PB, Jr, Cichutek K, von Messling V, Lopez M, Cattaneo R. 2011. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 480:530–533. 10.1038/nature10639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noyce RS, Bondre DG, Ha MN, Lin LT, Sisson G, Tsao MS, Richardson CD. 2011. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 7:e1002240. 10.1371/journal.ppat.1002240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noyce RS, Richardson CD. 2012. Nectin 4 is the epithelial cell receptor for measles virus. Trends Microbiol. 20:429–439. 10.1016/j.tim.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 11.Hsu EC, Sarangi F, Iorio C, Sidhu MS, Udem SA, Dillehay DL, Xu W, Rota PA, Bellini WJ, Richardson CD. 1998. A single amino acid change in the hemagglutinin protein of measles virus determines its ability to bind CD46 and reveals another receptor on marmoset B cells. J. Virol. 72:2905–2916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vongpunsawad S, Oezgun N, Braun W, Cattaneo R. 2004. Selectively receptor-blind measles viruses: identification of residues necessary for SLAM- or CD46-induced fusion and their localization on a new hemagglutinin structural model. J. Virol. 78:302–313. 10.1128/JVI.78.1.302-313.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coughlin MM, Bellini WJ, Rota PA. 2013. Contribution of dendritic cells to measles virus induced immunosuppression. Rev. Med. Virol. 23:126–138. 10.1002/rmv.1735 [DOI] [PubMed] [Google Scholar]
- 14.Kerdiles YM, Sellin CI, Druelle J, Horvat B. 2006. Immunosuppression caused by measles virus: role of viral proteins. Rev. Med. Virol. 16:49–63. 10.1002/rmv.486 [DOI] [PubMed] [Google Scholar]
- 15.Leonard VH, Hodge G, Reyes-Del Valle J, McChesney MB, Cattaneo R. 2010. Measles virus selectively blind to signaling lymphocytic activation molecule (SLAM; CD150) is attenuated and induces strong adaptive immune responses in rhesus monkeys. J. Virol. 84:3413–3420. 10.1128/JVI.02304-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leonard VH, Sinn PL, Hodge G, Miest T, Devaux P, Oezguen N, Braun W, McCray PB, Jr, McChesney MB, Cattaneo R. 2008. Measles virus blind to its epithelial cell receptor remains virulent in rhesus monkeys but cannot cross the airway epithelium and is not shed. J. Clin. Invest. 118:2448–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura T, Peng KW, Vongpunsawad S, Harvey M, Mizuguchi H, Hayakawa T, Cattaneo R, Russell SJ. 2004. Antibody-targeted cell fusion. Nat. Biotechnol. 22:331–336. 10.1038/nbt942 [DOI] [PubMed] [Google Scholar]
- 18.Peng KW, Pham L, Ye H, Zufferey R, Trono D, Cosset FL, Russell SJ. 2001. Organ distribution of gene expression after intravenous infusion of targeted and untargeted lentiviral vectors. Gene Ther. 8:1456–1463. 10.1038/sj.gt.3301552 [DOI] [PubMed] [Google Scholar]
- 19.Lawson ND, Stillman EA, Whitt MA, Rose JK. 1995. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. U. S. A. 92:4477–4481. 10.1073/pnas.92.10.4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayala-Breton C, Barber GN, Russell SJ, Peng KW. 2012. Retargeting vesicular stomatitis virus using measles virus envelope glycoproteins. Hum. Gene Ther. 23:484–491. 10.1089/hum.2011.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenks N, Myers R, Greiner SM, Thompson J, Mader EK, Greenslade A, Griesmann GE, Federspiel MJ, Rakela J, Borad MJ, Vile RG, Barber GN, Meier TR, Blanco MC, Carlson SK, Russell SJ, Peng KW. 2010. Safety studies on intrahepatic or intratumoral injection of oncolytic vesicular stomatitis virus expressing interferon-beta in rodents and nonhuman primates. Hum. Gene Ther. 21:451–462. 10.1089/hum.2009.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harcourt JL, Caidi H, Anderson LJ, Haynes LM. 2011. Evaluation of the Calu-3 cell line as a model of in vitro respiratory syncytial virus infection. J. Virol. Methods 174:144–149. 10.1016/j.jviromet.2011.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geys J, Coenegrachts L, Vercammen J, Engelborghs Y, Nemmar A, Nemery B, Hoet PH. 2006. In vitro study of the pulmonary translocation of nanoparticles: a preliminary study. Toxicol. Lett. 160:218–226. 10.1016/j.toxlet.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 24.Sinn PL, Williams G, Vongpunsawad S, Cattaneo R, McCray PB., Jr 2002. Measles virus preferentially transduces the basolateral surface of well-differentiated human airway epithelia. J. Virol. 76:2403–2409. 10.1128/jvi.76.5.2403-2409.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oglesby TJ, Allen CJ, Liszewski MK, White DJ, Atkinson JP. 1992. Membrane cofactor protein (CD46) protects cells from complement-mediated attack by an intrinsic mechanism. J. Exp. Med. 175:1547–1551. 10.1084/jem.175.6.1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson BD, Nakamura T, Russell SJ, Peng KW. 2004. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 64:4919–4926. 10.1158/0008-5472.CAN-04-0884 [DOI] [PubMed] [Google Scholar]
- 27.Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. 2003. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol. Immunol. 40:109–123. 10.1016/S0161-5890(03)00112-3 [DOI] [PubMed] [Google Scholar]
- 28.Loveland BE, Johnstone RW, Russell SM, Thorley BR, McKenzie IF. 1993. Different membrane cofactor protein (CD46) isoforms protect transfected cells against antibody and complement mediated lysis. Transpl. Immunol. 1:101–108. 10.1016/0966-3274(93)90002-P [DOI] [PubMed] [Google Scholar]
- 29.Mamidi S, Cinci M, Hasmann M, Fehring V, Kirschfink M. 2013. Lipoplex mediated silencing of membrane regulators (CD46, CD55 and CD59) enhances complement-dependent anti-tumor activity of trastuzumab and pertuzumab. Mol. Oncol. 7:580–594. 10.1016/j.molonc.2013.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ong HT, Timm MM, Greipp PR, Witzig TE, Dispenzieri A, Russell SJ, Peng KW. 2006. Oncolytic measles virus targets high CD46 expression on multiple myeloma cells. Exp. Hematol. 34:713–720. 10.1016/j.exphem.2006.03.002 [DOI] [PubMed] [Google Scholar]
- 31.Buettner R, Huang M, Gritsko T, Karras J, Enkemann S, Mesa T, Nam S, Yu H, Jove R. 2007. Activated signal transducers and activators of transcription 3 signaling induces CD46 expression and protects human cancer cells from complement-dependent cytotoxicity. Mol. Cancer Res. 5:823–832. 10.1158/1541-7786.MCR-06-0352 [DOI] [PubMed] [Google Scholar]
- 32.Hashiguchi T, Ose T, Kubota M, Maita N, Kamishikiryo J, Maenaka K, Yanagi Y. 2011. Structure of the measles virus hemagglutinin bound to its cellular receptor SLAM. Nat. Struct. Mol. Biol. 18:135–141. 10.1038/nsmb.1969 [DOI] [PubMed] [Google Scholar]
- 33.Santiago C, Celma ML, Stehle T, Casasnovas JM. 2010. Structure of the measles virus hemagglutinin bound to the CD46 receptor. Nat. Struct. Mol. Biol. 17:124–129. 10.1038/nsmb.1726 [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Lu G, Qi J, Li Y, He Y, Xu X, Shi J, Zhang CW, Yan J, Gao GF. 2013. Structure of measles virus hemagglutinin bound to its epithelial receptor nectin-4. Nat. Struct. Mol. Biol. 20:67–72. 10.1038/nsmb.2432 [DOI] [PubMed] [Google Scholar]
- 35.Mateo M, Navaratnarajah CK, Syed S, Cattaneo R. 2013. The measles virus hemagglutinin beta-propeller head beta4-beta5 hydrophobic groove governs functional interactions with nectin-4 and CD46 but not with the signaling lymphocytic activation molecule. J. Virol. 87:9208–9213. 10.1128/JVI.01210-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veillette A, Latour S. 2003. The SLAM family of immune-cell receptors. Curr. Opin. Immunol. 15:277–285. 10.1016/S0952-7915(03)00041-4 [DOI] [PubMed] [Google Scholar]
- 37.Dörig RE, Marcil A, Richardson CD. 1994. CD46, a primate-specific receptor for measles virus. Trends Microbiol. 2:312–318. 10.1016/0966-842X(94)90447-2 [DOI] [PubMed] [Google Scholar]
- 38.Tatsuo H, Ono N, Tanaka K, Yanagi Y. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893–897. 10.1038/35022579 [DOI] [PubMed] [Google Scholar]
- 39.de Vries RD, McQuaid S, van Amerongen G, Yuksel S, Verburgh RJ, Osterhaus AD, Duprex WP, de Swart RL. 2012. Measles immune suppression: lessons from the macaque model. PLoS Pathog. 8:e1002885. 10.1371/journal.ppat.1002885 [DOI] [PMC free article] [PubMed] [Google Scholar]