Abstract
A prophylactic vaccine for genital herpes disease remains an elusive goal. We report the results of two studies performed collaboratively in different laboratories that assessed immunogenicity and vaccine efficacy in herpes simplex virus 1 (HSV-1)-seropositive guinea pigs immunized and subsequently challenged intravaginally with HSV-2. In study 1, HSV-2 glycoproteins C (gC2) and D (gD2) were produced in baculovirus and administered intramuscularly as monovalent or bivalent vaccines with CpG and alum. In study 2, gD2 was produced in CHO cells and given intramuscularly with monophosphoryl lipid A (MPL) and alum, or gC2 and gD2 were produced in glycoengineered Pichia pastoris and administered intramuscularly as a bivalent vaccine with Iscomatrix and alum to HSV-1-naive or -seropositive guinea pigs. In both studies, immunization boosted neutralizing antibody responses to HSV-1 and HSV-2. In study 1, immunization with gC2, gD2, or both immunogens significantly reduced the frequency of genital lesions, with the bivalent vaccine showing the greatest protection. In study 2, both vaccines were highly protective against genital disease in naive and HSV-1-seropositive animals. Comparisons between gD2 and gC2/gD2 in study 2 must be interpreted cautiously, because different adjuvants, gD2 doses, and antigen production methods were used; however, significant differences invariably favored the bivalent vaccine. Immunization of naive animals with gC2/gD2 significantly reduced the number of days of vaginal shedding of HSV-2 DNA compared with that for mock-immunized animals. Surprisingly, in both studies, immunization of HSV-1-seropositive animals had little effect on recurrent vaginal shedding of HSV-2 DNA, despite significantly reducing genital disease.
INTRODUCTION
Genital herpes is one of the most common sexually transmitted infections (1, 2). An estimated 536 million people between the ages of 15 and 49 are infected globally, and about 23.6 million new infections develop annually (3). Herpes simplex virus 2 (HSV-2) genital infection leads to latency within lumbosacral dorsal root ganglia (DRG), and subsequent virus reactivation from DRG results in recurrences. In immunocompetent individuals, many primary and recurrent genital herpes virus infections are asymptomatic, which poses a risk for transmission between partners (4–6). Recurrent meningitis and neonatal herpes virus infection are serious sequelae of genital herpes (4, 7–9). Prevention of genital HSV infection and transmission is an important target for vaccine development because of the psychosocial and medical complications of genital herpes and because genital herpes is a significant risk factor for HIV acquisition and transmission (10–13). Antiviral therapy reduces the duration of HSV-2 symptomatic disease, and daily suppressive therapy decreases symptomatic recurrences and asymptomatic viral shedding (14); however, the protection is incomplete, since antiviral therapy does not totally prevent viral shedding or eliminate latency (15–17).
GlaxoSmithKline (GSK) clinical trials have evaluated the efficacy of an HSV-2 glycoprotein D (gD2) subunit antigen vaccine (18, 19). In the first publication, two studies reported no significant difference in genital lesions between vaccine and placebo groups; however, a subgroup analysis found that the vaccine was efficacious in HSV-1 and HSV-2 doubly seronegative women but not in HSV-1-seropositive women or in men, regardless of their prior exposure to HSV (18). To substantiate these results, a follow-up trial was performed in doubly seronegative women. The results demonstrated no significant protection against genital herpes disease; however, a subgroup analysis showed that the gD2 subunit vaccine protected against HSV-1 genital infection and disease but not against HSV-2 (19). Therefore, additional strategies are needed to develop a more effective HSV-2 vaccine.
We reported that HSV-1 glycoprotein C (gC1) and HSV-2 glycoprotein C (gC2), used in combination as subunit antigens with gD1 and gD2, respectively, prevented the virus from evading immune responses mediated by complement (20, 21). HSV-1 and HSV-2 gC proteins bind complement component C3b generated by activation of the classical, lectin, or alternative complement pathway. Complement activation leads to virus neutralization, lysis of infected cells, and enhancement of B- and T-cell responses (22–25). By binding to C3b, HSV-1 and HSV-2 gC proteins inhibit activation of the complement cascade (26–32). We previously reported that antibodies induced by immunization of animals with gC1 or gC2 bind to gC and block its ability to interact with C3b; thus, complement remains active in host defense against the virus (20, 21, 33). In mouse and guinea pig vaginal infection models, a bivalent gC2/gD2 subunit antigen vaccine provided significantly better protection than monovalent gC2 or gD2 subunit antigen vaccines in preventing DRG infection and recurrent vaginal shedding of HSV-2 DNA (21).
Here we evaluated the immunogenicity and protection against genital HSV-2 challenge provided by immunization with monovalent gC2 or gD2 or bivalent gC2/gD2 prepared in baculovirus and administered with CpG and alum adjuvants to HSV-1-seropositive guinea pigs. In a second study, we evaluated protection in both naive and HSV-1-seropositive animals provided by a monovalent gD2 antigen and adjuvant similar to those used in human trials or by a bivalent gC2/gD2 vaccine prepared in glycoengineered Pichia pastoris yeast and administered with Iscomatrix and alum as adjuvants. The rationale for evaluating HSV-1-seropositive guinea pigs is based on the epidemiology of HSV-1 and HSV-2 infections. HSV-1 infection generally occurs at a younger age than HSV-2 infection. More than 50% of the U.S. population is HSV-1 seropositive, and the seroprevalence of HSV-1 is even higher in resource-limited nations (34, 35). Prior HSV-1 infection reduces the severity of HSV-2 genital disease but does not totally prevent disease (8); therefore, one of the goals of an HSV-2 vaccine is to enhance protection against HSV-2 in HSV-1-seropositive individuals.
MATERIALS AND METHODS
Viruses and immunogens.
HSV-1 strain NS and HSV-2 strain MS were grown in Vero cells (20, 21). In study 1, glycoprotein subunit antigens were prepared in baculovirus. The bac-gC2(426t) construct extends from amino acids 27 to 426, where amino acid 27 is the first amino acid after the signal peptide (36). The bac-gD1(306t) and bac-gD2(306t) constructs extend from amino acids 26 to 331, where amino acid 26 is the first amino acid after the signal sequence (37–39). The methods used for these constructs resulted in aspartic acid and proline residues added at the N terminus. In study 2, gD2 was purified from supernatant fluids of CHO cells that were stably transfected with gD306NQ to express a truncated form of gD2 (referred to as CHO gD2). An asparagine and glutamine were added to the C terminus after gD2 amino acid 331 and linked to nine histidine residues by three glycine residues. The CHO gD2 protein was expressed from stably transfected cells and purified on a nickel column.
HSV-2 gC2 and gD2 subunit antigens were also prepared in glycoengineered Pichia pastoris (referred to as Pichia gC2 or Pichia gD2) at GlycoFi (40). Pichia gC2 contains gC2 amino acids 24 to 448 and a partial proteolytic cleavage fragment containing amino acids 75 to 448 that developed during purification. Pichia gC2 lacks the signal peptide and the transmembrane domain and contains an N-terminal nine-amino-acid His tag. It was produced in a glycoengineered version of Pichia pastoris (41), linked in frame to the Saccharomyces cerevisiae α-MFprepro signal peptide, and expressed using the P. pastoris AOX1 methanol-inducible promoter. The resulting plasmid was transformed into a glycoengineered and chaperone-engineered host strain of P. pastoris capable of secreting proteins with the human intermediate glycoform Man5GlcNAc2 (41–43) and that coexpresses human protein disulfide isomerase as an endoplasmic reticulum (ER) chaperone to aid in secretion (44). A single clone was selected, and the protein was purified in a two-step process because of the partial proteolysis of the N-terminal His tag. Purification first used standard Ni-chelating affinity resin (GE Healthcare, Piscataway, NJ) capture followed by Poros 50 HS anion exchange (Applied Biosystems). N-glycans were quantitatively analyzed by enzymatic release, 2-aminobenzamide labeling, and normal-phase high-pressure liquid chromatography (HPLC) (42) and revealed to be predominantly of the human intermediate form Man5GlcNAc2 (88.4%), with the remaining forms being a mixture of Man6-9GlcNAc2. Pichia gD2, containing amino acids 26 to 310 of HSV-2 gD2, lacks the signal peptide and the C-terminal transmembrane domain and contains a C-terminal nine-amino-acid His tag linked in frame to the S. cerevisiae α-MFpre signal peptide, and it was expressed using the P. pastoris AOX1 methanol-inducible promoter. The resulting plasmid was transformed into glycoengineered P. pastoris as described for Pichia gC2 (41). A single clone was selected, and the protein was purified using a three-step process starting with hydroxyapatite chromatography followed by Poros 50 HQ anion-exchange chromatography and SP Sepharose cation-exchange chromatography.
Guinea pig infection with HSV-1, immunizations, and HSV-2 challenge studies.
Laboratory animals were housed and handled in accordance with the guidelines of the Institutional Animal Care and Use Committees of the University of Pennsylvania and Merck Research Laboratories.
(i) Study 1.
Thirty-six female Hartley strain guinea pigs (Charles River) weighing 225 to 275 g were prebled and infected intranasally with 5 × 105 PFU of HSV-1 strain NS in 10 μl (45). Sixty-five days after HSV-1 infection, guinea pigs were immunized intramuscularly (i.m.) in the right hind calf muscle three times at 2-week intervals. Animals were mock immunized with the CpG oligonucleotide TCGTCGTTGTCGTTTTGTCGTT (Trilink Inc.) and alum (Alhydrogel; Accurate Chemical and Scientific Corp.) or immunized with 10 μg bac-gC2(426t) (gC2), 5 μg bac-gD2(306t) (gD2), or 10 μg gC2 combined with 5 μg gD2 (gC2/gD2). The doses of gC2 and gD2 were based on our prior studies indicating that higher doses of gC2 immunization are needed to generate robust C3b-blocking antibody responses (21). Subunit antigens were mixed with CpG at 100 μg/guinea pig and with alum at 20 μg/μg protein in a total volume of 50 μl per immunization (46). For the gC2/gD2 group, the individual antigens were first mixed with CpG and alum and combined just prior to immunization. Guinea pigs were bled from a hind limb saphenous vein 30 days after HSV-1 infection and 2 weeks after the third immunization (day 107). Eight days later, animals were challenged intravaginally with 5 × 105 PFU of HSV-2 strain MS and scored for 14 days, from days 115 to 129, for acute disease on a scale of 0 to 4, where 0 reflects no disease, 1 reflects redness, 2 reflects a single lesion, 3 reflects coalesced lesions, and 4 reflects ulcerated lesions (47). Urinary retention and hind leg weakness were recorded. Guinea pigs were swabbed for vaginal titers on days 1 to 6 after challenge and observed for genital lesions for 57 days between days 1 and 60 postinfection. Vaginal swabs for HSV-2 DNA copy number were obtained for 20 days, from days 28 to 49 postinfection. Swabs were stored at −80°C prior to culture or processing for HSV-2 DNA by quantitative PCR (qPCR) (48).
(ii) Study 2.
Forty-eight Hartley strain female guinea pigs (Charles River) weighing between 250 and 300 g were infected with 1 × 105 PFU of HSV-1 strain NS intranasally. Sixty days later, HSV-1-infected and age-matched HSV-1-naive guinea pigs (n = 16/group) were vaccinated intramuscularly on days 0, 7, and 21 with (i) 5 μg of CHO gD2 with monophosphoryl lipid A (MPL; InvivoGen), using 12.5 μg/dose, and alum (2% Alhydrogel; Accurate Chemical), using 125 μg/dose, in 125 μl; (ii) 20 μg of Pichia gC2 and 10 μg of Pichia gD2 with Iscomatrix (CSL Behring), using 40 μg/dose, and Merck alum (amorphous aluminum hydroxyphosphate sulfate), using 90 μg/dose, in 200 μl (49); or (iii) Iscomatrix and alum adjuvants alone (mock group). A goal of study 2 was to determine if the combination of gC2 and gD2 prepared in Pichia provided better protection than gD2 administered with MPL and alum at previously reported concentrations (50). Three weeks after the final immunization, animals were bled, and the following day guinea pigs were challenged intravaginally with 1 × 106 PFU of HSV-2 strain MS. At the conclusion of the experiment, lumbosacral DRG were harvested, and viral genome copy numbers were determined by qPCR.
ELISA and neutralizing antibody titers. (i) Study 1.
Enzyme-linked immunosorbent assay (ELISA) was performed to measure antibody responses following HSV-1 intranasal infection and immunization. Plates were coated with 50 ng of antigen, and serial dilutions of serum were added (21). The endpoint titer was considered the highest dilution of serum resulting in an optical density (OD) of ≥0.1 that was at least 2-fold higher than the OD of sera obtained from mock-immunized animals at that dilution. Neutralizing antibody titers were measured by heating guinea pig serum to 56°C for 30 min to inactivate guinea pig complement and then incubating 1 × 105 to 2 × 105 PFU/100 μl of HSV-1 or HSV-2 at 37°C for 1 h with a 1:40 dilution of guinea pig serum, with or without 10% human serum as a source of complement. The human serum was obtained from an HSV-1- and HSV-2-seronegative donor (51). Virus titers were determined by plaque assay on Vero cells and reported as log10 PFU/100 μl.
(ii) Study 2.
ELISA was performed as described for study 1, except that plates were coated with 50 ng/well of purified HSV-1 or HSV-2 lysate (Advanced Biotechnologies Inc.). The endpoint titer was the highest dilution that was ≥3-fold above background. Neutralizing antibody titers were evaluated using the ELVIS HSV cell reporter system (Diagnostic Hybrids Inc.). Heat-inactivated guinea pig serum was incubated with 1,800 PFU of HSV-2 strain MS for 1 h at 37°C in the presence of 6.7% Low-Tox-M rabbit complement (Cedarlane) and added to the ELVIS cell monolayer overnight. LacZ expression was measured using a Gal-Screen kit (Applied Biosystems). The endpoint titer was the highest serum dilution that reduced β-galactosidase activity by ≥50% compared with that in infected cells in the absence of serum.
Determination of HSV-2 DNA copy number by real-time qPCR. (i) Study 1.
DNA was isolated from guinea pig vaginal swabs by use of a DNeasy Blood kit (Qiagen). Viral DNA was quantified by real-time amplification of the Us9 gene product, using purified HSV-2 DNA (Advanced Biotechnologies), to establish a standard curve (21). Vaginal swab samples that contained ≥1.5 copies of HSV-2 DNA in duplicate wells by 40 cycles were considered positive, which established a cutoff for a positive sample at ≥150 copies/ml, based on amplifying 10 μl of swab DNA per assay (21). Amplification reactions were performed using TaqMan gene expression master mix (Applied Biosystems) and an ABI 7500 Fast machine.
(ii) Study 2.
Total DNA was isolated from daily vaginal swab samples or lumbosacral DRG taken 50 days after challenge by using a Qiamp DNA minikit (Qiagen) per the manufacturer's instructions. DNA samples and oligonucleotide primer-probe sets specific for gG2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were mixed with Quantitect Multiplex PCR no-Rox master mix (Qiagen). Reactions were carried out in a Stratagene Mx3005P real-time PCR system and analyzed with Stratagene MxPro software. Standard curves were constructed from 10-fold dilutions of a plasmid containing gG2 sequences and purified guinea pig DNA (BioChain Institute, Inc.), and numbers of HSV-2 DNA copies were normalized to 1 × 106 guinea pig GAPDH DNA copies.
Statistics.
Statistical significance for survival was calculated using the log rank (Mantel-Cox) test. The area under the curve was calculated, followed by one-way analysis of variance (ANOVA), to compare curves. Fisher's exact test was performed to compare the number of guinea pigs in each group that developed disease, the number of recurrent lesion days, and the number of DNA-positive days of shedding. ANOVA (nonparametric test) followed by Tukey's posttest analysis was performed for all possible pairwise comparisons.
RESULTS
Baculovirus gC2 and gD2 given with CpG alum to HSV-1-seropositive guinea pigs (study 1). (i) HSV-1 infection of guinea pigs.
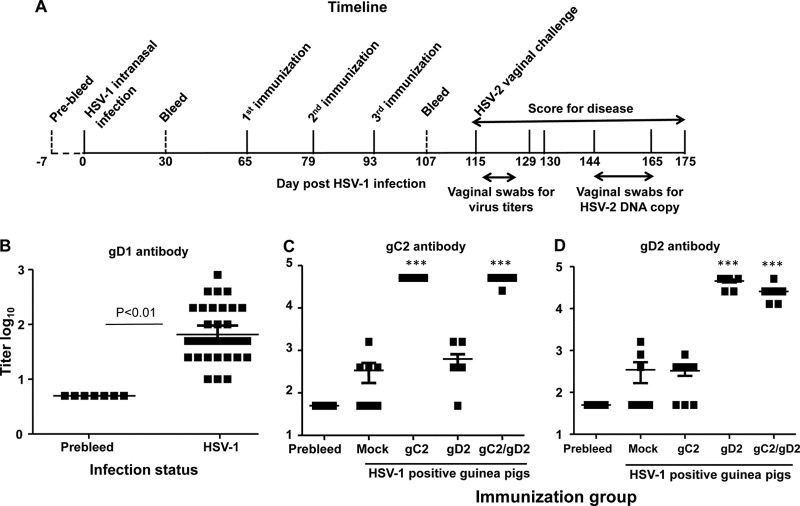
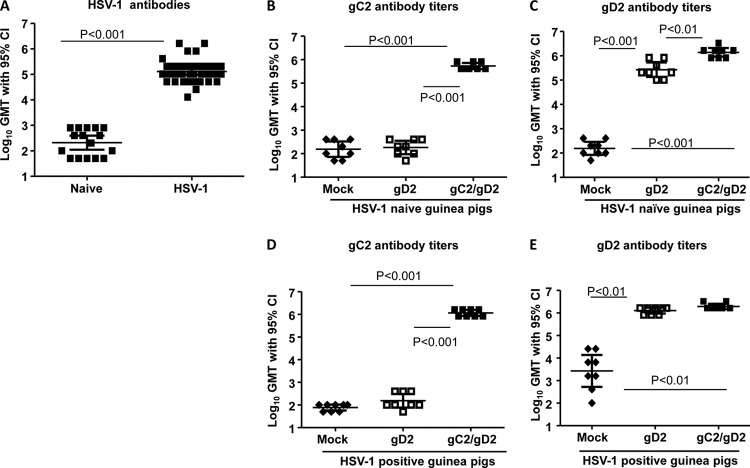
The timeline of the experiment is shown in Fig. 1A. Thirty-six female guinea pigs were infected intranasally with 5 × 105 PFU of HSV-1 strain NS. No animal died or showed signs of disease. Thirty days later, animals were bled and sera evaluated by ELISA for gD1 antibodies. All animals were HSV-1 infected based on gD1 antibody titers, although a range of responses was detected (Fig. 1B). Low, medium, and high antibody responders were distributed proportionally to mock, gC2, gD2, and gC2/gD2 immunization groups.
FIG 1.
Study 1 experimental timeline and ELISA titers. (A) Timeline. (B) HSV-1 gD1 ELISA titers of 7 guinea pigs bled prior to HSV-1 intranasal infection and 36 animals bled 30 days after infection. (C) Titers of antibody to gC2 in the same sera. ***, P < 0.001 for comparing gC2 or gC2/gD2 group with prebleed, mock, or gD2 group; P was not significant for comparing gC2 group with gC2/gD2 group or mock group with gD2 group. (D) Titers of antibody to gD2 in sera obtained 2 weeks after the third immunization (n = 7 for prebleed group and n = 9 for other groups). ***, P < 0.001 for comparing gD2 or gC2/gD2 group with prebleed, mock, or gC2 group; P was not significant for comparing gD2 group with gC2/gD2 group. Error bars in panels B to D represent 95% confidence intervals. Note that the y axis for panel B is different from those in panels C and D.
(ii) Immunization with bac-gC2(426t) and bac-gD2(306t) subunit antigens.
Sixty-five days after HSV-1 infection, guinea pigs were mock immunized with CpG and alum or immunized intramuscularly three times at 2-week intervals with individual gC2 or gD2 or the combined gC2/gD2 antigens administered with CpG and alum. The geometric mean antibody titer in mock-immunized guinea pigs was 2.5 log10 for gC2 (Fig. 1C) and 2.3 log10 for gD2 (Fig. 1D). The geometric mean gC2 antibody titer was 4.7 log10 in gC2- and gC2/gD2-immunized animals (Fig. 1C), while the gD2 antibody titer was 4.7 log10 in animals immunized with gD2 and 4.4 log10 in gC2/gD2-immunized animals (Fig. 1D). The gC2 and gD2 antibody titers were boosted in HSV-1-seropositive guinea pigs by immunization with gC2 alone, gD2 alone, and the bivalent gC2/gD2 vaccine. Mixing the two antigens in the bivalent vaccine did not significantly blunt the ELISA response to either antigen.
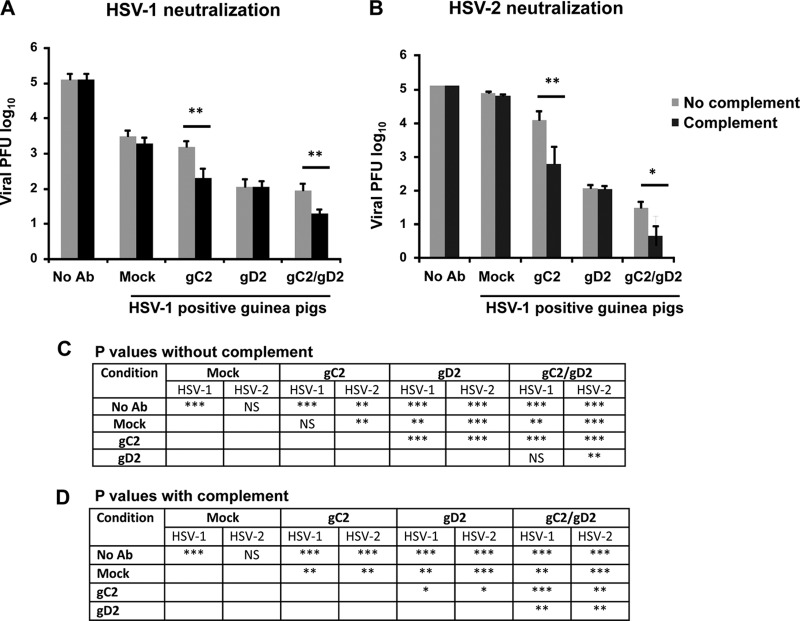
(iii) HSV-1 and HSV-2 neutralizing antibody titers with and without human complement.
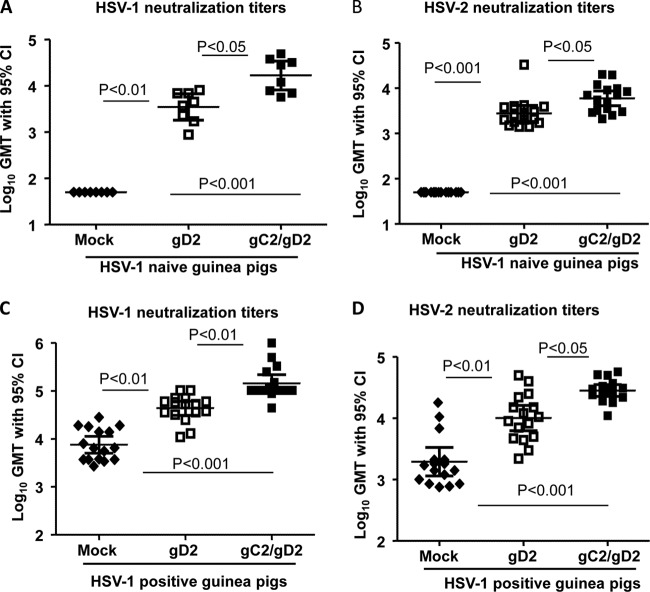
Guinea pigs were bled 2 weeks after the third immunization and tested for neutralizing antibodies to HSV-1 and HSV-2, using a 1:40 serum dilution, in the presence or absence of 10% human complement (Fig. 2A and B). Complement alone provided no neutralizing activity against either virus. Sera from mock-immunized HSV-1-infected animals neutralized HSV-1 approximately 1.5 log10, while having little effect on HSV-2. Sera from gC2-immunized animals added to the neutralization of HSV-2 in the absence of complement and to that of both viruses in the presence of complement. Sera from animals immunized with gD2 were more neutralizing against HSV-1 and HSV-2 than gC2 sera in the absence of complement, but complement did not enhance the neutralizing activity of gD2 sera. Animals immunized with gC2/gD2 had the highest titers of neutralizing antibody in the presence of complement, and antibody neutralized HSV-1 by approximately 2 log10 and HSV-2 by approximately 4 log10 compared with sera from mock-immunized animals. Therefore, gC2, gD2, and gC2/gD2 immunization of HSV-1-seropositive animals enhanced neutralization of HSV-1 and HSV-2, particularly in the presence of human complement.
FIG 2.
Study 1 neutralizing antibody titers. Neutralization in HSV-1-seropositive guinea pigs was tested at a 1:40 dilution against mock-, gC2-, gD2-, or gC2/gD2-immunized guinea pigs in the absence (gray bars) or presence (black bars) of 10% human complement. (A) Neutralization of HSV-1 strain NS. (B) Neutralization of HSV-2 strain MS. (C and D) P values comparing neutralizing titers of the immunization groups with or without complement. P values comparing gC2 or gC2/gD2 titers with and without complement are shown on the graphs. Ab, antibody. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
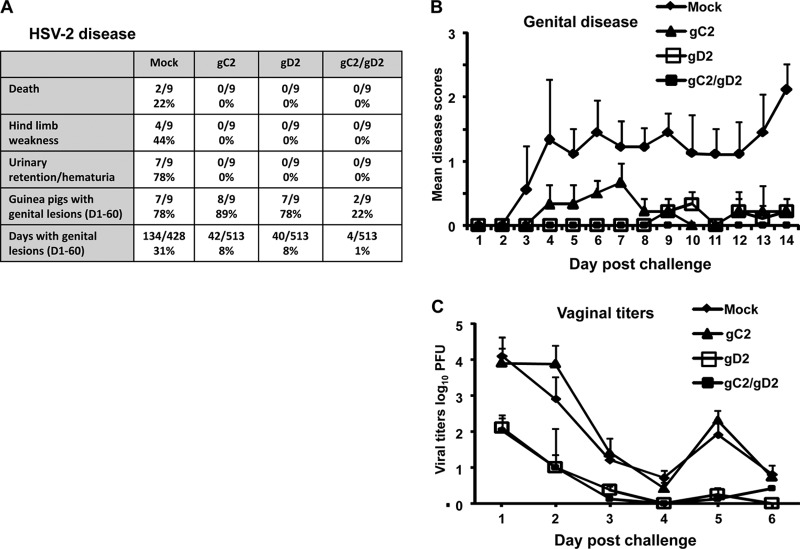
(iv) Combined bac-gC2(426t) and bac-gD2(306t) immunization protects guinea pigs from genital disease after HSV-2 vaginal challenge.
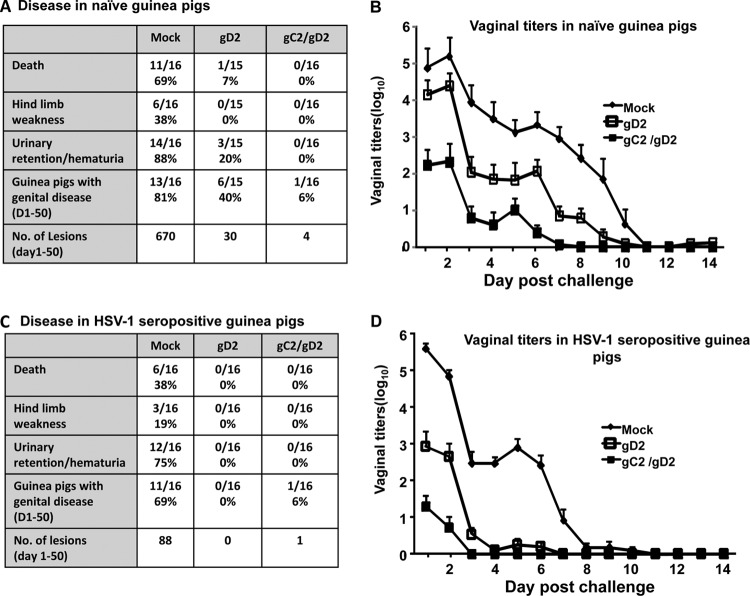
HSV-1-seropositive guinea pigs were challenged intravaginally with 5 × 105 PFU of HSV-2 strain MS. More animals died, developed hind limb weakness, and showed urinary retention/hematuria in the mock group than in groups immunized with monovalent gC2 or gD2 or the bivalent gC/gD2 vaccine (Fig. 3A). Genital disease developed in 7/9 mock-immunized HSV-1-seropositive animals; nevertheless, the acute disease scores on days 1 to 14 (Fig. 3B) were greatly reduced compared with our prior results for HSV-naive animals (21, 48). Guinea pigs immunized with gC2 or gD2 had less acute disease than mock-immunized animals, while the combined gC2/gD2 group had no disease (Fig. 3B). Vaginal titers were lower in the gD2 and gC2/gD2 groups than in mock- or gC2-immunized animals (Fig. 3C). Over the course of 60 days postinfection, mock-immunized animals had significantly more days with genital lesions than the other groups, while the gC2/gD2 group had the fewest days with genital lesions (Fig. 3A).
FIG 3.
Study 1 disease markers and vaginal titers. (A) Death and other indicators of disease. For urinary retention/hematuria, P < 0.01 for comparing gC2, gD2, or gC2/gD2 group with mock group; for guinea pigs with genital lesions, P < 0.05 for comparing gC2/gD2 group with gC2 group, and other comparisons were not significant; and for days with genital lesions, P < 0.001 for comparing gC2, gD2, or gC2/gD2 immunization with mock immunization, and P < 0.001 for comparing gC2/gD2 group with gC2 or gD2 group. (B) Severity scores for acute genital disease for surviving animals. P < 0.05 for comparing gC2 group with mock group; P < 0.02 for comparing gD2 group with mock group; P < 0.01 for comparing gC2/gD2 group with mock group; and the P value was not significant for comparing the gC2, gD2, and gC2/gD2 groups to one another. Results represent means ± standard errors of the means (SEM). (C) Vaginal titers at days 1 to 6 postchallenge. P < 0.05 for comparing mock or gC2 group with gD2 or gC2/gD2 group; the P value was not significant for comparing gD2 group with gC2/gD2 group. Results represent geometric mean titers ± SEM.
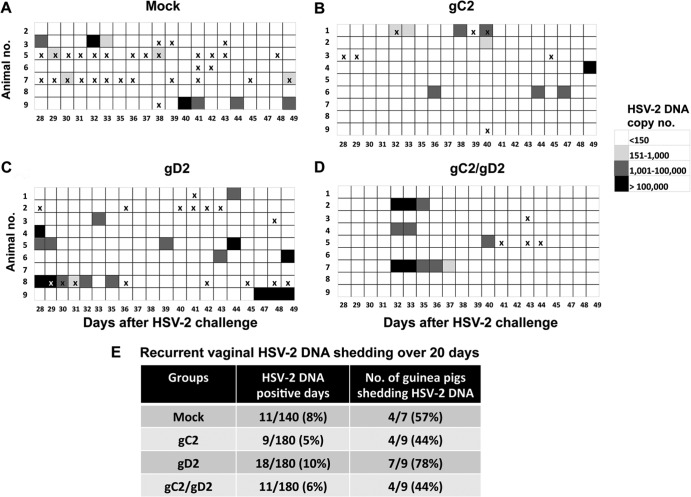
(v) Vaginal shedding of HSV-2 DNA during recurrent infection.
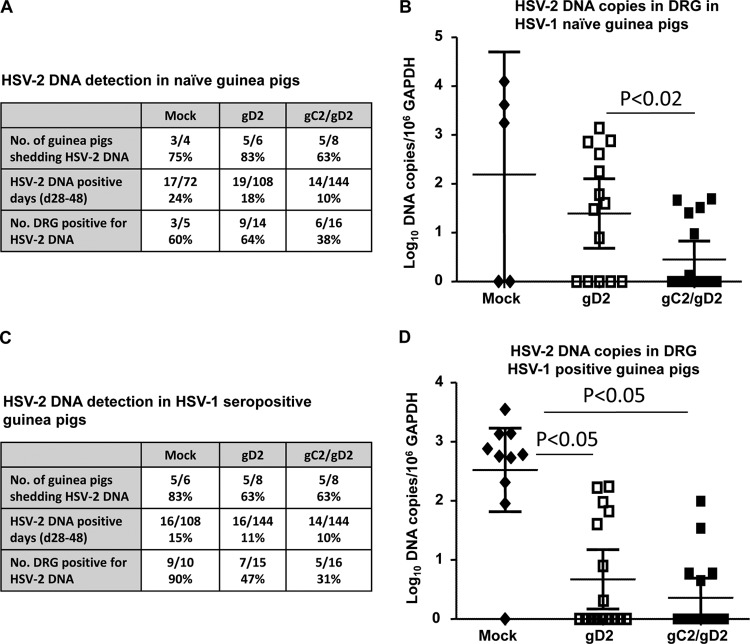
Vaginal swabs for determination of HSV-2 DNA copy number were obtained on 20 days, from days 28 to 49 after HSV-2 challenge (Fig. 4). Differences among all four groups were small in terms of the number of days with recurrent vaginal shedding of HSV-2 DNA or the number of guinea pigs that had at least 1 day of shedding (Fig. 4E). Days with genital disease (Fig. 4A to D) often did not coincide with days of vaginal shedding. Although gC2/gD2 immunization greatly reduced the number of days with genital lesions, it did not significantly alter vaginal shedding of HSV-2 DNA (Fig. 4A to D).
FIG 4.
Study 1 vaginal shedding of HSV-2 DNA. (A to D) Vaginal HSV-2 DNA shedding in mock-, gC2-, gD2-, and gC2/gD2-immunized animals. (E) Table showing the number of days (%) of vaginal shedding and the number of animals that shed HSV-2 DNA. P values were not significant for comparing gC2/gD2 group with the other groups. Each “x” indicates a day with genital disease.
Pichia gC2/gD2 given with Iscomatrix and Merck alum as adjuvants compared with CHO gD2 given with MPL and alum (study 2). (i) HSV-1 infection of guinea pigs.
The experimental timeline was similar to that for study 1 (Fig. 1A), except that 1 week separated the first and second immunizations and animals were observed for 50 days postchallenge rather than 60 days. Forty-eight female guinea pigs were infected intranasally with 1 × 105 PFU of HSV-1 strain NS. None of the guinea pigs showed signs of disease or died, and all animals seroconverted to HSV-1 (Fig. 5A). Prior to immunization, low, medium, and high antibody responders to HSV-1 antigen were distributed proportionally to mock, gD2, and gC2/gD2 immunization groups.
FIG 5.
Study 2 ELISA titers. (A) HSV-1 antibody titers in uninfected (naive) and HSV-1-infected animals (n = 16 for naive group and n = 48 for HSV-1-infected group). (B and D) ELISA responses to gC2 in naive (B) and HSV-1-seropositive (D) animals immunized with gD2 or gC2/gD2 (n = 8 per group). (C and E) ELISA responses to gD2 in the same animals as those in panels B and D. GMT, geometric mean titer; 95% CI, 95% confidence interval.
(ii) ELISA and neutralizing antibody responses.
HSV-1-naive and HSV-1-seropositive guinea pigs developed robust gC2 ELISA responses following three immunizations with Pichia gC2/gD2 (Fig. 5B and D) and gD2 ELISA responses after immunization with CHO gD2 or Pichia gC2/gD2 (Fig. 5C and E). Prior HSV-1 infection did not blunt the gC2 or gD2 ELISA response. The gD2 ELISA titers in naive animals were significantly higher in the Pichia gC2/gD2 group than the CHO gD2 group (Fig. 5C). Three weeks after the third immunization, sera were evaluated for HSV-2 neutralizing antibodies in the presence of rabbit complement. Neutralizing antibody titers of naive and HSV-1-seropositive animals were boosted after immunization with CHO gD2 and Pichia gC2/gD2 (Fig. 6). Pichia gC2/gD2 produced significantly higher HSV-1 and HSV-2 neutralizing antibody titers than CHO gD2 in naive and HSV-1-seropositive animals.
FIG 6.
Study 2 neutralizing antibody titers. (A and C) HSV-1 neutralizing titers in naive (n = 8) (A) and HSV-1-seropositive (n = 16) (C) guinea pigs. (B and D) HSV-2 titers in naive (B) and HSV-1-seropositive (D) guinea pigs (n = 16 per group).
(iii) gC2/gD2 immunization protects guinea pigs from genital disease after HSV-2 vaginal challenge of naive and HSV-1-seropositive guinea pigs.
Prior HSV-1 infection partially protected guinea pigs from genital disease, since genital lesions were far more abundant in mock-immunized naive animals than in mock-immunized HSV-1-seropositive animals (Fig. 7A and C). Immunization with Pichia gC2/gD2 totally protected naive and HSV-1-seropositive animals against death, hind limb weakness, and urinary retention/hematuria, while one animal immunized with CHO gD2 died and three developed urinary retention/hematuria (Fig. 7A and C). CHO gD2 or Pichia gC2/gD2 protected naive and HSV-1-seropositive animals against genital disease (Fig. 7A and C), with better protection noted in the naive animals immunized with Pichia gC2/gD2 than in those immunized with CHO gD2 (Fig. 7A). Vaginal titers were lower in naive and HSV-1-seropositive animals immunized with Pichia gC2/gD2 than in mock-immunized or CHO gD2-immunized animals (Fig. 7B and D). The number of days that naive guinea pigs shed HSV-2 DNA was significantly lower for the Pichia gC2/gD2 group than for mock-immunized animals (Fig. 8A). The HSV-2 DNA copy number in DRG was significantly lower in naive animals immunized with Pichia gC2/gD2 than in those immunized with CHO gD2 (Fig. 8B). Among HSV-1-seropositive animals, the CHO gD2- and Pichia gC2/gD2-immunized animals had significantly fewer DRG that contained HSV-2 DNA and lower DRG HSV-2 DNA copy numbers than the mock-immunized animals (Fig. 8C and D).
FIG 7.
Study 2 disease markers and vaginal titers. (A) Death and other disease markers in naive animals. For death, P < 0.001 for comparing gD2 or gC2/gD2 group with mock group; for hind limb weakness, P < 0.05 for comparing gD2 or gC2/gD2 group with mock group; for urinary retention/hematuria, P < 0.001 for comparing gD2 or gC2/gD2 group with mock group; for guinea pigs with genital disease, P < 0.05 for comparing gD2 group with mock group, P < 0.001 for comparing gC2/gD2 group with mock group, and P < 0.05 for comparing gC2/gD2 group with gD2 group; and for number of lesions, P < 0.001 for comparing gD2 or gC2/gD2 group with mock group, and P < 0.01 for comparing gD2 group with gC2/gD2 group. (B) Vaginal titers at days 1 to 14 postchallenge in naive animals, P < 0.01 for comparing gD2 or gC2/gD2 group with mock group, and P < 0.05 for comparing gD2 group with gC2/gD2 group. (C) Death and other disease markers in HSV-1-seropositive animals. For death, P < 0.05 for comparing gD2 or gC2/gD2 group with mock group; for hind limb weakness, P values were not significant; for urinary retention/hematuria, P < 0.001 for comparing gD2 or gC2/gD2 group with mock group; for guinea pigs with genital disease, P < 0.001 for comparing gD2 or gC2/gD2 group with mock group; for number of lesions, P < 0.001 for comparing gD2 or gC2/gD2 group with mock group. (D) Vaginal titers at days 1 to 14 postchallenge in HSV-1-seropositive animals (P < 0.01 for comparing gD2 or gC2/gD2 group with mock group, and P < 0.01 for comparing gD2 group with gC2/gD2 group. Results in panels B and D represent geometric mean titers ± SEM (n = 16 per group, except n = 15 in mock-immunized naive group).
FIG 8.
Study 2 HSV-2 DNA detection in vaginal secretions and DRG. (A) HSV-2 DNA detection in naive animals. For HSV-2 DNA-positive days, P < 0.05 for comparing gC2/gD2 and mock groups. (B) HSV-2 DNA copy number in DRG of naive animals (n = 5 for mock group, 15 for gD2 group, and 16 for gC2/gD2 group). (C) HSV-2 DNA detection in HSV-1-seropositive animals. For the number of DRG positive for HSV-2 DNA, P < 0.05 for comparing gD2 group with mock group, and P < 0.01 for comparing gC2/gD2 group with mock group. (D) HSV-2 DNA copy number in DRG of HSV-1-seropositive animals (n = 10 for mock group, 15 for gD2 group, and 16 for gC2/gD2 group).
DISCUSSION
Protection against genital disease in HSV-1-seropositive guinea pigs was reported previously using a replication-defective live virus, dl5-29, and the gD2 subunit antigen (45). Our results confirm and extend this important finding to include the impact of immunization on recurrent vaginal shedding of HSV-2 DNA. In study 1, we compared mock-immunized HSV-1-seropositive animals with animals immunized with gC2 and gD2 monovalent vaccines or a gC2/gD2 bivalent vaccine, while in study 2 we compared mock-immunized HSV-1-naive and HSV-1-seropositive animals with animals immunized with a gD2 monovalent or gC2/gD2 bivalent vaccine. In both experiments, the subunit antigen vaccines, including gD2 alone, reduced the incidence of hind limb paralysis, urinary retention, and genital disease in HSV-1-seropositive animals. These guinea pig results contrast with findings in human trials, in which gD2 given with MPL or alum failed to protect HSV-1-seropositive subjects against HSV-2 genital disease (18). The human trial in HSV-1-seropositive individuals was powered to detect a genital disease attack rate of 7%; however, the actual attack rate in the trial was 1.2% in HSV-1-seropositive women, which was significantly less than the 11.9% attack rate in HSV-1-seronegative women and may explain the negative result for seropositive subjects (18).
Other possible explanations for the discrepancy between the human trial in HSV-1-seropositive subjects and the animal studies include the possibility that immunity may have waned in the human trial, since the interval between immunization and challenge may have been longer than that in the animal study. In addition, the dose and virulence of strains acquired during infection in humans may differ from conditions in the guinea pig model. A final consideration is that the animal model may poorly predict outcomes in humans, in part because HSV has coevolved with humans and has adapted strategies to evade human immunity more effectively than guinea pig immunity. Nevertheless, the guinea pig genital infection model accurately reproduces many features of genital disease in humans, including the gross and microscopic appearance of genital lesions, establishment of latency in DRG, development of recurrent disease, subclinical genital shedding of HSV-2 DNA, and protection against genital disease by prior HSV-1 infection (45, 47, 52). Another disease manifestation that is reproduced in the guinea pig model is that subclinical vaginal shedding of HSV-2 DNA occurs in the absence of genital lesions, as reported here. In study 1, vaginal shedding was detected on 49/680 (7%) days, combining the results from all four HSV-1-seropositive groups. Forty of the 49 (82%) shedding days occurred when no genital lesions were detected, which reproduces an important feature of genital herpes virus infection in humans (6, 53).
HSV-1-seropositive animals had fewer genital lesions than HSV-1-naive animals, confirming that prior HSV-1 infection is protective. Although immunization reduced disease severity in HSV-1-seropositive animals, immunization did not reduce vaginal shedding of HSV-2 DNA in these animals. For example, in study 1, HSV-1-seropositive guinea pigs in the mock group had recurrent genital disease on 31% of days, compared with 1% of days for the gC2/gD2 group, yet HSV-2 DNA vaginal shedding was detected on 8% of days for the mock group and 6% of days for the gC2/gD2 group. The explanation for the discordance between protection against genital disease and vaginal shedding of HSV-2 DNA in HSV-1-seropositive animals is unclear. A role for CD8+ T cells in controlling HSV-2 in DRG and at peripheral sites has been proposed (54, 55). Recently, therapeutic immunization of guinea pigs with gD2 and ICP4 subunit antigens given with Matrix M-2 adjuvant was shown to induce humoral immune responses and CD4+ and CD8+ T-cell responses and to reduce recurrent genital lesions and recurrent vaginal shedding of HSV-2 DNA (56). Based on our prior flow cytometry results for mice, it seems likely that the gC2 and gD2 subunit antigens stimulated CD4+ T-cell responses in guinea pigs (21). Virus was present in vaginal secretions of immunized animals, which likely stimulated additional CD4+ and CD8+ T-cell responses; however, the immune boost from immunization and vaginal challenge was not sufficient to prevent vaginal shedding of HSV-2 DNA. Therefore, additional approaches to boost immunity, particularly CD8+ T-cell responses and mucosal immunity, may be required to reduce vaginal HSV-2 shedding in HSV-1-seropositive animals (56, 57). In contrast, immunization with gC2/gD2 was effective at reducing the number of days of vaginal shedding of HSV-2 DNA in naive animals in both the current study and our prior study (21).
Study 1 used the same antigens and adjuvants individually or combined to facilitate comparisons between groups. Comparing gC2/gD2 immunization with gD2 alone, the bivalent gC2/gD2 vaccine significantly outperformed gD2 alone in multiple immunologic and disease parameters in HSV-1-seropositive animals, as we previously reported for naive guinea pigs (21). In study 2, the CHO gD2 experiments were designed to evaluate gD2 concentrations and adjuvants similar to those used by others (50), which differed from the gD2 concentration and adjuvants used in Pichia experiments. Therefore, comparisons between the gD2 and gC2/gD2 groups are confounded in study 2; nevertheless, it is interesting that gC2/gD2 significantly outperformed gD2 alone in multiple variables, while in no assessment did gD2 significantly outperform gC2/gD2. Comparing studies 1 and 2 for immunogenicity and vaccine efficacy yielded many similar results; however, some differences were detected, which may be attributable to the adjuvants, antigen concentrations, or expression systems used to prepare immunogens in the two studies.
Immunization of naive and HSV-1-seropositive guinea pigs boosted neutralizing antibody titers to both HSV-1 and HSV-2. Complement enhanced the neutralization in gC2- or gC2/gD2-immunized animals in study 1, which can be attributed at least in part to the observation that gC antibodies block the ability of the glycoprotein to interact with C3b (20, 21, 33). Complement did not enhance antibody neutralization of gD2-immunized animals, which was expected, since gC1 and gC2 were available to inhibit complement activation. The HSV-1 neutralizing antibody titers in naive guinea pigs are of particular interest, since many first-time genital infections are caused by HSV-1 (19, 58). The high neutralizing titers to HSV-1 induced by immunization with gD2 in naive animals may explain the protection against HSV-1 genital disease reported for humans using the GSK gD2 vaccine (19). It will be interesting to evaluate whether immunization with gC2/gD2 protects against HSV-1 vaginal challenge.
The observation that subunit antigens were immunogenic and protective in HSV-1-seropositive animals is encouraging, since screening to exclude HSV-1-infected individuals prior to immunization may not be required. The gC2/gD2 combination was efficacious in preventing genital disease in naive and HSV-1-seropositive guinea pigs, although it reduced the number of days of vaginal shedding of HSV-2 DNA only in naive animals. Preventing genital disease was the primary endpoint in several large human vaccine trials (18, 19). Our results indicate that immunization with gC2/gD2 achieved this important endpoint and suggest that HSV-1-seropositive individuals may benefit from an HSV-2 vaccine.
ACKNOWLEDGMENTS
This work was supported by NIH grants RO1 HL 028220 and R21/R33 AI 105959 and by a grant from Merck and Co., Inc.
We thank Gary Cohen and Roselyn Eisenberg for providing purified baculovirus immunogens. We also thank Sarah Ratcliffe (Center for AIDS Research Epidemiology and Biostatistics Core) and Farida Shaheen (Center for AIDS Research Viral and Molecular Core) for expert advice on statistics and quantitative PCR, respectively. We thank Marc d'Anjou, Stefan Wildt, Erin Giaccone, and Rachel Xoconostle for supporting immunogen production and purification, and we thank members of Merck Laboratory Animal Resources, especially Patty Rebbeck and Cindy O'Neill, who assisted us with animal handling and serum collection.
Footnotes
Published ahead of print 27 November 2013
REFERENCES
- 1.Gupta R, Warren T, Wald A. 2007. Genital herpes. Lancet 370:2127–2137. 10.1016/S0140-6736(07)61908-4 [DOI] [PubMed] [Google Scholar]
- 2.Mertz KJ, Trees D, Levine WC, Lewis JS, Litchfield B, Pettus KS, Morse SA, St Louis ME, Weiss JB, Schwebke J, Dickes J, Kee R, Reynolds J, Hutcheson D, Green D, Dyer I, Richwald GA, Novotny J, Weisfuse I, Goldberg M, O'Donnell JA, Knaup R. 1998. Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 US cities. The Genital Ulcer Disease Surveillance Group. J. Infect. Dis. 178:1795–1798 [DOI] [PubMed] [Google Scholar]
- 3.Looker KJ, Garnett GP, Schmid GP. 2008. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull. World Health Organ. 86:805–812. 10.2471/BLT.07.046128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corey L, Adams HG, Brown ZA, Holmes KK. 1983. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann. Intern. Med. 98:958–972. 10.7326/0003-4819-98-6-958 [DOI] [PubMed] [Google Scholar]
- 5.Koutsky LA, Stevens CE, Holmes KK, Ashley RL, Kiviat NB, Critchlow CW, Corey L. 1992. Underdiagnosis of genital herpes by current clinical and viral-isolation procedures. N. Engl. J. Med. 326:1533–1539. 10.1056/NEJM199206043262305 [DOI] [PubMed] [Google Scholar]
- 6.Tronstein E, Johnston C, Huang ML, Selke S, Magaret A, Warren T, Corey L, Wald A. 2011. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 305:1441–1449. 10.1001/jama.2011.420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown ZA, Vontver LA, Benedetti J, Critchlow CW, Sells CJ, Berry S, Corey L. 1987. Effects on infants of a first episode of genital herpes during pregnancy. N. Engl. J. Med. 317:1246–1251. 10.1056/NEJM198711123172002 [DOI] [PubMed] [Google Scholar]
- 8.Brown ZA, Selke S, Zeh J, Kopelman J, Maslow A, Ashley RL, Watts DH, Berry S, Herd M, Corey L. 1997. The acquisition of herpes simplex virus during pregnancy. N. Engl. J. Med. 337:509–515. 10.1056/NEJM199708213370801 [DOI] [PubMed] [Google Scholar]
- 9.Kimberlin DW, Lin CY, Jacobs RF, Powell DA, Corey L, Gruber WC, Rathore M, Bradley JS, Diaz PS, Kumar M, Arvin AM, Gutierrez K, Shelton M, Weiner LB, Sleasman JW, de Sierra TM, Weller S, Soong SJ, Kiell J, Lakeman FD, Whitley RJ, National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group 2001. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics 108:230–238. 10.1542/peds.108.2.230 [DOI] [PubMed] [Google Scholar]
- 10.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73–83. 10.1097/01.aids.0000198081.09337.a7 [DOI] [PubMed] [Google Scholar]
- 11.Renzi C, Douglas JM, Jr, Foster M, Critchlow CW, Ashley-Morrow R, Buchbinder SP, Koblin BA, McKirnan DJ, Mayer KH, Celum CL. 2003. Herpes simplex virus type 2 infection as a risk factor for human immunodeficiency virus acquisition in men who have sex with men. J. Infect. Dis. 187:19–25. 10.1086/345867 [DOI] [PubMed] [Google Scholar]
- 12.Reynolds SJ, Risbud AR, Shepherd ME, Zenilman JM, Brookmeyer RS, Paranjape RS, Divekar AD, Gangakhedkar RR, Ghate MV, Bollinger RC, Mehendale SM. 2003. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J. Infect. Dis. 187:1513–1521. 10.1086/368357 [DOI] [PubMed] [Google Scholar]
- 13.Wald A, Link K. 2002. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J. Infect. Dis. 185:45–52. 10.1086/338231 [DOI] [PubMed] [Google Scholar]
- 14.Corey L, Wald A, Patel R, Sacks SL, Tyring SK, Warren T, Douglas JM, Jr, Paavonen J, Morrow RA, Beutner KR, Stratchounsky LS, Mertz G, Keene ON, Watson HA, Tait D, Vargas-Cortes M, Valacyclovir HSV Transmission Study Group 2004. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N. Engl. J. Med. 350:11–20. 10.1056/NEJMoa035144 [DOI] [PubMed] [Google Scholar]
- 15.Bryson YJ, Dillon M, Lovett M, Acuna G, Taylor S, Cherry JD, Johnson BL, Wiesmeier E, Growdon W, Creagh-Kirk T, Keeney R. 1983. Treatment of first episodes of genital herpes simplex virus infection with oral acyclovir. A randomized double-blind controlled trial in normal subjects. N. Engl. J. Med. 308:916–921 [DOI] [PubMed] [Google Scholar]
- 16.Sawtell NM, Bernstein DI, Stanberry LR. 1999. A temporal analysis of acyclovir inhibition of induced herpes simplex virus type 1 in vivo reactivation in the mouse trigeminal ganglia. J. Infect. Dis. 180:821–823. 10.1086/314958 [DOI] [PubMed] [Google Scholar]
- 17.Sawtell NM, Thompson RL, Stanberry LR, Bernstein DI. 2001. Early intervention with high-dose acyclovir treatment during primary herpes simplex virus infection reduces latency and subsequent reactivation in the nervous system in vivo. J. Infect. Dis. 184:964–971. 10.1086/323551 [DOI] [PubMed] [Google Scholar]
- 18.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapeliere P, Dubin G. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652–1661. 10.1056/NEJMoa011915 [DOI] [PubMed] [Google Scholar]
- 19.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD. 2012. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 366:34–43. 10.1056/NEJMoa1103151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Awasthi S, Lubinski JM, Friedman HM. 2009. Immunization with HSV-1 glycoprotein C prevents immune evasion from complement and enhances the efficacy of an HSV-1 glycoprotein D subunit vaccine. Vaccine 27:6845–6853. 10.1016/j.vaccine.2009.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awasthi S, Lubinski JM, Shaw CE, Barrett SM, Cai M, Wang F, Betts M, Kingsley S, Distefano DJ, Balliet JW, Flynn JA, Casimiro DR, Bryan JT, Friedman HM. 2011. Immunization with a vaccine combining herpes simplex virus 2 (HSV-2) glycoprotein C (gC) and gD subunits improves the protection of dorsal root ganglia in mice and reduces the frequency of recurrent vaginal shedding of HSV-2 DNA in guinea pigs compared to immunization with gD alone. J. Virol. 85:10472–10486. 10.1128/JVI.00849-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carroll MC. 2004. The complement system in regulation of adaptive immunity. Nat. Immunol. 5:981–986. 10.1038/ni1113 [DOI] [PubMed] [Google Scholar]
- 23.Kopf M, Abel B, Gallimore A, Carroll M, Bachmann MF. 2002. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat. Med. 8:373–378. 10.1038/nm0402-373 [DOI] [PubMed] [Google Scholar]
- 24.Fang C, Miwa T, Shen H, Song WC. 2007. Complement-dependent enhancement of CD8+ T cell immunity to lymphocytic choriomeningitis virus infection in decay-accelerating factor-deficient mice. J. Immunol. 179:3178–3186 [DOI] [PubMed] [Google Scholar]
- 25.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME. 2008. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 28:425–435. 10.1016/j.immuni.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kostavasili I, Sahu A, Friedman HM, Eisenberg RJ, Cohen GH, Lambris JD. 1997. Mechanism of complement inactivation by glycoprotein C of herpes simplex virus. J. Immunol. 158:1763–1771 [PubMed] [Google Scholar]
- 27.Eisenberg RJ, Ponce de Leon M, Friedman HM, Fries LF, Frank MM, Hastings JC, Cohen GH. 1987. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb. Pathog. 3:423–435. 10.1016/0882-4010(87)90012-X [DOI] [PubMed] [Google Scholar]
- 28.Seidel-Dugan C, Ponce de Leon M, Friedman HM, Fries LF, Frank MM, Cohen GH, Eisenberg RJ. 1988. C3b receptor activity on transfected cells expressing glycoprotein C of herpes simplex virus types 1 and 2. J. Virol. 62:4027–4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung SL, Peng C, Kostavasili I, Friedman HM, Lambris JD, Eisenberg RJ, Cohen GH. 1994. The interaction of glycoprotein C of herpes simplex virus types 1 and 2 with the alternative complement pathway. Virology 203:299–312. 10.1006/viro.1994.1488 [DOI] [PubMed] [Google Scholar]
- 30.Fries LF, Friedman HM, Cohen GH, Eisenberg RJ, Hammer CH, Frank MM. 1986. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J. Immunol. 137:1636–1641 [PubMed] [Google Scholar]
- 31.Rux AH, Lou H, Lambris JD, Friedman HM, Eisenberg RJ, Cohen GH. 2002. Kinetic analysis of glycoprotein C of herpes simplex virus types 1 and 2 binding to heparin, heparan sulfate, and complement component C3b. Virology 294:324–332. 10.1006/viro.2001.1326 [DOI] [PubMed] [Google Scholar]
- 32.Hook LM, Huang J, Jiang M, Hodinka R, Friedman HM. 2008. Blocking antibody access to neutralizing domains on glycoproteins involved in entry as a novel mechanism of immune evasion by herpes simplex virus type 1 glycoproteins C and E. J. Virol. 82:6935–6941. 10.1128/JVI.02599-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Judson KA, Lubinski JM, Jiang M, Chang Y, Eisenberg RJ, Cohen GH, Friedman HM. 2003. Blocking immune evasion as a novel approach for prevention and treatment of herpes simplex virus infection. J. Virol. 77:12639–12645. 10.1128/JVI.77.23.12639-12645.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JS, Robinson NJ. 2002. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J. Infect. Dis. 186:S3–S28. 10.1086/343739 [DOI] [PubMed] [Google Scholar]
- 35.Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296:964–973. 10.1001/jama.296.8.964 [DOI] [PubMed] [Google Scholar]
- 36.Tal-Singer R, Peng C, Ponce De Leon M, Abrams WR, Banfield BW, Tufaro F, Cohen GH, Eisenberg RJ. 1995. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J. Virol. 69:4471–4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tengvall S, Lundqvist A, Eisenberg RJ, Cohen GH, Harandi AM. 2006. Mucosal administration of CpG oligodeoxynucleotide elicits strong CC and CXC chemokine responses in the vagina and serves as a potent Th1-tilting adjuvant for recombinant gD2 protein vaccination against genital herpes. J. Virol. 80:5283–5291. 10.1128/JVI.02013-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canziani G, Zhang W, Cines D, Rux A, Willis S, Cohen G, Eisenberg R, Chaiken I. 1999. Exploring biomolecular recognition using optical biosensors. Methods 19:253–269. 10.1006/meth.1999.0855 [DOI] [PubMed] [Google Scholar]
- 39.Sisk WP, Bradley JD, Leipold RJ, Stoltzfus AM, Ponce de Leon M, Hilf M, Peng C, Cohen GH, Eisenberg RJ. 1994. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J. Virol. 68:766–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beck A, Cochet O, Wurch T. 2010. GlycoFi's technology to control the glycosylation of recombinant therapeutic proteins. Expert Opin. Drug Discov. 5:95–111. 10.1517/17460440903413504 [DOI] [PubMed] [Google Scholar]
- 41.Wildt S, Gerngross TU. 2005. The humanization of N-glycosylation pathways in yeast. Nat. Rev. Microbiol. 3:119–128. 10.1038/nrmicro1087 [DOI] [PubMed] [Google Scholar]
- 42.Potgieter TI, Cukan M, Drummond JE, Houston-Cummings NR, Jiang Y, Li F, Lynaugh H, Mallem M, McKelvey TW, Mitchell T, Nylen A, Rittenhour A, Stadheim TA, Zha D, d'Anjou M. 2009. Production of monoclonal antibodies by glycoengineered Pichia pastoris. J. Biotechnol. 139:318–325. 10.1016/j.jbiotec.2008.12.015 [DOI] [PubMed] [Google Scholar]
- 43.Choi BK, Bobrowicz P, Davidson RC, Hamilton SR, Kung DH, Li H, Miele RG, Nett JH, Wildt S, Gerngross TU. 2003. Use of combinatorial genetic libraries to humanize N-linked glycosylation in the yeast Pichia pastoris. Proc. Natl. Acad. Sci. U. S. A. 100:5022–5027. 10.1073/pnas.0931263100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shusta EV, Raines RT, Pluckthun A, Wittrup KD. 1998. Increasing the secretory capacity of Saccharomyces cerevisiae for production of single-chain antibody fragments. Nat. Biotechnol. 16:773–777. 10.1038/nbt0898-773 [DOI] [PubMed] [Google Scholar]
- 45.Hoshino Y, Pesnicak L, Dowdell KC, Burbelo PD, Knipe DM, Straus SE, Cohen JI. 2009. Protection from herpes simplex virus (HSV)-2 infection with replication-defective HSV-2 or glycoprotein D2 vaccines in HSV-1-seropositive and HSV-1-seronegative guinea pigs. J. Infect. Dis. 200:1088–1095. 10.1086/605645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao Y, Aldaz-Carroll L, Ortiz AM, Whitbeck JC, Alexander E, Lou H, Davis HL, Braciale TJ, Eisenberg RJ, Cohen GH, Isaacs SN. 2007. A protein-based smallpox vaccine protects mice from vaccinia and ectromelia virus challenges when given as a prime and single boost. Vaccine 25:1214–1224. 10.1016/j.vaccine.2006.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanberry LR, Kern ER, Richards JT, Abbott TM, Overall JC., Jr 1982. Genital herpes in guinea pigs: pathogenesis of the primary infection and description of recurrent disease. J. Infect. Dis. 146:397–404. 10.1093/infdis/146.3.397 [DOI] [PubMed] [Google Scholar]
- 48.Awasthi S, Zumbrun EE, Si H, Wang F, Shaw CE, Cai M, Lubinski JM, Barrett SM, Balliet JW, Flynn JA, Casimiro DR, Bryan JT, Friedman HM. 2012. Live attenuated herpes simplex virus 2 glycoprotein E deletion mutant as a vaccine candidate defective in neuronal spread. J. Virol. 86:4586–4598. 10.1128/JVI.07203-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morelli AB, Becher D, Koernig S, Silva A, Drane D, Maraskovsky E. 2012. Iscomatrix: a novel adjuvant for use in prophylactic and therapeutic vaccines against infectious diseases. J. Med. Microbiol. 61:935–943. 10.1099/jmm.0.040857-0 [DOI] [PubMed] [Google Scholar]
- 50.Bourne N, Bravo FJ, Francotte M, Bernstein DI, Myers MG, Slaoui M, Stanberry LR. 2003. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J. Infect. Dis. 187:542–549. 10.1086/374002 [DOI] [PubMed] [Google Scholar]
- 51.Hook LM, Lubinski JM, Jiang M, Pangburn MK, Friedman HM. 2006. Herpes simplex virus type 1 and 2 glycoprotein C prevents complement-mediated neutralization induced by natural immunoglobulin M antibody. J. Virol. 80:4038–4046. 10.1128/JVI.80.8.4038-4046.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langenberg AG, Corey L, Ashley RL, Leong WP, Straus SE. 1999. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. N. Engl. J. Med. 341:1432–1438 [DOI] [PubMed] [Google Scholar]
- 53.Wald A, Zeh J, Selke S, Ashley RL, Corey L. 1995. Virologic characteristics of subclinical and symptomatic genital herpes infections. N. Engl. J. Med. 333:770–775. 10.1056/NEJM199509213331205 [DOI] [PubMed] [Google Scholar]
- 54.Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, Wald A, Corey L. 2007. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med. 204:595–603. 10.1084/jem.20061792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. 2008. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 322:268–271. 10.1126/science.1164164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skoberne M, Cardin R, Lee A, Kazimirova A, Zielinski V, Garvie D, Lundberg A, Larson S, Bravo FJ, Bernstein DI, Flechtner JB, Long D. 2013. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in guinea pigs. J. Virol. 87:3930–3942. 10.1128/JVI.02745-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin H, Iwasaki A. 2012. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491:463–467. 10.1038/nature11522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wald A. 2006. Genital HSV-1 infections. Sex. Transm. Infect. 82:189–190. 10.1136/sti.2006.019935 [DOI] [PMC free article] [PubMed] [Google Scholar]