Abstract
DNA vaccines offer advantage over conventional vaccines, as they are safer to use, easier to produce, and able to induce humoral as well cellular immune responses. Unfortunately, no DNA vaccines have been licensed for human use for the difficulties in developing an efficient and safe in vivo gene delivery system. In vivo electroporation (EP)-based DNA delivery has attracted great attention for its potency to enhance cellular uptake of DNA vaccines and function as an adjuvant. Minicircle DNA (a new form of DNA containing only a gene expression cassette and lacking a backbone of bacterial plasmid DNA) is a powerful candidate of gene delivery in terms of improving the levels and the duration of transgene expression in vivo. In this study, as a novel vaccine delivery system, we combined in vivo EP and the minicircle DNA carrying a codon-optimized HIV-1 gag gene (minicircle-gag) to evaluate the immunogenicity of this system. We found that minicircle-gag conferred persistent and high levels of gag expression in vitro and in vivo. The use of EP delivery further increased minicircle-based gene expression. Moreover, when delivered by EP, minicircle-gag vaccination elicited a 2- to 3-fold increase in cellular immune response and a 1.5- to 3-fold augmentation of humoral immune responses compared with those elicited by a pVAX1-gag positive control. Increased immunogenicity of EP-assisted minicircle-gag may benefit from increasing local antigen expression, upregulating inflammatory genes, and recruiting immune cells. Collectively, in vivo EP of minicircle DNA functions as a novel vaccine platform that can enhance efficacy and immunogenicity of DNA vaccines.
INTRODUCTION
Vaccination, one of the greatest achievements of modern medicine, is the optimal solution for controlling the spread of major infectious diseases (1). However, the conventional vaccines cover only a small number of diseases, while other deadly and debilitating disorders, such as AIDS, hepatitis C, and malaria, still have no effective vaccines to be introduced into clinical use. DNA vaccines are third-generation vaccines and have evolved significantly over the last 20 years (2, 3). Compared to first-generation vaccines (whole-organism vaccines) and second-generation vaccines (subunit vaccines), DNA vaccines have more safety, flexibility, and stability and can readily elicit both humoral and broad cellular responses (4–6). The first human trial of a DNA-based vaccine is for the treatment of human HIV infection, and it was initiated almost 20 years ago (7). In fact, no DNA vaccines have yet been licensed for human use because of their low immunogenicity in large animals and in humans (8). Several approaches have been investigated to enhance vaccine immunogenicity, including plasmid design to increase antigen expression (9), the use of new delivery techniques (10), the addition of adjuvant (11), and the prime-boost strategy (12).
Minicircle DNA is a novel form of supercoiled DNA that contains only a gene expression cassette, without plasmid backbone sequences (e.g., the bacterial origin of replication and antibiotic resistance sequences) (13, 14). It is produced in Escherichia coli by att site-specific recombination catalyzed by the phage Φ31 integrase (15). Minicircle DNA has great advantages over conventional DNA vectors for biosafety and robust and persistent gene expression, which have been demonstrated in muscle, liver, heart, human carcinoma xenograft tumors, and iPS cells (16–21). The unique feature of minicircle DNA to enhance levels and duration of protein expression allows us to investigate whether minicircle DNA functions as an innovative vaccine delivery platform.
pVAX1 is a vector specifically designed to meet FDA regulations on the rapid development of DNA vaccines. The features of this vector allow high-copy-number replication in E. coli and high-level transient expression in most mammalian cells. Almost all known genes of HIV-1, including gag, vif, and nef, were inserted into this vector as DNA vaccines and were capable of inducing strong HIV-specific cellular and humoral immune responses in BALB/c mice (22, 23). Therefore, we used pVAX1 carrying the gene of interest as the positive control in our experiments.
It is clear that immunogenicity of DNA vaccines greatly depends upon the delivery methods used for immunization (24). Improvements in delivery methods are required to make DNA vaccines sufficiently effective. Several strategies, such as jet injection, gene guns, and in vivo electroporation (EP), are under investigation (8). Among these methods, in vivo EP has great potential and has been proven to enhance cellular uptake of DNA vaccines in muscle, skin, and tumors (25–30). Moreover, EP itself works as an adjuvant to induce significant immune responses by causing local inflammation and recruiting lymphocytes to the injection sites (31, 32). A wide range of independent clinical studies have proven the safety and efficacy of in vivo EP in patients (33, 34). The effects of in vivo EP on minicircle DNA vaccines have not been studied.
In this work, we present a novel vaccine delivery method to enhance HIV-1-specific immune responses using in vivo EP delivery of minicircle DNA carrying a codon-optimized gag gene (minicircle-gag). We show that minicircle DNA confers higher levels and longer duration of antigen expression than pVAX1 DNA. When minicircle DNA was delivered by EP, its immunogenicity significantly enhanced. The high efficiency of EP-assisted minicircle DNA may be explained in part by increasing local antigen expression, upregulating inflammatory genes, and recruiting immune cells.
MATERIALS AND METHODS
Reagents, plasmids, and strains.
HIV-1 gag peptides P1 (AMQMLKETI), P2 (TTSTLQEQI) and P3 (EPFRDYVDRF) and the control peptide (IGPGRAFYAR) were synthesized by SBS Genetech Co., Ltd. (Beijing, China), at a purity of >95%. Plasmid p2ΦC31 and minicircle producer strain ZYCY10P3S2T were provided as a gift by Zhiying Chen (Shenzhen Institute of Advanced Technology, Guangdong, China).
Immunization of mice.
BALB/c mice were housed at the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, according to protocols approved by the Institutional Animal Care and Use Committee. For immunization, 6- to 8-week-old female BALB/c mice were given intradermal (i.d.) injection with or without EP, intramuscular (i.m.) injection with or without EP, or hydrodynamic delivery (HD) alone. Hydrodynamic DNA was administered as previously described (35). For i.m. or i.d. injection with EP, the minicircle DNA in 50 μl of phosphate-buffered saline (PBS) was administered by i.m. or i.d. injection. Immediately after i.m. or i.d. injection, two silver needles 6 mm apart were inserted over the injection site, and electric pulses were applied (6 pulses, 100 V/cm, 50 ms) using the TERESA-EPI medicine delivery system (Terasha Healthcare Sci-Tech, Shanghai, China). The procedure was repeated up to three times at 3-week internals.
Western blot analysis.
The samples were subjected to SDS-PAGE and Western blot analysis using specific antibodies (Abs). The expression of gag was determined using anti-p24 antibody (Santa Cruz Biotech, Santa Cruz, CA). The activation of extracellular signal-regulated kinase (ERK) and Jun N-terminal protein kinase (JNK) was determined using anti-phospho-ERK and anti-phospho-JNK antibodies (Cell Signaling, Beverly, MA). IκBα was assayed using anti-IκBα antibody (Santa Cruz Biotech).
IFN-γ ELISPOT assays.
Enzyme-linked immunosorbent spot (ELISPOT) assays for gamma interferon (IFN-γ) release were performed using ELISPOT kits from BD-Pharmingen (San Diego, CA). The 96-well ELISPOT plate was coated with diluted purified anti-mouse IFN-γ monoclonal antibody in PBS overnight at 4°C. The plates were then blocked, and 1 × 105 fresh splenocytes were added into each well and incubated with H-2d-restricted cytotoxic T lymphocyte (CTL) epitope peptides (the final concentration of each peptide was 1 μM)—P1 (AMQMLKETI), P2 (TTSTLQEQI), and P3 (EPFRDYVDRF)—for 20 h in a 37°C incubator (5% CO2). Concanavalin A (ConA; 2.5 μg/ml) was used as a positive control, and an irrelevant peptide (IGPGRAFYAR) was used as a negative control. The plates were washed and incubated with diluted biotinylated anti-mouse IFN-γ antibody for 2 h at 37°C. After a washing with PBS-Tween (PBST), the plates were incubated with diluted streptavidin-horseradish peroxidase (HRP) for 1 h at 37°C. The spots were developed by adding 100 μl of 3′-amino-9-ethylcarbazole (AEC) substrate and analyzed with an Immunospot reader (CTL, Cleveland, OH).
ELISA.
The serum antibodies against HIV-1 gag were assessed using an enzyme-linked immunosorbent assay (ELISA) as previously described (31). The endpoint antibody titers were defined as the last reciprocal serial serum dilution at which the absorbance at 450 nm was greater than two times the background signal detected.
Intracellular cytokine staining.
Freshly isolated splenocytes were plated into round-bottom 96-well plates (2 × 106 cells per well) and incubated with either stimulation peptides (HIV-1 gag peptides) or negative peptides and 3 μg/ml of brefeldin A. The surface markers were stained with peridinin chlorophyll protein (PerCP)-Cy5.5-labeled anti-mouse CD3, fluorescein isothiocyanate (FITC)-labeled anti-mouse CD8, and phycoerythrin (PE)-conjugated anti-mouse CD4. The internal molecules were stained with allophycocyanin (APC)-labeled anti-mouse IFN-γ as previously described (36). Stained samples were analyzed using BD FACSCalibur.
Histology and immunohistochemical analysis.
BALB/c mice received minicircle DNA, pVAX1, or PBS by i.m. injection with or without in vivo EP. Four days after injection, the mice were sacrificed, and the muscle tissues were processed for histological analysis. The tissues were embedded in paraffin, sectioned at 7 μm, and stained with hematoxylin and eosin (H&E). For immunohistochemical staining, the muscle samples were embedded in OCT. The serial cross sections, measuring 7 μm in thickness, were prepared and stained with antibodies specific for Gr-1, CD11b, F4/80, CD4, CD8, and B220 (BD Biosciences, San Jose, CA).
Quantitative RT-PCR analysis.
The mice were treated with or without in vivo EP and sacrificed at 12 h postinjection. Immediately after the mice were sacrificed, the muscle tissue was removed, the total RNA was extracted to perform quantitative reverse transcription-PCR (RT-PCR) analyses with a SYBR green real-time PCR kit according to the manufacturer's instructions (Applied Biosystems, Life Technologies Corporation, Carlsbad, CA).
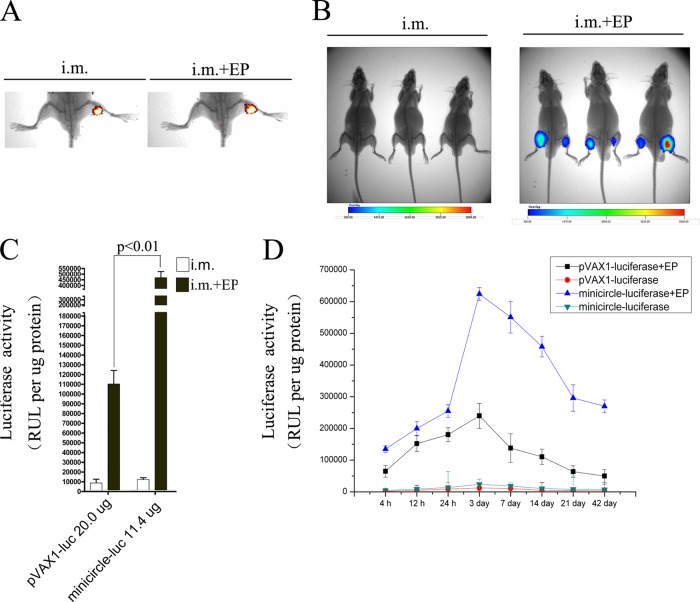
In vivo imaging of minicircle distribution and minicircle-mediated luciferase expression.
For in vivo tracking of the distribution of minicircle DNA, the mice were injected with 20 μg of ethidium monoazide (EMA)-labeled minicircle-luciferase with or without in vivo EP. Ten minutes later, the mice were imaged using the In Vivo Imaging System FX Pro (Carestream Molecular Imaging). For in vivo imaging of minicircle-mediated luciferase reporter gene expression, the mice were injected with 20 μg of minicircle-luciferase with or without in vivo EP. At 7 days postinjection, the mice were injected intraperitoneally with d-luciferin potassium salt in PBS and imaged using an In Vivo Imaging System FX Pro 5 to 10 min later.
Statistical analysis.
The data are presented as means ± standard deviations (SDs). Statistical comparisons were performed using SPSS 11.5 (SPSS, Inc., Chicago, IL). Parametrical data were compared using Student's t test. One-way analysis of variance (ANOVA) was used to determine the difference between independent groups. The differences between the variants were considered to be statistically significant at a P value of <0.05.
RESULTS
Minicircle DNA mediates a higher level of gag expression than pVAX1 in vitro and in vivo.
We cloned the codon-optimized HIV-1 gag gene into the p2ΦC31 vector to obtain the construct p2ΦC31-gag. This parent plasmid was then transformed into the novel minicircle producer strain ZYCY10P3S2T to produce high-quality minicircle-gag (Fig. 1A) (37). pVAX1-gag was constructed as the positive control (Fig. 1B). Each vector contains the same sequences of gag expression cassettes. To evaluate the expression and immune potency of minicircle DNA vaccines, we transfected mouse C2C12 cells with p2ΦC31-gag (12 kb), minicircle-gag (2.6 kb), or pVAX1-gag (4.5 kb) (Fig. 1). As expected, higher levels of gag were produced in minicircle-gag-transfected cells than in those transfected with p2ΦC31-gag (9.9-fold at 48 h and 6.5-fold at 96 h) or pVAX1-gag (1.6-fold at 48 h and 1.4-fold at 96 h) (Fig. 2A and B). To evaluate the expression of gag in vivo further, BALB/c mice were intramuscularly injected with p2ΦC31-gag, minicircle-gag, or pVAX1-gag. Seven days later, the mice were sacrificed and the muscle tissue samples were subjected to Western blot analysis. The gag expression mediated by minicircle DNA was 3-fold higher than that mediated by p2ΦC31 and 1.3-fold higher than that mediated by pVAX1 (Fig. 2C and D). Together, these results indicate that minicircle DNA achieves higher expression efficiency than conventional plasmids.
FIG 1.
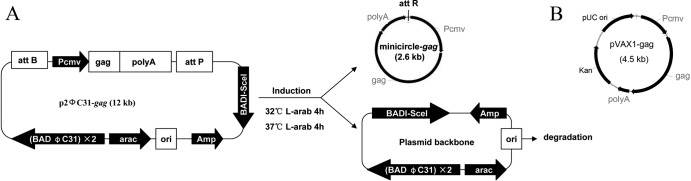
Production of minicircle-gag. (A) A codon-optimized gag gene was inserted into the p2ΦC31 vector. This parent plasmid was then transformed into E. coli strain ZYCY10P3S2T. After the induction of l-arabinose, minicircle-gag was produced and purified. Pcmv, immediate-early human cytomegalovirus enhancer/promoter; polyA, bovine growth factor polyadenylation signal; Ampr, ampicillin resistance gene; ori, pUC origin of DNA replication; BAD, araBAD promoter; araC, araC repressor; attB, bacterial attachment site; attP, phage attachment site; attR, right hybrid sequence; I-SceI, I-Sce I gene. (B) Construct of pVAX1-gag. Each vector carries the same gag expression cassette (Pcmv-gag-polyA).
FIG 2.
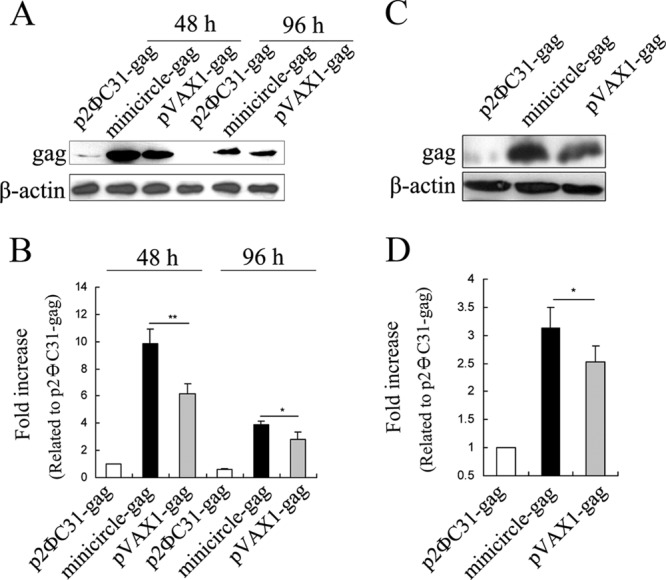
Determination of HIV-1 gag gene expression mediated by minicircle DNA. (A) C2C12 cells were transfected with 2.0 μg of p2ΦC31-gag, 2.0 μg of pVAX1-gag, or 1.14 μg of minicircle-gag (equimolar with pVAX1-gag) and harvested at 48 h and 96 h posttransfection, respectively. Expression of gag was monitored by Western blotting. (C) BALB/c mice were intramuscularly injected with 20.0 μg of p2ΦC31-gag, 20.0 μg of pVAX1-gag, or 11.4 μg of minicircle-gag (equimolar with pVAX1-gag). The samples were harvested 7 days later for Western blot analysis. (B and D) The histograms indicate the levels of the protein determined from 3 independent experiments expressed as the fold change relative to that in the p2ΦC31-gag control after normalization to β-actin. Values are means ± SDs. *, P < 0.05 versus the pVAX1-gag control.
Optimization of in vivo minicircle-gag delivery.
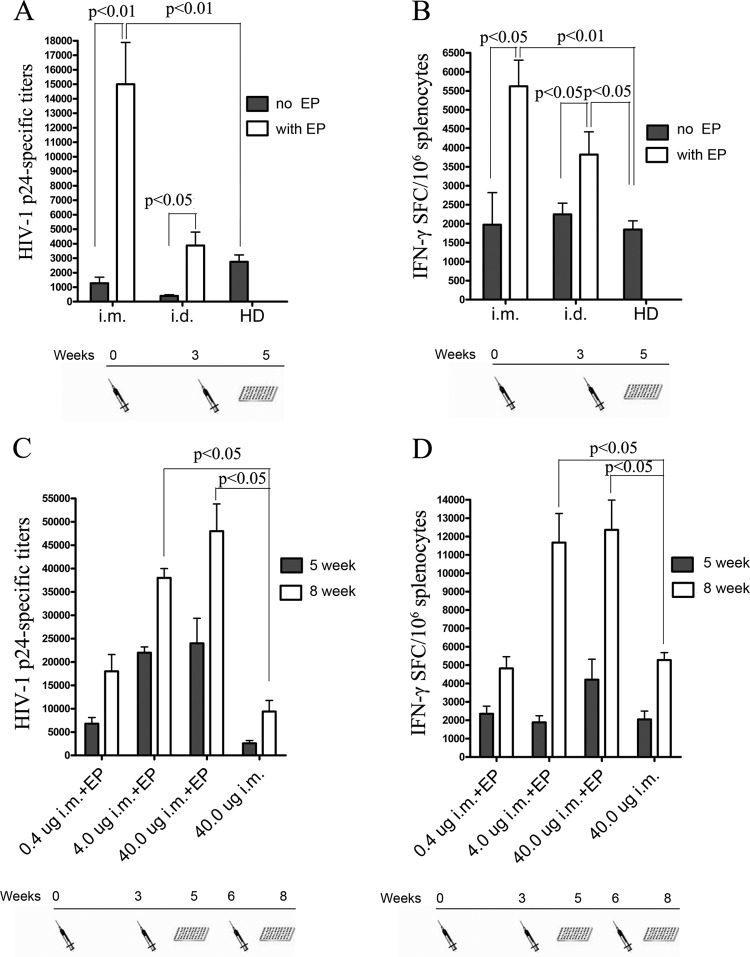
Because the immunogenicity of DNA vaccines greatly depends upon the delivery methods used for immunization (24), we investigated the immunogenicity of different routes of minicircle-gag delivery. Groups of 10 mice were given 20 μg of minicircle-gag delivered by intramuscular (i.m.) injection with or without in vivo EP, intradermal (i.d.) injection with or without in vivo EP, or hydrodynamic delivery (HD) alone. gag-specific humoral and cellular immune responses were assessed by p24-specific ELISAs and IFN-γ ELISPOT assays, respectively. The results in Fig. 3A and B clearly showed that in vivo EP enhanced the immunogenicity of minicircle-gag delivered by i.m. or i.d. injection. In vivo EP-assisted i.m. injection of minicircle-gag induced a 12.3-fold increase in p24-specific antibody titers (P < 0.05) and a 2.8-fold increase in IFN-γ-secreting cytotoxic T lymphocytes (CTLs) (P < 0.05) compared with i.m. injection alone at weeks 5 after the prime immunization. In vivo EP-assisted i.d. injection stimulated a 12-fold increase in p24-specific antibody titers (P < 0.05) and a 1.7-fold increase in the IFN-γ-secreting CTLs (P < 0.05) compared with those obtained with i.d. injection alone. Also, EP-based delivery showed a higher magnitude of immune response than HD. Notably, i.m. injection with in vivo EP induced the strongest humoral and cellular immune responses of the delivery methods used in this study, and hence, this strategy was selected for the following study.
FIG 3.
Detection of gag-specific humoral and cellular immune responses following immunization with minicircle-gag by different delivery strategies. Groups of five BALB/c mice were immunized with 20 μg of minicircle-gag at weeks 0 and 3 by i.m. injection with or without EP, i.d. injection with or without EP, or HD alone. At 5 weeks after the prime immunization, gag-specific humoral and cellular immune responses were assessed by p24-specific ELISAs (A) and gag epitope peptide IFN-γ ELISPOT assays (B). (C and D) gag-specific humoral and cellular immune responses induced by different doses of minicircle-gag administered i.m. with or without EP at 5 weeks and 8 weeks after the prime immunization. P values of <0.05 were considered significant and were determined using one-way ANOVA followed by Tukey multiple-comparison tests.
We investigated the dose response to minicircle-gag delivered with in vivo EP-assisted i.m. injection. BALB/c mice were immunized with 0.4, 4.0, or 40 μg of minicircle-gag by i.m. injection with EP at weeks 0, 3, and 6. Groups of 10 mice given 40 μg of minicircle-gag by i.m. injection not receiving in vivo EP were used as controls. With respect to p24 Ab titers, a clear dose-dependent response relationship was observed at weeks 8 (Fig. 3C). More importantly, even the lowest minicircle dose (0.4 μg) delivered i.m. with EP resulted in the induction of high anti-p24 titers, comparable to those elicited by a 100-fold-higher dose of minicircle-gag (40 μg) delivered by i.m. injection alone (P < 0.05). These results suggest that EP-based delivery dramatically enhanced the dose efficiency of the DNA vaccine. Similar results were also obtained in the cell-mediated immune response assays. The 0.4-μg dose given with in vivo EP induced the same mean peptide-specific CTL response levels as the 40-μg dose delivered without in vivo EP. There was no significant difference in the levels of CTL response between the groups receiving 4-μg or 40-μg doses of minicircle-gag when using in vivo EP (Fig. 3D). Taken together, the results show that in vivo EP dramatically enhances the immunogenicity of minicircle-gag, supporting the benefits of the adjuvant effects mediated by in vivo EP.
Comparison of the immuogenicities of in vivo EP-assisted minicircle-gag and conventional plasmid vector.
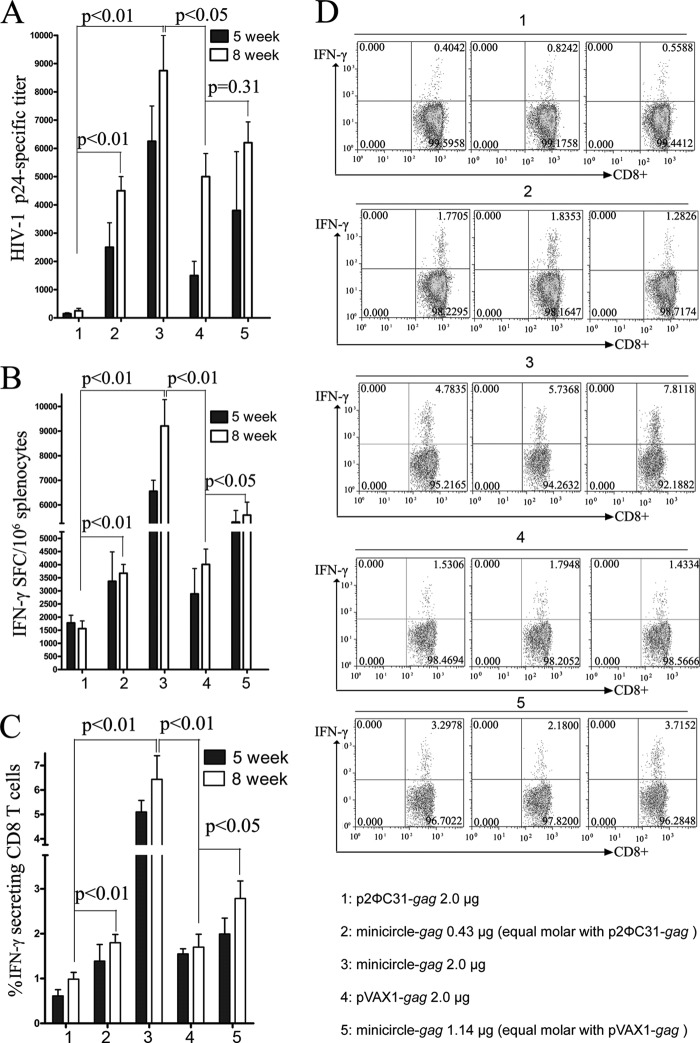
To determine if the enhanced expression of minicircle-encoded gag corresponded to increased immunogenicity in vivo, we immunized BALB/c mice with equal weights or equimolar amounts of p2ΦC31-gag, minicircle-gag, or pVAX1-gag via in vivo EP. As shown in Fig. 4A, mice immunized with 2 μg of minicircle-gag (bars 3) showed significantly higher anti-p24 titers than the equal weight of p2ΦC31-gag group (15 to 16-fold [bars 1]) or the equal weight of pVAX1-gag group (1.5 to 2.1-fold [bars 4]) at weeks 8. The anti-p24 titers were also comparable between mice immunized with equimolar amounts of p2ΦC31-gag and minicircle-gag. The latter is statistically comparable to the 9-fold increase in p24 titers at weeks 8 (Fig. 4A, bars 2 and 1). Equimolar amounts of minicircle-gag also showed higher anti-p24 titers than equimolar amounts of pVAX1-gag at weeks 5 (bars 4 and 5).
FIG 4.
Comparison of the immunogenicity of in vivo EP-assisted minicircle-gag, p2ΦC31-gag, and pVAX1-gag. Groups of 10 BALB/c mice were immunized with p2ΦC31-gag, minicircle-gag, or pVAX1-gag at the indicated doses two or three times at 3-week intervals i.m. with in vivo EP. The immunized mice were sacrificed at 5 weeks and 8 weeks after the prime administration. The humoral and cellular immune responses were evaluated by p24-specific ELISA (A), gag epitope peptide IFN-γ ELISPOT assays (B), and intracellular cytokine staining assays (C and D). P values of <0.05 were considered significant using one-way ANOVA followed by Tukey multiple-comparison tests.
The levels of T cell responses were determined by IFN-γ ELISPOT assay and intracellular cytokine staining. With respect to the IFN-γ-producing spot-forming cells (SFCs) and CD8+ T cells, the results were consistent with observations from p24 Ab titers. Compared to those obtained with p2ΦC31-gag, an equal weight of minicircle-gag elicited a 4- to 6-fold increase in IFN-γ-producing SFCs and CD8+ T cells at weeks 8 (Fig. 4B to D, bars 3 and 1). Equimolar amounts of minicircle-gag elicited a 2- to 3-fold increase in T cell responses (bars 2 and 1). Compared to those obtained with pVAX1-gag, an equal weight of minicircle-gag elicited a 2.5- to 3-fold increase in IFN-γ-producing SFCs and CD8+ T cells at weeks 8 (Fig. 4B to D, lanes 3 and 4). Equimolar amounts of minicircle-gag elicited a 1.5- to 2.1-fold increase in T cell responses (lanes 5 and 4). Thus, it is clear that EP delivered minicircle-gag exhibited better immunogenicity than pVAX1-gag, which is specifically designed and licensed by the FDA for the development of DNA vaccines.
In vivo EP facilitates inflammatory cell infiltration at the injection site.
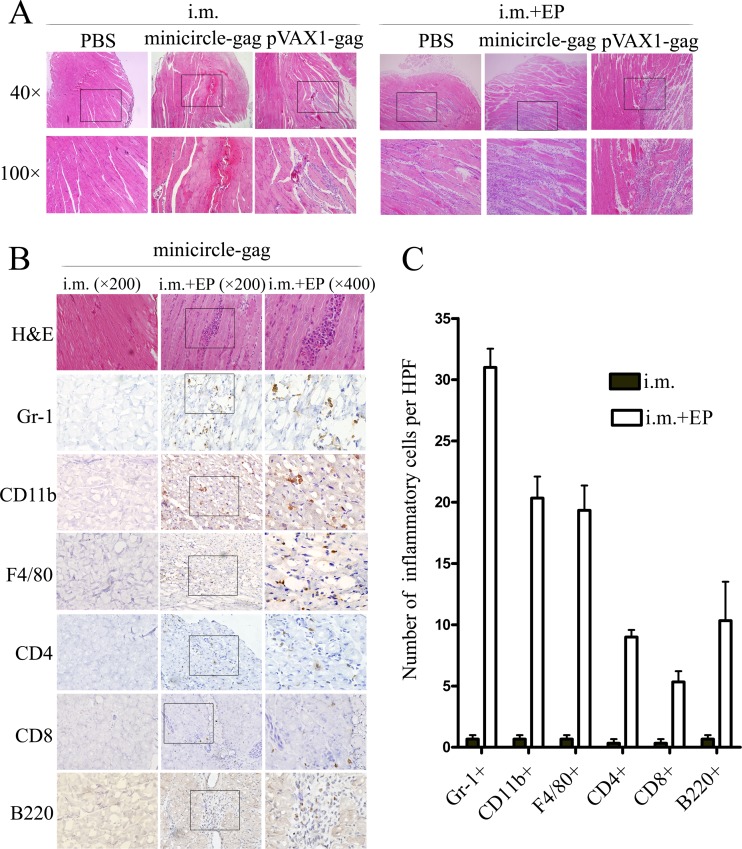
To determine the mechanism by which in vivo EP of minicircle induced a higher level of immune responses, we investigated the effects of this strategy on the local inflammatory response at the site of injection. BALB/c mice were injected with PBS, minicircle-gag, or pVAX1-gag i.m. with or without in vivo EP. Four days later, the muscles were obtained for hematoxylin and eosin (H&E) staining and immunohistochemical analysis. As expected, a local inflammatory response was not detected at the injection site following i.m. injection of PBS, minicircle-gag, or pVAX1-gag. In contrast, mice from EP-treated groups showed moderate muscle degeneration and pronounced mononuclear cell infiltration, clearly showing that in vivo EP causes a local tissue damage and inflammatory response (Fig. 5A). There were no significant differences in the degree of local inflammation in mice treated with EP of minicircle-gag or pVAX1-gag. A systematic evaluation of the inflammatory infiltrate at the injected site revealed that the infiltrates contained a large proportion of Gr-1+ granulocytes, CD11b+ macrophages or dendritic cells, F4/80+ macrophages, CD4+ and CD8+ T lymphocytes, and B220+ B lymphocytes (Fig. 5B and C). The infiltration of these cells may additively assist in the priming of immune responses.
FIG 5.
Immune cell infiltration at the i.m. injection site induced by EP. Groups of five BALB/c mice were injected with PBS, 20 μg of minicircle-gag, or 20 μg of pVAX1-gag i.m. with or without EP. Four days later, the injected muscles were obtained for H&E staining (A) and immunohistochemical analysis (B) with the antibodies as indicated. (C) The mean numbers of inflammatory cells per high-power field (HPF) (×200). Data shown are representative of three independent experiments.
In vivo EP results in local activation of JNK, ERK, and NF-κB pathways and upregulation of immune regulatory genes.
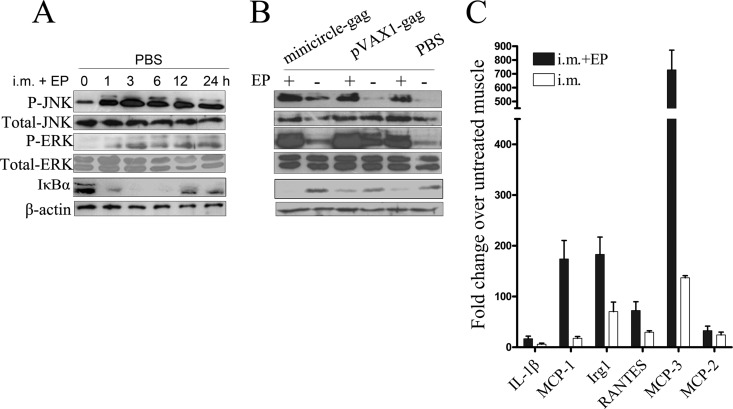
To explore the potential mechanisms by which EP causes inflammation and recruits macrophages and lymphocytes to the injection site, we examined the induction of inflammatory markers, including JNK, ERK, and NF-κB pathways, after in vivo EP treatment. BALB/c mice were injected with PBS i.m. with EP, and the injection site were surgically removed 0, 3, 6, 12, and 24 h after administration. The phosphorylation of the key mitogen-activated protein kinase (MAPK) family members and the degradation of IκBα, an inhibitor of the NF-κB pathway, were analyzed by Western blot analysis. As shown in Fig. 6A, in vivo EP rapidly induced the phosphorylation of JNK and ERK1/2 but not that of p38 (data not shown), which reached the highest level at 3 h postadministration. In vivo EP also significantly reduced IκBα protein accumulation, which, in turn, led to NF-κB activation. Moreover, the results in Fig. 6B suggested that the phosphorylation of JNK and ERK1/2 and degradation of IκBα were an EP-dependent but plasmid DNA-independent response.
FIG 6.
Effects of in vivo EP on the activation of JNK, ERK, and NF-κB pathways and the expression of immune regulatory genes. (A) BALB/c mice were injected with PBS i.m., with in vivo EP. Animals were sacrificed at the indicated time points and the involved muscles were removed. Total cell lysates were prepared and analyzed for p-JNK, p-ERK1/2, and IκBα by Western blotting. Total JNK, ERK1/2, and β-actin levels were used as loading controls. (B) BALB/c mice were injected with 20 μg of minicircle-gag, 20 μg of pVAX1-gag, or PBS i.m., with or without in vivo EP. At 6 h postinjection, the involved muscles were removed and total cell lysates were prepared for Western blotting with the antibodies indicated. (C) BALB/c mice were injected with PBS i.m. with or without in vivo EP. At 12 h postdelivery, the injected muscles were obtained for real-time PCR analysis. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA values were used for normalization. Each sample was run in triplicates. Bars represent means ± SDs of three independent experiments.
A quantitative PCR (qPCR) analysis of the MAPK- and NF-κB-directed inflammatory cytokines was performed, including interleukin 1β (IL-1β), immunoresponsive gene 1 (Irg1), regulated upon activation normal T-cell expression and secreted (RANTES), monocyte chemotactic protein 1 (MCP-1), monocyte chemotactic protein 2 (MCP-2), and monocyte chemotactic protein 3 (MCP-3). As shown in Fig. 6C, the expression of these genes was significantly increased in EP-treated mice. Together, the results show that in vivo EP, regardless of the DNA construct used, is capable of facilitating the recruitment of inflammatory cells at the injection site by activating JNK, ERK1/2, and NF-κB pathways, thereby promoting the expression of inflammatory cytokine-related genes. These factors are important for the priming of immune responses.
In vivo EP enhances the distribution and expression of minicircle DNA in muscle.
Previous studies have demonstrated that EP-based delivery dramatically enhances the biodistribution and antigen expression of conventional DNA vaccines (31, 32, 38, 39). We wanted to detect if the same was true for EP-delivered minicircle DNA. Minicircle DNA was labeled with EMA and injected into mouse muscles by i.m. with or without EP. As determined by in vivo imaging, the combination of in vivo EP facilitated the distribution of minicircle DNA in muscle tissue (Fig. 7A). We then evaluated the effect of EP-based minicircle delivery on protein expression. Minicircle carrying the firefly luciferase reporter gene was delivered into mice by i.m. injection with or without in vivo EP. At 7 days postinjection, the expression of luciferase reporter was analyzed using in vivo imaging. The signal intensity from the EP-treated group was significantly stronger than that from the group receiving i.m. injection alone, suggesting that EP increases minicircle-mediated protein expression sufficiently (Fig. 7B).
FIG 7.
Detection of the biodistribution and expression of minicircle DNA delivered i.m. with or without in vivo EP at the injection site. (A) BALB/c mice were injected with 20 μg of EMA-labeled minicircle-luciferase with or without EP. Ten minutes later, the biodistribution of minicircle DNA was determined using an in vivo imager. (B) BALB/c mice were injected with 20 μg of minicircle-luciferase with or without EP. Seven days later, the expression of luciferase was measured in vivo using an in vivo image system. (C) BALB/c mice were injected with 20 μg of pVAX1-luciferase, or 11.4 μg of minicircle-luciferase (equimolar with pVAX1-luciferase) i.m. with or without EP. At 7 days postinjection, the involved muscles were surgically removed; total cell lysates were prepared and analyzed with the luciferase assays. (D) BALB/c mice were injected as for panel C. The involved muscles were surgically removed at the indicated time points; total cell lysates were prepared and analyzed with the luciferase assays.
To further evaluate the expression levels of minicircle DNA compared with conventional pVAX1 vector when delivered by EP, luciferase activity in muscle tissues was determined in mice injected with 20 μg of pVAX1-luciferase or 11.4 μg of minicircle-luciferase (equimolar with pVAX1-luciferase). As shown in Fig. 7C, in vivo EP increased the luciferase activity of minicircle DNA and pVAX1 vector 39.5-fold and 13.4-fold, respectively, compared with i.m. injection alone. Furthermore, equimolar amounts of minicircle-luciferase elicited a 4-fold increase in luciferase activity over that elicited by equimolar amounts of pVAX1-luciferase at days 7 after EP delivery. To test whether in vivo EP promoted long-term persistence of antigen expression at the site of injection, luciferase activity was analyzed from 4 h to 42 days after injection. In the in vivo EP-treated pVAX1-luciferase group, luciferase activity peaked at 3 days and was much decreased by day 21. EP-delivered minicircle increased luciferase activity significantly. It is noteworthy that minicircle-luciferase activity on day 42 was comparable with the pVAX1-luciferase peak response on day 3 (Fig. 7D). Together, these data indicate that in vivo EP promotes longer and higher levels of reporter genes expression than does i.m. injection alone. In vivo EP of minicircle DNA presented more sustained antigen expression than pVAX1 vector.
DISCUSSION
Due to its safety, flexibility, stability, and cost-effectiveness, DNA vaccination has entered into a variety of human clinical trials (7, 40, 41). Although a proof of concept was demonstrated in a recent trial conducted in Thailand, significant scientific obstacles remain in improving the antigen expression and developing an efficient and safe in vivo gene delivery system (42–44). In this work, we present a novel vaccine delivery system by in vivo EP delivery of minicircle DNA carrying a codon-optimized gag gene. Our data indicate that minicircle DNA is more efficient in mediating antigen expression than conventional plasmid DNA in vitro and in vivo. EP-delivered minicircle-gag vaccination efficiently induces gag-specific humoral and cellular responses. The enhanced immunogenicity of EP-assisted minicircle DNA vaccination is most likely to benefit from increasing local cellular uptake, augmenting antigen expression, recruiting immune cells, and upregulating inflammatory genes.
The use of DNA vectors represents an attractive platform for gene delivery in vivo (8, 45). Conventional plasmid DNA platforms suffer from low transgene expression in situ (46, 47). Concerns have been raised regarding the bacterial backbone sequences including the bacterial origin of replication and antibiotic resistance genes constructed in the plasmid DNA. Antibiotic resistance markers have been shown to hinder transgene expression in vivo (48, 49). The bacterial backbone sequence can cause transgene silencing via covalent attachment to the expression cassette (50). In addition, the bacterial backbone sequence may cause undesirable immune responses (51, 52). Minicircle DNA may minimize the adverse effects described. First, minicircle DNA is devoid of essentially all prokaryotic sequence elements. This approach therefore avoids transgene silencing and increases the safety of DNA vaccines. Second, the lack of these backbone sequences reduces minicircle size significantly. The small size improves the transfection efficiency of DNA and entry into the nucleus. Moreover, Molnar et al. have shown that plasmid size has an inverse relationship with the level of transgene expression (53). Third, minicircle DNA vectors achieve sustained expression reflected by active chromatin and the transcriptional level (54). The robust and persistent gene expression delivered by minicircle DNA has been shown in vitro and in vivo (14, 15, 20, 37, 55). Because of this, we hypothesized that minicircle DNA may be an attractive platform for DNA vaccine. We constructed minicircle DNA carrying HIV-1 gag to assess the potential of minicircle DNA as a vaccine vector. Our data showed that minicircle DNA is more efficient in mediating HIV-1 gag expression than the parent plasmid p2ΦC31 or the licensed DNA vaccine vector pVAX1 in vitro and in vivo.
Despite the advantages described, a weakness of minicircle DNA is low immnunogenicity as a vaccine vector, since most of the unmethylated CpG motifs carried in the conventional plasmid backbone have been eliminated, which could act as an intrinsic adjuvant for DNA vaccines (56, 57). It is clear that the immunogenicity of DNA vaccines greatly depends upon the delivery methods used for immunization (24, 58). Therefore, we tested different routes of administration, including i.m. injection, i.m. injection with EP, i.d. injection, i.d. injection with EP, and hydrodynamic delivery (HD). HD of HIV-1 DNA vaccine to the liver has been shown to induce high and long-lasting humoral immune responses (35). In our experiment, HD delivery of minicircle-gag did induce higher anti-p24 titers than i.m. or i.d. injection alone. But no difference in CTL response was observed between the HD group and i.m. group or i.d. group. Alternatively, EP-assisted i.m. injection induced the strongest humoral and cellular immune responses of all the delivery methods used in this study. It is clear from the dose-response experiments that even the lowest minicircle dose (0.4 μg) delivered i.m. with EP resulted in the induction of high anti-p24 titers, comparable to those elicited by a 100-fold-higher dose of minicircle (40 μg) delivered by i.m. alone (P < 0.05). Similar results were also obtained in the cellular immune response assays. Collectively, the results show that EP is a more effective means of administering minicircle DNA.
EP-based delivery has been used for humans and animals to enhance cellular uptake of both drugs and DNA plasmids (59, 60). EP administration transiently opens pores in the myocyte membranes, allowing plasmid entry into the nucleus and expression (61). This was also true for the delivery of minicircle DNA, as indicated in our experiments showing that EP enhanced the biodistribution and expression of minicircle DNA in the injection site. Another major benefit of EP is that it works as an adjuvant. “Danger signals” released from the moderate tissue injury recruit antigen-presenting cells to the injection site, inducing a significant immune response (62). The detailed mechanisms need to be further characterized. In this study, we demonstrated that inflammatory cells, including Gr-1+ granulocytes, CD11b+ macrophages or dendritic cells, F4/80+ macrophages, CD4+ and CD8+ T lymphocytes, and B220+ B lymphocytes, can be recruited after EP administration. The antigen-presenting cell recruitment most likely is triggered by the activation of JNK, ERK1/2, and NF-κB pathways and upregulation of critical inflammatory genes (MCP-1, MCP-2, MCP-3, RANTES, IL-1β, and Irg1). The activation of a danger proinflammatory pathway and the recruitment of inflammatory cells by EP were DNA injection independent. Although the adjuvant effect of EP is DNA independent, in vivo EP-assisted minicircle-gag still shows a greater ability to induce Gag-specific humoral and cellular immune response than pVAX1-gag. This may be explained by the higher transfection efficiency and longer-lasting gag expression in vivo mediated by minicircle DNA than by pVAX1.
The major obstacle to widespread use of minicircle DNA has been its time-consuming and labor-intensive production. Kay et al. presented a robust system for production of minicircle DNA by transformation of modified bacterial strain ZYCY10P3S2T with a minicircle producer plasmid. The procedure was greatly simplified compared to a routine plasmid preparation (37). In this study, we used this novel bacterial strain to produce minicircle DNA. To our knowledge, no systematic research exists addressing the immunogenicity of minicircle DNA as a vaccine. When we were preparing the manuscript, Dietz et al. used tattooing to deliver minicircle DNA intradermally to mice (63). They showed that minicircle DNA was superior to plasmid DNA in eliciting antigen-specific CD8+ T cell responses and conferred protection against bacterial infection in a model of listeriosis. It should be recognized that persistence of antigen expression delivered by minicircle DNA with in vivo EP of minicircle-gag did not dramatically enhance its immunogenicity compared with that of pVAX1-gag. Thus, further studies to determine the magnitude and quality of HIV-I-specific CD4+ and CD8+ T cell adaptive and memory immune responses and levels of antibody to gag in the memory phase of the immune response are clearly warranted. Regardless, EP-based delivery significantly enhanced the dose efficiency of minicircle DNA. The results also suggested that the immunogenicity of minicircle DNA greatly depends on the delivery methods used for immunization.
In conclusion, we show for the first time that the combination of i.m. injection with in vivo EP is a more efficient route for minicircle delivery. In vivo EP of minicircle DNA may function as a novel vaccine platform that enhances efficiency and immunogenicity of DNA vaccines.
ACKNOWLEDGMENTS
We thank Zhiying Chen (Shenzhen Institute of Advanced Technology, Guangdong, People's Republic of China) for his generous gift of p2ΦC31 and the minicircle producer strain ZYCY10P3S2T; we also thank Linqi Zhang (Tsinghua University, Beijing, People's Republic of China) for his advice on this work.
This work was supported by the National Basic Research Program of China (973 Program; no. 2012CB518900, no. 2011CB504706, no. 2010CB529904, and no. 2011CB504805), the National Natural Science Foundation of China (no. 30901751, no. 81171572, and no. 30870745), National Major New Drugs Innovation and Development (no. 2012ZX09401015), and Guangdong Innovative Research Team Program (no. 2009010058).
Footnotes
Published ahead of print 27 November 2013
REFERENCES
- 1.Plotkin SA. 1999. Vaccination against the major infectious diseases. C. R. Acad. Sci. III 322:943–951 [DOI] [PubMed] [Google Scholar]
- 2.Liu MA, Wahren B, Karlsson Hedestam GB. 2006. DNA vaccines: recent developments and future possibilities. Hum. Gene Ther. 17:1051–1061. 10.1089/hum.2006.17.1051 [DOI] [PubMed] [Google Scholar]
- 3.Saha R, Killian S, Donofrio RS. 2011. DNA vaccines: a mini review. Recent Pat. DNA Gene Seq. 5:92–96. 10.2174/187221511796392114 [DOI] [PubMed] [Google Scholar]
- 4.Fioretti D, Iurescia S, Fazio VM, Rinaldi M. 2010. DNA vaccines: developing new strategies against cancer. J. Biomed. Biotechnol. 2010:174378. 10.1155/2010/174378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toda M, Kasai M, Hosokawa H, Nakano N, Taniguchi Y, Inouye S, Kaminogawa S, Takemori T, Sakaguchi M. 2002. DNA vaccine using invariant chain gene for delivery of CD4+ T cell epitope peptide derived from Japanese cedar pollen allergen inhibits allergen-specific IgE response. Eur. J. Immunol. 32:1631–1639. [DOI] [PubMed] [Google Scholar]
- 6.Wingard JB, Anderson B, Weissman D. 2008. Induction of HIV-specific T and B cell responses with a replicating and conditionally infectious lentiviral vaccine. Eur. J. Immunol. 38:1310–1320. 10.1002/eji.200738069 [DOI] [PubMed] [Google Scholar]
- 7.MacGregor RR, Boyer JD, Ugen KE, Lacy KE, Gluckman SJ, Bagarazzi ML, Chattergoon MA, Baine Y, Higgins TJ, Ciccarelli RB, Coney LR, Ginsberg RS, Weiner DB. 1998. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J. Infect. Dis. 178:92–100. 10.1086/515613 [DOI] [PubMed] [Google Scholar]
- 8.Kutzler MA, Weiner DB. 2008. DNA vaccines: ready for prime time? Nat. Rev. Genet. 9:776–788. 10.1038/nrg2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garmory HS, Brown KA, Titball RW. 2003. DNA vaccines: improving expression of antigens. Genet. Vaccines Ther. 1:2. 10.1186/1479-0556-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luckay A, Sidhu MK, Kjeken R, Megati S, Chong SY, Roopchand V, Garcia-Hand D, Abdullah R, Braun R, Montefiori DC, Rosati M, Felber BK, Pavlakis GN, Mathiesen I, Israel ZR, Eldridge JH, Egan MA. 2007. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J. Virol. 81:5257–5269. 10.1128/JVI.00055-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivory C, Chadee K. 2004. DNA vaccines: designing strategies against parasitic infections. Genet. Vaccines Ther. 2:17. 10.1186/1479-0556-2-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radcliffe JN, Roddick JS, Friedmann PS, Stevenson FK, Thirdborough SM. 2006. Prime-boost with alternating DNA vaccines designed to engage different antigen presentation pathways generates high frequencies of peptide-specific CD8+ T cells. J. Immunol. 177:6626–6633 [DOI] [PubMed] [Google Scholar]
- 13.Bigger BW, Tolmachov O, Collombet JM, Fragkos M, Palaszewski I, Coutelle C. 2001. An araC-controlled bacterial cre expression system to produce DNA minicircle vectors for nuclear and mitochondrial gene therapy. J. Biol. Chem. 276:23018–23027. 10.1074/jbc.M010873200 [DOI] [PubMed] [Google Scholar]
- 14.Darquet AM, Cameron B, Wils P, Scherman D, Crouzet J. 1997. A new DNA vehicle for nonviral gene delivery: supercoiled minicircle. Gene Ther. 4:1341–1349. 10.1038/sj.gt.3300540 [DOI] [PubMed] [Google Scholar]
- 15.Chen ZY, He CY, Ehrhardt A, Kay MA. 2003. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol. Ther. 8:495–500. 10.1016/S1525-0016(03)00168-0 [DOI] [PubMed] [Google Scholar]
- 16.Yoon CS, Jung HS, Kwon MJ, Lee SH, Kim CW, Kim MK, Lee M, Park JH. 2009. Sonoporation of the minicircle-VEGF(165) for wound healing of diabetic mice. Pharm. Res. 26:794–801. 10.1007/s11095-008-9778-x [DOI] [PubMed] [Google Scholar]
- 17.Chang CW, Christensen LV, Lee M, Kim SW. 2008. Efficient expression of vascular endothelial growth factor using minicircle DNA for angiogenic gene therapy. J. Control. Release 125:155–163. 10.1016/j.jconrel.2007.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang M, Chen Z, Hu S, Jia F, Li Z, Hoyt G, Robbins RC, Kay MA, Wu JC. 2009. Novel minicircle vector for gene therapy in murine myocardial infarction. Circulation 120:S230–S237. 10.1161/CIRCULATIONAHA.108.841155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, Panetta NJ, Chen ZY, Robbins RC, Kay MA, Longaker MT, Wu JC. 2010. A nonviral minicircle vector for deriving human iPS cells. Nat. Methods 7:197–199. 10.1038/nmeth.1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J, Xiao X, Zhao P, Xue G, Zhu Y, Zhu X, Zheng L, Zeng Y, Huang W. 2006. Minicircle-IFNγ induces antiproliferative and antitumoral effects in human nasopharyngeal carcinoma. Clin. Cancer Res. 12:4702–4713. 10.1158/1078-0432.CCR-06-0520 [DOI] [PubMed] [Google Scholar]
- 21.Zuo Y, Wu J, Xu Z, Yang S, Yan H, Tan L, Meng X, Ying X, Liu R, Kang T, Huang W. 2011. Minicircle-oriP-IFNγ: a novel targeted gene therapeutic system for EBV positive human nasopharyngeal carcinoma. PLoS One 6:e19407. 10.1371/journal.pone.0019407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang WZ, Jin NY, Li ZJ, Zhang LS. 2004. Study on the immunogenicity of HIV-1 gag vaccine. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 20:272–273 (In Chinese.) [PubMed] [Google Scholar]
- 23.Ribeiro SP, Rosa DS, Fonseca SG, Mairena EC, Postol E, Oliveira SC, Guilherme L, Kalil J, Cunha-Neto E. 2010. A vaccine encoding conserved promiscuous HIV CD4 epitopes induces broad T cell responses in mice transgenic to multiple common HLA class II molecules. PLoS One 5:e11072. 10.1371/journal.pone.0011072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu S, Wang S, Grimes-Serrano JM. 2008. Current progress of DNA vaccine studies in humans. Expert Rev. Vaccines 7:175–191. 10.1586/14760584.7.2.175 [DOI] [PubMed] [Google Scholar]
- 25.Dujardin N, Preat V. 2004. Delivery of DNA to skin by electroporation. Methods Mol. Biol. 245:215–226. 10.1007/978-1-59745-194-9_16 [DOI] [PubMed] [Google Scholar]
- 26.Hartikka J, Sukhu L, Buchner C, Hazard D, Bozoukova V, Margalith M, Nishioka WK, Wheeler CJ, Manthorp M, Sawdey M. 2001. Electroporation-facilitated delivery of plasmid DNA in skeletal muscle: plasmid dependence of muscle damage and effect of poloxamer 188. Mol. Ther. 4:407–415. 10.1006/mthe.2001.0483 [DOI] [PubMed] [Google Scholar]
- 27.Shimao K, Takayama T, Enomoto K, Saito T, Nagai S, Miyazaki J, Ogawa K, Tahara H. 2005. Cancer gene therapy using in vivo electroporation of Flt3-ligand. Int. J. Oncol. 27:457–463 [PubMed] [Google Scholar]
- 28.Tamura T, Sakata T. 2003. Application of in vivo electroporation to cancer gene therapy. Curr. Gene Ther. 3:59–64. 10.2174/1566523033347462 [DOI] [PubMed] [Google Scholar]
- 29.Tollefsen S, Tjelle T, Schneider J, Harboe M, Wiker H, Hewinson G, Huygen K, Mathiesen I. 2002. Improved cellular and humoral immune responses against Mycobacterium tuberculosis antigens after intramuscular DNA immunisation combined with muscle electroporation. Vaccine 20:3370–3378. 10.1016/S0264-410X(02)00289-X [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Nolan E, Kreitschitz S, Rabussay DP. 2002. Enhanced delivery of naked DNA to the skin by non-invasive in vivo electroporation. Biochim. Biophys. Acta 1572:1–9. 10.1016/S0304-4165(02)00270-2 [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Kjeken R, Mathiesen I, Barouch DH. 2008. Recruitment of antigen-presenting cells to the site of inoculation and augmentation of human immunodeficiency virus type 1 DNA vaccine immunogenicity by in vivo electroporation. J. Virol. 82:5643–5649. 10.1128/JVI.02564-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roos AK, Eriksson F, Timmons JA, Gerhardt J, Nyman U, Gudmundsdotter L, Brave A, Wahren B, Pisa P. 2009. Skin electroporation: effects on transgene expression, DNA persistence and local tissue environment. PLoS One 4:e7226. 10.1371/journal.pone.0007226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bodles-Brakhop AM, Heller R, Draghia-Akli R. 2009. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol. Ther. 17:585–592. 10.1038/mt.2009.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, Munster PN, Sullivan DM, Ugen KE, Messina JL, Heller R. 2008. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J. Clin. Oncol. 26:5896–5903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raska M, Moldoveanu Z, Novak J, Hel Z, Novak L, Bozja J, Compans RW, Yang C, Mestecky J. 2008. Delivery of DNA HIV-1 vaccine to the liver induces high and long-lasting humoral immune responses. Vaccine 26:1541–1551. 10.1016/j.vaccine.2008.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roos AK, Moreno S, Leder C, Pavlenko M, King A, Pisa P. 2006. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol. Ther. 13:320–327. 10.1016/j.ymthe.2005.08.005 [DOI] [PubMed] [Google Scholar]
- 37.Kay MA, He CY, Chen ZY. 2010. A robust system for production of minicircle DNA vectors. Nat. Biotechnol. 28:1287–1289. 10.1038/nbt.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahlén G, Soderholm J, Tjelle T, Kjeken R, Frelin L, Hoglund U, Blomberg P, Fons M, Mathiesen I, Sallberg M. 2007. In vivo electroporation enhances the immunogenicity of hepatitis C virus nonstructural 3/4A DNA by increased local DNA uptake, protein expression, inflammation, and infiltration of CD3+ T cells. J. Immunol. 179:4741–4753 [DOI] [PubMed] [Google Scholar]
- 39.Dupuis M, Denis-Mize K, Woo C, Goldbeck C, Selby MJ, Chen M, Otten GR, Ulmer JB, Donnelly JJ, Ott G, McDonald DM. 2000. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J. Immunol. 165:2850–2858 [DOI] [PubMed] [Google Scholar]
- 40.Mehendale S, Thakar M, Sahay S, Kumar M, Shete A, Sathyamurthi P, Verma A, Kurle S, Shrotri A, Gilmour J, Goyal R, Dally L, Sayeed E, Zachariah D, Ackland J, Kochhar S, Cox JH, Excler JL, Kumaraswami V, Paranjape R, Ramanathan VD. 2013. Safety and immunogenicity of DNA and MVA HIV-1 subtype C vaccine prime-boost regimens: a phase I randomised trial in HIV-uninfected Indian volunteers. PLoS One 8:e55831. 10.1371/journal.pone.0055831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Connell RJ, Kim JH, Corey L, Michael NL. 2012. Human immunodeficiency virus vaccine trials. Cold Spring Harb Perspect Med. 2:a007351. 10.1101/cshperspect.a007351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Excler JL, Tomaras GD, Russell ND. 2013. Novel directions in HIV-1 vaccines revealed from clinical trials. Curr. Opin. HIV AIDS 8:420–430. 10.1097/COH.0b013e3283632c26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pitisuttithum P, Rerks-Ngarm S, Bussaratid V, Dhitavat J, Maekanantawat W, Pungpak S, Suntharasamai P, Vanijanonta S, Nitayapan S, Kaewkungwal J, Benenson M, Morgan P, O'Connell RJ, Berenberg J, Gurunathan S, Francis DP, Paris R, Chiu J, Stablein D, Michael NL, Excler JL, Robb ML, Kim JH. 2011. Safety and reactogenicity of canarypox ALVAC-HIV (vCP1521) and HIV-1 gp120 AIDSVAX B/E vaccination in an efficacy trial in Thailand. PLoS One 6:e27837. 10.1371/journal.pone.0027837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220. 10.1056/NEJMoa0908492 [DOI] [PubMed] [Google Scholar]
- 45.Cai Y, Rodriguez S, Hebel H. 2009. DNA vaccine manufacture: scale and quality. Expert Rev. Vaccines 8:1277–1291. 10.1586/erv.09.84 [DOI] [PubMed] [Google Scholar]
- 46.Barouch DH. 2008. Challenges in the development of an HIV-1 vaccine. Nature 455:613–619. 10.1038/nature07352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hokey DA, Weiner DB. 2006. DNA vaccines for HIV: challenges and opportunities. Springer Semin. Immunopathol. 28:267–279. 10.1007/s00281-006-0046-z [DOI] [PubMed] [Google Scholar]
- 48.Hartikka J, Sawdey M, Cornefert-Jensen F, Margalith M, Barnhart K, Nolasco M, Vahlsing HL, Meek J, Marquet M, Hobart P, Norman J, Manthorpe M. 1996. An improved plasmid DNA expression vector for direct injection into skeletal muscle. Hum. Gene Ther. 7:1205–1217. 10.1089/hum.1996.7.10-1205 [DOI] [PubMed] [Google Scholar]
- 49.Valera A, Perales JC, Hatzoglou M, Bosch F. 1994. Expression of the neomycin-resistance (neo) gene induces alterations in gene expression and metabolism. Hum. Gene Ther. 5:449–456. 10.1089/hum.1994.5.4-449 [DOI] [PubMed] [Google Scholar]
- 50.Chen ZY, Riu E, He CY, Xu H, Kay MA. 2008. Silencing of episomal transgene expression in liver by plasmid bacterial backbone DNA is independent of CpG methylation. Mol. Ther. 16:548–556. 10.1038/sj.mt.6300399 [DOI] [PubMed] [Google Scholar]
- 51.Bloquel C, Bourges JL, Touchard E, Berdugo M, BenEzra D, Behar-Cohen F. 2006. Non-viral ocular gene therapy: potential ocular therapeutic avenues. Adv. Drug Deliv Rev. 58:1224–1242. 10.1016/j.addr.2006.07.023 [DOI] [PubMed] [Google Scholar]
- 52.Faurez F, Dory D, Le Moigne V, Gravier R, Jestin A. 2010. Biosafety of DNA vaccines: new generation of DNA vectors and current knowledge on the fate of plasmids after injection. Vaccine 28:3888–3895. 10.1016/j.vaccine.2010.03.040 [DOI] [PubMed] [Google Scholar]
- 53.Molnar MJ, Gilbert R, Lu Y, Liu AB, Guo A, Larochelle N, Orlopp K, Lochmuller H, Petrof BJ, Nalbantoglu J, Karpati G. 2004. Factors influencing the efficacy, longevity, and safety of electroporation-assisted plasmid-based gene transfer into mouse muscles. Mol. Ther. 10:447–455. 10.1016/j.ymthe.2004.06.642 [DOI] [PubMed] [Google Scholar]
- 54.Gracey Maniar LE, Maniar JM, Chen ZY, Lu J, Fire AZ, Kay MA. 2013. Minicircle DNA vectors achieve sustained expression reflected by active chromatin and transcriptional level. Mol. Ther. 21:131–138. 10.1038/mt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X, Epperly MW, Kay MA, Chen ZY, Dixon T, Franicola D, Greenberger BA, Komanduri P, Greenberger JS. 2008. Radioprotection in vitro and in vivo by minicircle plasmid carrying the human manganese superoxide dismutase transgene. Hum. Gene Ther. 19:820–826. 10.1089/hum.2007.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klinman DM, Yamshchikov G, Ishigatsubo Y. 1997. Contribution of CpG motifs to the immunogenicity of DNA vaccines. J. Immunol. 158:3635–3639 [PubMed] [Google Scholar]
- 57.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen MD, Silverman GJ, Lotz M, Carson DA, Raz E. 1996. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science 273:352–354. 10.1126/science.273.5273.352 [DOI] [PubMed] [Google Scholar]
- 58.Fuller DH, Loudon P, Schmaljohn C. 2006. Preclinical and clinical progress of particle-mediated DNA vaccines for infectious diseases. Methods 40:86–97. 10.1016/j.ymeth.2006.05.022 [DOI] [PubMed] [Google Scholar]
- 59.Eriksson F, Totterman T, Maltais AK, Pisa P, Yachnin J. 2013. DNA vaccine coding for the rhesus prostate specific antigen delivered by intradermal electroporation in patients with relapsed prostate cancer. Vaccine 31:3843–3848. 10.1016/j.vaccine.2013.06.063 [DOI] [PubMed] [Google Scholar]
- 60.Rabussay DP, Nanda GS, Goldfarb PM. 2002. Enhancing the effectiveness of drug-based cancer therapy by electroporation (electropermeabilization). Technol. Cancer Res. Treat. 1:71–82 [DOI] [PubMed] [Google Scholar]
- 61.Grønevik E, von Steyern FV, Kalhovde JM, Tjelle TE, Mathiesen I. 2005. Gene expression and immune response kinetics using electroporation-mediated DNA delivery to muscle. J. Gene Med. 7:218–227. 10.1002/jgm.650 [DOI] [PubMed] [Google Scholar]
- 62.Chiarella P, Massi E, De Robertis M, Sibilio A, Parrella P, Fazio VM, Signori E. 2008. Electroporation of skeletal muscle induces danger signal release and antigen-presenting cell recruitment independently of DNA vaccine administration. Expert Opin. Biol. Ther. 8:1645–1657. 10.1517/14712598.8.11.1645 [DOI] [PubMed] [Google Scholar]
- 63.Dietz WM, Skinner NE, Hamilton SE, Jund MD, Heitfeld SM, Litterman AJ, Hwu P, Chen ZY, Salazar AM, Ohlfest JR, Blazar BR, Pennell CA, Osborn MJ. 2013. Minicircle DNA is superior to plasmid DNA in eliciting antigen-specific CD8(+) T-cell responses. Mol. Ther. 21:1526–1535. 10.1038/mt.2013.85 [DOI] [PMC free article] [PubMed] [Google Scholar]