ABSTRACT
Persistent viral infections are associated with host and viral factors that impair effective antiviral immunity. Natural killer (NK) cells contribute to establishment of persistent lymphocytic choriomeningitis virus (LCMV) infection in mice through suppression of virus-specific T cell responses during the first few days of infection, but NK cell depletion during those early time points can enable severe T cell-mediated immune pathology and death of the host. Here we show that long after their peak in cytolytic activation, NK cells continue to support viral persistence at later times of infection. Delayed depletion of NK cells, 2 to 3 weeks after infection, enhanced virus-specific T cell responses and viral control. This enhancing effect of delayed NK cell depletion on antiviral immunity, in contrast to early NK cell depletion, was not associated with increased morbidity and mortality, and mice quickly regained weight after treatment. The efficacy of the depletion depended in part upon the size of the original virus inoculum, the viral load at the time of depletion, and the presence of CD4 T cells. Each of these factors is an important contributor to the degree of CD8 T cell dysfunction during viral persistence. Thus, NK cells may continuously contribute to exhaustion of virus-specific T cells during chronic infection, possibly by depleting CD4 T cells. Targeting of NK cells could thus be considered in combination with blockade of other immunosuppressive pathways, such as the interleukin-10 (IL-10) and programmed death 1 (PD-1) pathways, as a therapy to cure chronic human infections, including those with HIV or hepatitis C virus.
IMPORTANCE
INTRODUCTION
Persistent infections with HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV) are major threats to human health. A number of host and viral mechanisms cooperate to suppress effective antiviral immunity and facilitate viral persistence during these types of infections. An important focus of ongoing research concerns the targeting of specific host immunosuppressive factors in order to reinvigorate the immune response. In murine models of persistent lymphocytic choriomeningitis virus (LCMV) infection, the blockade of interleukin-10 (IL-10) (1, 2) or programmed death 1 (PD-1) (3) signaling can enhance LCMV-specific T cell responses and enable improved control of virus infection. In large part, these mechanisms may have evolved to protect the host from an overexuberant immune response, as evidenced by the severe immunopathological diseases associated with complete ablation of PD-1 or its ligands during LCMV infection (3, 4).
Immune suppression during later stages of persistent LCMV infection has been attributed in part to the expansion of particular innate immune suppressor cells, including myeloid tissue-derived suppressor cells (5) and IL-10-expressing antigen-presenting cells (6). Recent work by our group and others has suggested that natural killer (NK) cells can act at a very early stage of LCMV infection to curtail the development of a protective and potentially pathogenic population of virus-specific T cells (7–9). It was proposed that NK cells lysed CD4 (7) or CD8 (8) T cells during the initial days of infection, when type I interferon (IFN) was prevalent and when the NK cells were thus cytolytically activated. This resulted in a weaker antiviral T cell response that could not effect viral clearance (7–9) or cause fatal immune pathology (7).
The potential link between type I IFN expression and NK cell-mediated suppression of antiviral T cell responses (7, 8) is notable given the relationship between an elevated type I IFN signature and disease pathogenesis during chronic infections. In contrast to rhesus macaques, which develop an AIDS-like syndrome after simian immunodeficiency virus (SIV) infection, reduced IFN-associated inflammation is associated with modest disease in either sooty mangabeys or African green monkeys (10, 11). Progression of HIV infection has also been linked to both type I IFN (12) and expression of particular NK cell receptors (13). Similarly, the activation state of NK cells and type I IFN have been linked to both chronicity of HCV infection and refractoriness to antiviral therapy (14, 15). Recently, two groups demonstrated that blockade of type I IFN signaling during persistent LCMV infection in mice could facilitate viral clearance (16, 17). If type I IFN contributes to maintenance of persistent LCMV infection, and in consideration of our previous findings that IFN activates NK cells in the LCMV system (18), we reasoned that perhaps IFN-activated NK cells continue to contribute to immune dysfunction and viral persistence at later time points of infection.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from The Jackson Laboratories (Bar Harbor, ME). IL-21 receptor knockout (IL-21R KO) mice on a C57BL/6 background were obtained from Warren Leonard (19). Male mice at 6 to 12 weeks of age were routinely used for experiments. Mice were maintained under specific-pathogen-free conditions, and experiments were conducted in compliance with guidelines approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School (UMMS).
Virus infections and in vivo cell depletion.
The clone 13 variant strain of LCMV was titrated by plaque assay on Vero cells after propagation in baby hamster kidney BHK21 cells (20). Mice were infected intravenously (i.v.) via the lateral tail vein with 2 × 105 to 5 × 105 (medium dose), 2 × 106 (high dose), or 4 × 106 (very high dose) PFU of LCMV. To selectively deplete NK cells, mice were injected intraperitoneally (i.p.) once or twice with 25 μg of anti-NK1.1 monoclonal antibody (MAb) (PK136) or a control mouse IgG2a produced by Bio-X-Cell (West Lebanon, NH), as previously described (7). Mice were depleted of CD4 T cells by i.p. injection of 200 μg of anti-CD4 (GK1.5), produced by Bio-X-Cell, at days −1 and +1 of infection.
Peptides.
T-cell epitopes encoded by LCMV included NP396–404 (FQPQNGQFI), GP33–41 (KAVYNFATC), GP61–80 (GLKGPDIYKGVYQFKSVEFD), and GP276–286 (SGVENPGGYCL). Peptides were purchased from 21st Century Biochemicals and purified by reverse-phase high-pressure liquid chromatography (HPLC) to 90% purity.
Antibodies and fluorescence-activated cell sorter (FACS) analysis.
Fluorochrome-tagged antibodies were purchased from BD Biosciences (San Jose, CA), eBioscience (San Diego, CA), and BioLegend (San Diego, CA). Data collection was performed on an LSR II cytometer (BD Biosciences) equipped with FACSDiva software, and data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Lymphocyte preparation and intracellular cytokine assay.
Single-cell leukocyte suspensions were prepared from spleens or peripheral blood and plated at up to 2 × 106 cells per well in 96-well plates. Cells were stimulated for 5 h at 37°C with either 1 μM viral peptide or 2.5 μg/ml anti-CD3 antibody (BD Biosciences) in the presence of brefeldin A and 0.2 U/ml recombinant human IL-2 (rhIL-2). After stimulation, cells were preincubated with a 1:200 dilution of Fc Block (2.4G2) (BD Biosciences) in FACS buffer (Hanks balanced salt solution [HBSS], 2% fetal calf serum [FCS], 0.1% NaN3) and stained for 20 min at 4°C with various combinations of tagged antibodies. Cells were washed and then permeabilized using BD Cytofix/Cytoperm solution prior to intracellular staining in BD Permwash (BD Biosciences).
Statistical analysis.
Results are routinely displayed as means ± standard errors of the means (SEM), with statistical differences between experimental groups determined using the Mann-Whitney U test, in which a P value of <0.05 was deemed significant. The limit of detection in viral plaque assays was a log10 PFU value of 1 for the spleen and 2 for other organs. Viral titers below the limit of detection were assigned a “less than” value. Therefore, statistical analyses including viral infectivity determinations below the level of detection will undervalue the true difference between groups. Data are presented as geometric mean titers, which are the arithmetic means of the log10 viral titers. Graphs were produced and statistical analyses were performed using either Microsoft Excel or GraphPad Prism (San Diego, CA).
RESULTS
Timing of NK cell immunoregulatory activity.
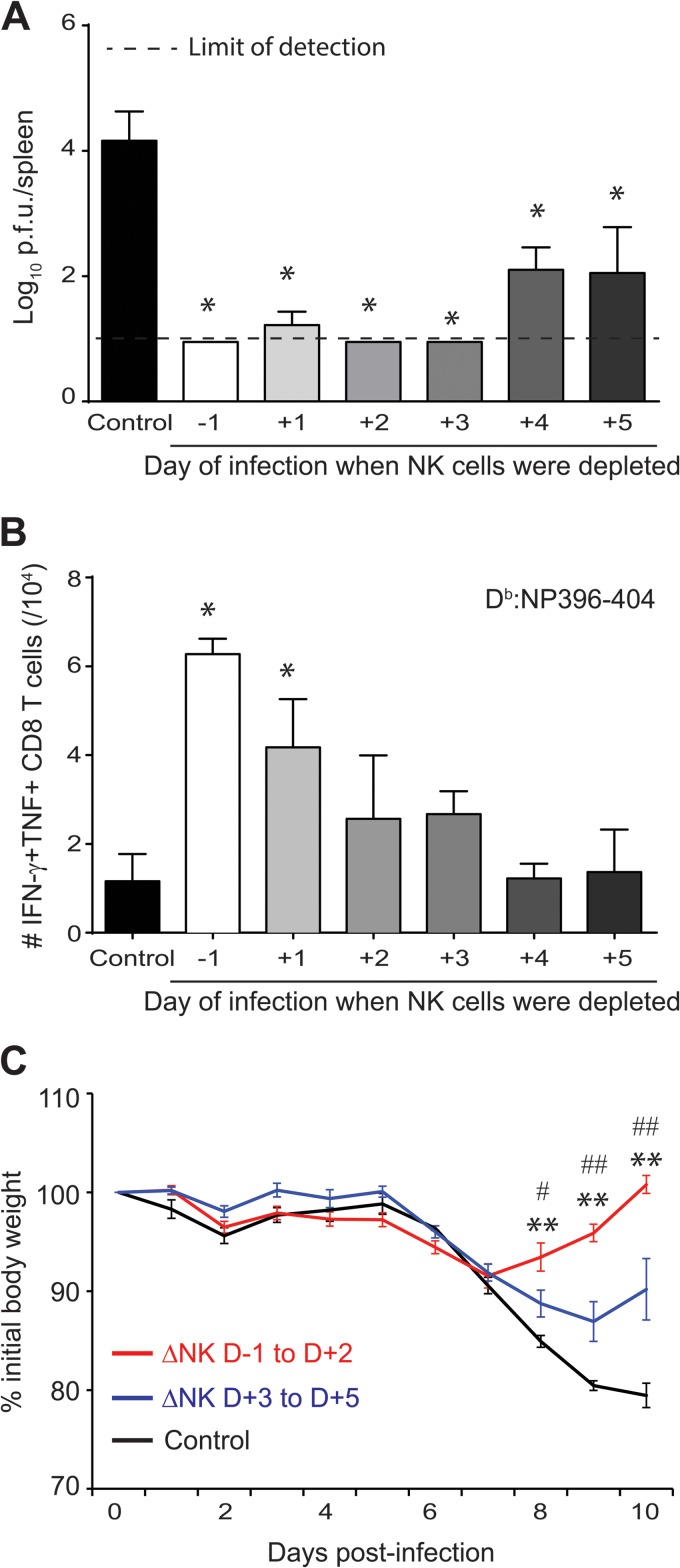
We previously reported that whereas a high dose of LCMV clone 13 established a persistent infection associated with clonal exhaustion of T cells, a medium dose resulted in severe T cell-mediated immune pathology (7). NK cell depletion prior to infection enhanced T cell responses at both doses, resulting in more rapid clearance of virus and less pathology at the medium dose but, paradoxically, lethal immune pathology at the higher dose, probably due to the failure of the elevated T cell response to be exhausted. Notably, while delayed depletion of NK cells on day 2 or 3 of medium-dose infection could still enhance LCMV-specific CD8 T cell responses measured on day 15 postinfection (p.i.), depletion at day 4 or 5 of infection had little, if any, effect (7). Others have similarly observed that NK cell suppression of LCMV-specific CD4 T cells occurred prior to day 4 of infection (9).
Nonetheless, we show here that delayed depletion of NK cells with anti-NK1.1 antibody on day 4 or 5 after infection with a medium dose of LCMV clone 13 still resulted in a substantial (120-fold) reduction in splenic viral load at day 15 of infection compared to that in control mice treated with isotype control antibody (Fig. 1A), despite the absence of an apparent effect on LCMV NP396–404-specific CD8 T cell responses (Fig. 1B). In addition, NK cell depletion at any point during the first 5 days of infection alleviated the severe weight loss normally caused by the immunopathological T cell response to medium-dose LCMV clone 13 infection. This reduced pathology was most pronounced in mice depleted of NK cells during the initial 2 days of infection but was still apparent in those depleted at day 3, 4, or 5 of infection (Fig. 1C). Taken together, these experiments using a medium dose of virus demonstrated that NK cells may continue to play an additional yet less prominent role in viral pathogenesis beyond the first few days of infection.
FIG 1.
Timing of NK cell immunoregulatory activities during pathogenic medium-dose infection. C57BL/6 mice (n = 3/group) were treated with either NK cell-depleting anti-NK1.1 antibody or a nondepleting isotype control at various time points relative to i.v. inoculation with a medium dose (2 × 105 PFU) of LCMV clone 13. (A) At day 15 p.i., viral titers in the spleen were determined. Values below the lower limit of detection (log10 PFU = 1.0; dotted line) were assigned a “less than” value. *, P ≤ 0.05 versus nondepleted group. (B) Numbers of splenic IFN-γ+ TNF+ LCMV NP396–404-specific CD8 T cells were determined at day 15 p.i. by intracellular cytokine staining. *, P ≤ 0.05 versus nondepleted group. TNF, tumor necrosis factor. (C) Body weight was measured daily, and three patterns of weight loss were observed. The mean % of initial body weight was graphed as a function of time for nondepleted mice (black line; n = 3) and for pooled (phenotypically) groups of mice depleted at very early (day −1 [D−1], D+1, or D+2) (red line; n = 8) and later (D+3, D+4, or D+5) (blue line; n = 9) times of infection. **, P ≤ 0.01 versus nondepleted group; #, P ≤ 0.05 versus D+3 to D+5 group; ##, P ≤ 0.01 versus D+3 to D+5 group. Data are presented as means ± SEM and are representative of two independent experiments.
Delayed NK cell depletion improves control of persistent LCMV infection.
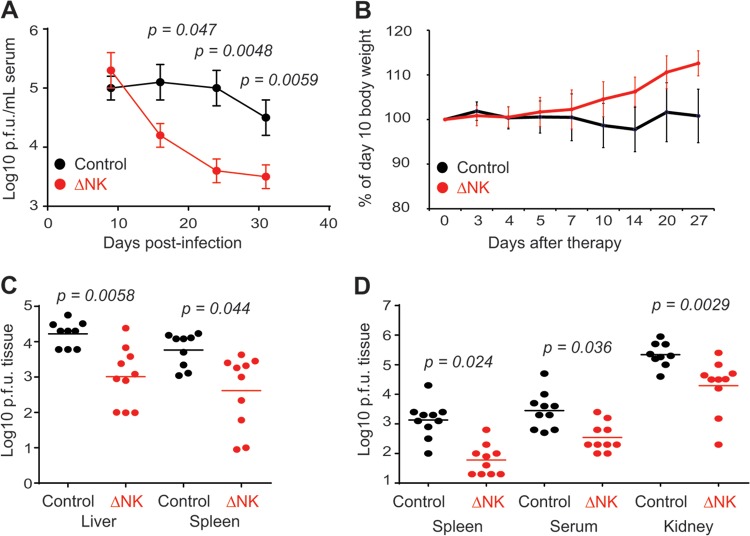
The fact that NK cells continued to impair viral control at later times of medium-dose LCMV clone 13 infection suggested a possible role for NK cells in the maintenance of viral persistence during infection with a high dose of virus, which normally causes a persistent infection due to substantial T cell clonal exhaustion. In order to assess whether NK cells supported sustained virus replication during persistence, we first attempted to deplete NK cells at a time point of high-dose LCMV clone 13 infection (day 10) just subsequent to the clonal exhaustion and depletion of virus-specific T cells (21, 22). Remarkably, administration of anti-NK1.1 antibody at days 10 and 13 of high-dose infection caused a 10-fold reduction in levels of viremia at day 15 p.i. relative to those in isotype control-treated mice (Fig. 2A). This enhanced control of virus replication was maintained at least 10 to 20 days after the last administration of NK cell-depleting antibody. Thus, NK cells continue to suppress viral control late in high-dose LCMV clone 13 infection, and depletion of NK cells has a therapeutic benefit.
FIG 2.
Therapeutic depletion of NK cells enhances control of persistent LCMV infection. Groups of C57BL/6 mice were inoculated i.v. with a high dose (2 × 106 PFU) of LCMV clone 13. (A and B) At days 10 and 13 p.i., mice were either treated with an isotype control antibody (n = 8) or depleted of NK cells (n = 9). (A) Viral titers in serum. (B) Body weights (n = 6/group) relative to weights prior to NK cell depletion on day 10 p.i. (C and D) Other groups of mice were treated with isotype control antibody or given a single injection of anti-NK1.1 antibody on days 20 and 21 (C) or days 28 to 31 (D) of infection. Viral titers in various tissues of isotype control (n = 9 or 10)- or anti-NK1.1 (n = 10)-treated mice were determined 10 to 13 (C) or 12 to 14 (D) days after NK cell depletion. Values below the limit of detection for the spleen (log10 PFU = 1) and other tissues (log10 PFU = 2) were assigned a “less than” value. Data in panels A, C, and D were pooled from two independent experiments. Data in panel B are representative of two similar experiments.
Late NK cell depletion is less pathogenic than early NK cell depletion.
Of particular concern to the concept of therapeutic NK cell depletion is our previous observation that the early depletion of NK cells prior to high-dose LCMV clone 13 infection resulted in severe immunopathology and heightened mortality during the second week of infection (7). In contrast, therapeutic depletion of NK cells at days 10 and 13 of high-dose LCMV clone 13 infection did not result in either death or increased weight loss. In fact, NK cell-depleted mice had gained more weight than control mice 3 to 4 weeks after anti-NK1.1 antibodies were administered (Fig. 2B).
Therapeutic removal of NK cells enhances control of established persistent infection.
Three weeks after infection with a high dose of LCMV clone 13, viral persistence is firmly established, coincident with virtually undetectable CD8 T cell responses to the Db-restricted NP396–404 epitope of LCMV and highly dysfunctional CD8 T cell responses to other viral epitopes in regard to cytokine production (21, 22). Therefore, we sought to determine whether NK cell depletion at even later time points of established persistent infection could also enhance viral control. At day 21 of high-dose LCMV clone 13 infection, mice were administered a single dose of anti-NK1.1 antibody or an isotype control i.p. The efficacy of depletion was evident in the reduced number of splenic CD3− NKp46+ NK cells 6 days after the administration of NK cell-depleting antibody on day 20 p.i. (control group [n = 9], 2.3 × 105 ± 0.38 × 105 NK cells; and ΔNK group [n = 8], 0.68 × 105 ± 0.093 × 105 NK cells; P = 0.0016) and 14 days after anti-NK1.1 therapy on day 30 p.i. (control group [n = 5], 1.0 × 105 ± 0.14 × 105 NK cells; and ΔNK group [n = 5], 0.27 × 105 ± 0.015 × 105 NK cells; P = 0.00086). Viral load was reduced 14- and 16-fold in the spleen and liver, respectively, of therapeutically NK cell-depleted mice relative to isotype-treated mice 10 to 12 days after administration of a single inoculation of depleting antibody (Fig. 2C). Furthermore, single injections of NK cell-depleting antibodies could be administered at very late time points, i.e., 28 to 31 days after infection, and still cause 11- to 29-fold reductions of viral loads in the spleen and serum 2 weeks after therapy onset relative to those in control mice (Fig. 2D). Notably, viral titers were also reduced 11-fold in the kidneys, a tissue where LCMV clone 13 usually persists indefinitely (23).
Control of LCMV replication is predominately mediated by class I major histocompatibility complex (MHC)-restricted virus-specific CD8 T cells (24). In line with the reduced levels of replicating virus after NK cell depletion therapy, we observed an increase in the frequency of Db-restricted LCMV GP33–41-specific CD8 T cells in the blood and spleen of therapeutically NK cell-depleted mice relative to isotype-treated mice at days 41 to 44 of infection, 12 to 14 days after the onset of therapy (Fig. 3A and B). The responses of CD4 T cells specific for the I-Ab-restricted LCMV GP61–81 epitope were marginally increased after therapeutic NK cell depletion, but the total number of IFN-γ-producing CD4 T cells in response to anti-CD3 stimulation was more dramatically enhanced (Fig. 3C). Together, these studies suggest that NK cells continue to suppress LCMV-specific T cell responses and viral control at very late time points of persistent infection.
FIG 3.
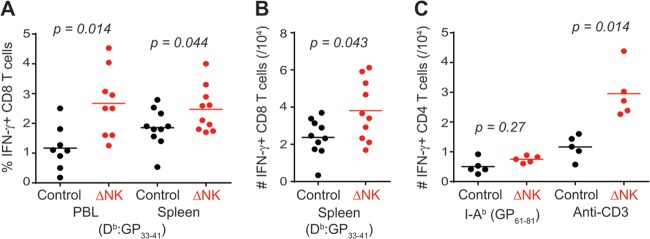
Therapeutic depletion of NK cells augments virus-specific T cell responses. Groups of C57BL/6 mice were inoculated i.v. with a high dose (2 × 106 PFU) of LCMV clone 13. At days 28 to 31 of infection, mice were either treated with isotype control antibody (n = 8) or depleted of NK cells (n = 9). Proportions (A) and numbers (B) of LCMV GP33–41-specific CD8 T cells in the blood and spleen were determined by intracellular cytokine staining after ex vivo stimulation with peptide or anti-CD3 antibody. (C) Numbers of IFN-γ+ CD4 T cells (n = 5/group) in the spleen. Data were pooled from two independent experiments.
Effect of NK cell depletion is sensitive to virus dose and degree of exhaustion.
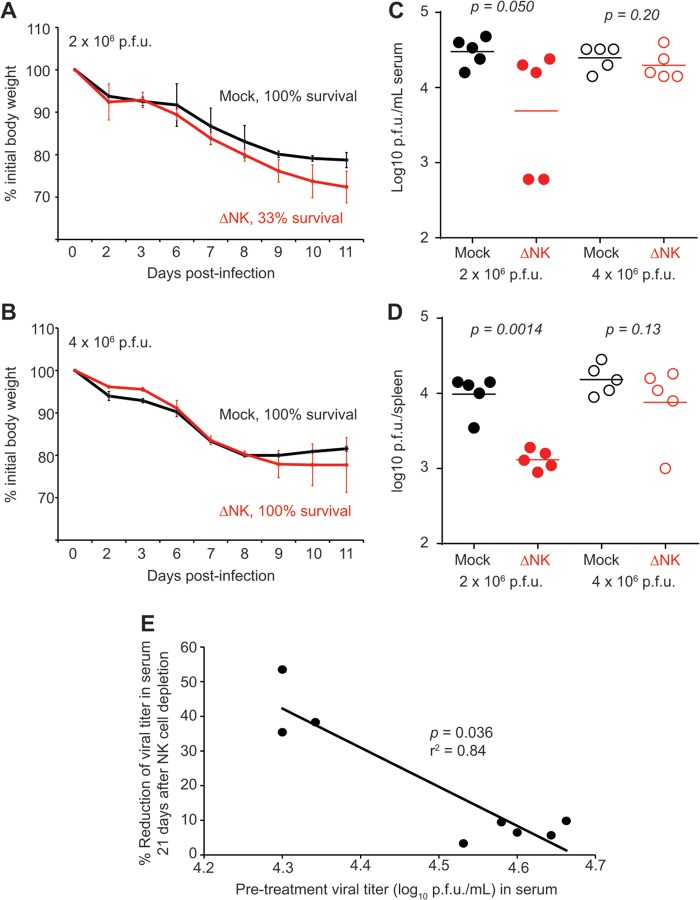
In contrast to our findings, a recent report suggested that depletion of NK cells at day 4, 7, 11, or 14 of high-dose LCMV clone 13 infection had no measurable effect on virus-specific T cell numbers as measured by cytokine production (9). In total, we detected measurable increases in virus-specific T cell responses and reduced viral loads as a result of therapeutic NK cell depletion in 10 independent experiments. Nevertheless, in three experiments, we observed that the effects of NK cell depletion at late time points of infection were minimal or statistically insignificant with regard to either viral burden or virus-specific T cell responses (data not shown). In these cases, virus-specific cytokine-producing T cell responses appeared weaker than those in typical high-dose LCMV clone 13-infected mice at similar time points. For example, the frequency of splenic LCMV-specific IFN-γ+ CD8 T cells on day 40 p.i. was higher in a nondepleted group of control mice (2.2 × 104 ± 0.58 × 104 GP33–41-specific IFN-γ+ CD8 T cells) from an experiment where NK cell depletion enhanced viral control than in the control group (1.3 × 104 ± 0.46 × 104 GP33–41-specific IFN-γ+ CD8 T cells) from an experiment where there was no measurable benefit of NK cell depletion.
To determine whether experimental variation in viral load could affect the efficacy of NK cell depletion-based therapy, we first examined whether the inoculum dose could be increased to overcome the effects of NK cell depletion. In agreement with our previous work (7), intravenous infection with a normally nonlethal high dose (2 × 106 PFU) of LCMV clone 13 caused substantial weight loss that was more severe if NK cells were depleted at the time of infection, resulting in 66% mortality (Fig. 4A). We show here that increasing the original virus inoculum further, to 4 × 106 PFU, overcame the effects of NK cell depletion, resulting in weight loss that was comparable to that of isotype-treated mice, as well as 100% survival (Fig. 4B). These features were likely related to the increased degree of functional exhaustion (data not shown) of virus-specific CD8 T cells at the higher inocula.
FIG 4.
Effect of NK cell depletion is sensitive to size of LCMV inoculum. C57BL/6 mice (n = 3 or 4/group) were depleted of NK cells or treated with isotype control antibody 1 day prior to infection with a high (2 × 106 PFU) (A) or very high (4 × 106 PFU) (B) dose of LCMV clone 13 i.v. Body weight and survival were monitored for 11 days. (C and D) Mice (n = 5/group) were infected for 10 days with a high (2 × 106 PFU) or very high (4 × 106 PFU) dose of LCMV clone 13 prior to isotype control treatment or administration of anti-NK1.1 antibody. At day 31 p.i., 21 days after treatment, viral titers in the sera (C) and spleens (D) of these mice were determined. (E) For a group of mice infected with the high (2 × 106 PFU) dose of LCMV clone 13, viral titers in sera just prior to depletion of NK cells (day 10) were plotted against the % reduction in viral titers in sera 21 days later (endpoint viral titer/initial viral titer × 100 = % reduction), revealing an inverse relationship between these parameters.
Based on these findings, we compared the efficacies of NK cell depletion therapy during persistent infection with a high (2 × 106 PFU) or very high (4 × 106 PFU) dose of LCMV clone 13. Whereas depletion of NK cells at day 10 of high-dose infection resulted in reduced viral burdens in the serum (Fig. 4C) and spleen (Fig. 4D) at day 31 p.i., the same regimen given after very-high-dose infection had little effect on viral control. Notably, the ability of NK cell depletion to effect a reduction in viremia was inversely correlated with the levels of viremia at the time of NK cell depletion in a group of mice infected with 2 × 106 PFU of virus (Fig. 4E). Thus, the value of therapeutic depletion of NK cells in enhancing control of persistent virus infection may depend on viral burden and the extent of immune exhaustion.
Potential role for CD4 T cells in NK cell depletion therapy.
Enhancement of LCMV-specific CD8 T cell responses and immunopathology after high-dose LCMV clone 13 infection in the absence of NK cells was shown by us to be dependent on CD4 T cells (7). To examine whether CD4 T cells were required for therapeutic NK cell depletion-mediated enhancement of LCMV-specific CD8 T cell responses and viral control, mice were depleted of CD4 T cells by use of an antibody to CD4 and then given high-dose LCMV clone 13. At days 26 to 29 p.i., mice were given either anti-NK1.1 or an isotope control i.p. In contrast to NK cell depletion in CD4-replete mice, NK cell depletion did not enhance LCMV-specific CD8 T cell responses (Fig. 5A) or viral control (Fig. 5B) in mice infected with a high dose of LCMV clone 13 in the absence of CD4 T cells.
FIG 5.
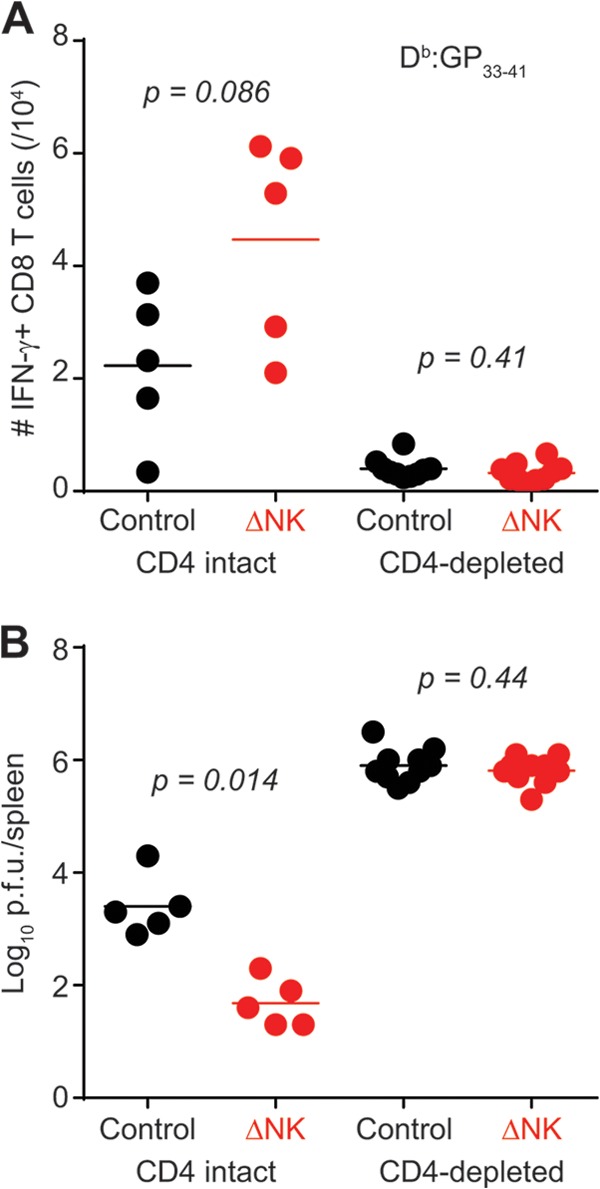
CD4 T cells are required for efficacy of NK cell depletion therapy. C57BL/6 mice were either depleted of CD4 T cells (n = 10/group) or treated with isotype control antibody (n = 5/group) at days −1 and +1 of high-dose LCMV clone 13 infection. At days 26 to 29 of infection, groups of mice were either treated with isotype control antibody or depleted of NK cells. LCMV-specific CD8 T cell responses (A) and viral titers (B) were measured in the spleen. These results were pooled from two independent experiments.
DISCUSSION
A number of studies have now demonstrated that NK cells at a very early stage of infection, when they are highly activated by high levels of IFN and other cytokines, can suppress antiviral T cell responses (reviewed in reference 25). Here we describe a role for NK cells in continuing to impair virus-specific T cell responses and viral control well after viral persistence has been established. In fact, depletion of NK cells in mice persistently infected with LCMV represents a viable therapeutic approach to reinvigorate virus-specific T cell responses and reduce viral burdens. In contrast to the fatal disease associated with depletion of NK cells at the onset of high-dose LCMV clone 13 infection (7), delayed depletion of NK cells during established persistence did not result in exacerbated morbidity and mortality. This suggests that NK cell depletion therapy may be a safe approach to increase immune function. This parallels recently published studies showing that early blockade of type 1 IFN activity enhanced viral loads but blockade of IFN activity later on, during persistence, reduced viral loads (16, 17).
Whereas removal of immunoregulatory NK cells seemed to be a safe and effective strategy to enhance immunity during persistent infection, the success of this approach was dependent upon the severity of immune dysfunction. Functional exhaustion of virus-specific CD8 T cells during LCMV infection is a consequence of persistently high levels of viral antigen (26, 27). CD4 T cells can act to sustain virus-specific CD8 T cell function under conditions of high virus load (28), and this activity involves production of the cytokine IL-21 (29–31). In this study, immune dysfunction was amplified by increasing the viral burden via a very high inoculum of virus or through elimination of CD4 T cells. Under these conditions, NK cell depletion by itself was no longer an effective means of enhancing immune-mediated control of LCMV infection. Furthermore, our preliminary data indicate that NK cell depletion of IL-21 receptor-deficient mice infected with a high dose of LCMV clone 13 also did not enhance LCMV-specific CD8 T cell responses or reduce the viral burden in the spleen (data not shown). Thus, CD4 T cells and IL-21 are important components of the ability of NK cell depletion therapy to enhance control of a persistent virus infection.
In the current study, a relationship was evident between the size of the viral inoculum and the ability of NK cell depletion to enhance immune-mediated control of persistent LCMV infection. Preliminary data indicated that administration of a very high dose of virus was associated with a slightly more pronounced exhaustion phenotype of activated CD8 T cells, i.e., reduced expression of KLRG1 (32), than that observed after infection with the “normal” high dose of virus (data not shown). A greater degree of exhaustion may have contributed to the negligible immune-enhancing efficacy of NK cell depletion during very-high-dose LCMV infections and could potentially account for differences in the efficacy of this therapeutic regimen among individual mice infected with the normal high dose of LCMV. In this regard, the level of viremia at the onset of NK cell depletion therapy was inversely correlated with the effectiveness of this regimen in enhancing viral control. Although more substantial parallels must be drawn between immune status and the amenability of individual of mice to this therapy, these findings provide a basis from which to explore parameters that may be clinically relevant to the determination of when NK cell depletion would be an appropriate treatment for chronic viral infection.
Recent studies from several groups have highlighted the existence of long-lived NK cells that display characteristics of immunological memory, including more robust responsiveness upon secondary stimulation (33). Such “memory” NK cells have been postulated to contribute to immunity in the context of cytomegalovirus infections in both mice (34) and humans (35). The existence of a memory-like population of NK cells in mice persistently infected with LCMV is not known, and if they are present, it might be difficult to distinguish them from activated NK cells in this cytokine-rich environment. Furthermore, our NK cell depletion strategy was sufficient to eliminate NK cells in the liver, which is a reservoir for putative NK memory cells, so if they were there, they should still have been depleted by our approach.
NK cell depletion during persistent LCMV infection likely enhances the proliferation or antiviral activity of a population of partially exhausted virus-specific T cells. Our data are consistent with but do not fully prove the hypothesis that the NK cell inhibition of T cell functionality is mediated through suppression of CD4 T cell-derived IL-21. Alternatively, CD4 T cells and IL-21 may act separately from NK cells, though we have clearly established that NK cell-mediated lysis of CD4 T cells controls CD8 responses earlier in infection (7). A separate study recently identified a similar suppressive activity for NK cells at late stages of persistent retrovirus infection (36). Thus, depletion of NK cells may augment immune-mediated control of a persistent virus infection, with an apparent lack of detrimental health consequences associated with such depletion. This suggests that NK cell depletion or a regimen to inhibit NK cell function could be combined with other therapeutic strategies, including PD-1 or IL-10 blockade (2, 3), which has been shown to be partially effective at clearing persistent LCMV infection.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH) training grant AI07349 (S.N.W.), by a New Scholar Award from The Ellison Medical Foundation (S.N.W.), and by research grants AI-17672, AI-081675, and CA34461 (R.M.W.).
We thank Carey Zammitti, Steven Hatfield, and Laurie Kenney for providing technical and administrative support; Liisa Selin and Eva Szomolanyi-Tsuda for insightful discussions; and Heather Ducharme as well as Amanda Cyr for mouse husbandry. We thank Warren Leonard and Susan Swain for IL-21R KO mice.
The views expressed are those of the authors and do not necessarily express the views of the NIH.
The authors declare that there are no financial conflicts of interest.
Footnotes
Published ahead of print 27 November 2013
REFERENCES
- 1.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. 2006. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 203:2461–2472. 10.1084/jem.20061462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12:1301–1309. 10.1038/nm1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687. 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- 4.Frebel H, Nindl V, Schuepbach RA, Braunschweiler T, Richter K, Vogel J, Wagner CA, Loffing-Cueni D, Kurrer M, Ludewig B, Oxenius A. 2012. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J. Exp. Med. 209:2485–2499. 10.1084/jem.20121015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris BA, Uebelhoer LS, Nakaya HI, Price AA, Grakoui A, Pulendran B. 2013. Chronic but not acute virus infection induces sustained expansion of myeloid suppressor cell numbers that inhibit viral-specific T cell immunity. Immunity 38:309–321. 10.1016/j.immuni.2012.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson EB, Kidani Y, Elsaesser H, Barnard J, Raff L, Karp CL, Bensinger S, Brooks DG. 2012. Emergence of distinct multiarmed immunoregulatory antigen-presenting cells during persistent viral infection. Cell Host Microbe 11:481–491. 10.1016/j.chom.2012.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waggoner SN, Cornberg M, Selin LK, Welsh RM. 2012. Natural killer cells act as rheostats modulating antiviral T cells. Nature 481:394–398. 10.1038/nature10624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang PA, Lang KS, Xu HC, Grusdat M, Parish IA, Recher M, Elford AR, Dhanji S, Shaabani N, Tran CW, Dissanayake D, Rahbar R, Ghazarian M, Brustle A, Fine J, Chen P, Weaver CT, Klose C, Diefenbach A, Haussinger D, Carlyle JR, Kaech SM, Mak TW, Ohashi PS. 2012. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc. Natl. Acad. Sci. U. S. A. 109:1210–1215. 10.1073/pnas.1118834109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook KD, Whitmire JK. 2013. The depletion of NK cells prevents T cell exhaustion to efficiently control disseminating virus infection. J. Immunol. 190:641–649. 10.4049/jimmunol.1202448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, Silvestri G, Giavedoni LD, Lebon P, Barre-Sinoussi F, Benecke A, Muller-Trutwin MC. 2009. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J. Clin. Invest. 119:3544–3555. 10.1172/JCI40093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, Zeng M, Masopust D, Carlis JV, Ran L, Vanderford TH, Paiardini M, Isett RB, Baldwin DA, Else JG, Staprans SI, Silvestri G, Haase AT, Kelvin DJ. 2009. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J. Clin. Invest. 119:3556–3572. 10.1172/JCI40115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbeuval JP, Nilsson J, Boasso A, Hardy AW, Kruhlak MJ, Anderson SA, Dolan MJ, Dy M, Andersson J, Shearer GM. 2006. Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc. Natl. Acad. Sci. U. S. A. 103:7000–7005. 10.1073/pnas.0600363103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bashirova AA, Thomas R, Carrington M. 2011. HLA/KIR restraint of HIV: surviving the fittest. Annu. Rev. Immunol. 29:295–317. 10.1146/annurev-immunol-031210-101332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M, Cox S, Little AM, Alexander GJ, Cramp ME, O'Brien SJ, Rosenberg WM, Thomas DL, Carrington M. 2004. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 305:872–874. 10.1126/science.1097670 [DOI] [PubMed] [Google Scholar]
- 15.Edlich B, Ahlenstiel G, Zabaleta Azpiroz A, Stoltzfus J, Noureddin M, Serti E, Feld JJ, Liang TJ, Rotman Y, Rehermann B. 2012. Early changes in interferon signaling define natural killer cell response and refractoriness to interferon-based therapy of hepatitis C patients. Hepatology 55:39–48. 10.1002/hep.24628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. 2013. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340:207–211. 10.1126/science.1235214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. 2013. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340:202–207. 10.1126/science.1235208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welsh RM., Jr 1978. Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J. Exp. Med. 148:163–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, Liu C, Schwartzberg PL, Leonard WJ. 2002. A critical role for IL-21 in regulating immunoglobulin production. Science 298:1630–1634. 10.1126/science.1077002 [DOI] [PubMed] [Google Scholar]
- 20.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927. 10.1128/JVI.77.8.4911-4927.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205–2213. 10.1084/jem.188.12.2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed R, Jamieson BD, Porter DD. 1987. Immune therapy of a persistent and disseminated viral infection. J. Virol. 61:3920–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zinkernagel RM, Welsh RM. 1976. H-2 compatibility requirement for virus-specific T cell-mediated effector functions in vivo. I. Specificity of T cells conferring antiviral protection against lymphocytic choriomeningitis virus is associated with H-2K and H-2D. J. Immunol. 117:1495–1502 [PubMed] [Google Scholar]
- 25.Welsh RM, Waggoner SN. 2013. NK cells controlling virus-specific T cells: Rheostats for acute vs. persistent infections. Virology 435:37–45. 10.1016/j.virol.2012.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mueller SN, Ahmed R. 2009. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. U. S. A. 106:8623–8628. 10.1073/pnas.0809818106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuller MJ, Zajac AJ. 2003. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 170:477–486 [DOI] [PubMed] [Google Scholar]
- 28.Matloubian M, Concepcion RJ, Ahmed R. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056–8063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elsaesser H, Sauer K, Brooks DG. 2009. IL-21 is required to control chronic viral infection. Science 324:1569–1572. 10.1126/science.1174182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi JS, Du M, Zajac AJ. 2009. A vital role for interleukin-21 in the control of a chronic viral infection. Science 324:1572–1576. 10.1126/science.1175194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. 2009. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324:1576–1580. 10.1126/science.1172815 [DOI] [PubMed] [Google Scholar]
- 32.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. 2007. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27:670–684. 10.1016/j.immuni.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 33.Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. 2013. Natural killer cells: walking three paths down memory lane. Trends Immunol. 34:251–258. 10.1016/j.it.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun JC, Beilke JN, Lanier LL. 2009. Adaptive immune features of natural killer cells. Nature 457:557–561. 10.1038/nature07665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang SM, Norris PJ, Nixon DF, Lanier LL. 2011. Expansion of a unique CD57NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. U. S. A. 108:14725–14732. 10.1073/pnas.1110900108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Littwitz E, Francois S, Dittmer U, Gibbert K. 2013. Distinct roles of NK cells in viral immunity during different phases of acute Friend retrovirus infection. Retrovirology 10:127. 10.1186/1742-4690-10-127 [DOI] [PMC free article] [PubMed] [Google Scholar]