Abstract
Alphaviruses are mosquito-borne viruses that cause significant disease in animals and humans. Western equine encephalitis virus (WEEV) and eastern equine encephalitis virus (EEEV), two New World alphaviruses, can cause fatal encephalitis, and EEEV is a select agent of concern in biodefense. However, we have no antiviral therapies against alphaviral disease, and current vaccine strategies target only a single alphavirus species. In an effort to develop new tools for a broader response to outbreaks, we designed and tested a novel alphavirus vaccine comprised of cationic lipid nucleic acid complexes (CLNCs) and the ectodomain of WEEV E1 protein (E1ecto). Interestingly, we found that the CLNC component, alone, had therapeutic efficacy, as it increased survival of CD-1 mice following lethal WEEV infection. Immunization with the CLNC-WEEV E1ecto mixture (lipid-antigen-nucleic acid complexes [LANACs]) using a prime-boost regimen provided 100% protection in mice challenged with WEEV subcutaneously, intranasally, or via mosquito. Mice immunized with LANACs mounted a strong humoral immune response but did not produce neutralizing antibodies. Passive transfer of serum from LANAC E1ecto-immunized mice to nonimmune CD-1 mice conferred protection against WEEV challenge, indicating that antibody is sufficient for protection. In addition, the LANAC E1ecto immunization protocol significantly increased survival of mice following intranasal or subcutaneous challenge with EEEV. In summary, our LANAC formulation has therapeutic potential and is an effective vaccine strategy that offers protection against two distinct species of alphavirus irrespective of the route of infection. We discuss plausible mechanisms as well the potential utility of our LANAC formulation as a pan-alphavirus vaccine.
INTRODUCTION
Alphaviruses are distributed globally and are responsible for serious outbreaks of mosquito-borne disease in animals, including humans. Geographically, the genus Alphavirus (Togavirdae) can be divided into Old World viruses (OWAs) and New World viruses (NWAs) (1). Many OWAs cause debilitating arthralgic disease in humans, while NWAs are often associated with encephalitis in humans and equids. Western equine encephalitis virus (WEEV), eastern equine encephalitis virus (EEEV), and Venezuelan equine encephalitis virus (VEEV) are the most medically important NWAs. WEEV, EEEV, and VEEV can cause severe central nervous system (CNS) infection of animal models following respiratory exposure, which is one reason that VEEV and EEEV are classified as select agents of concern in biodefense. Currently, no specific therapies are available for alphaviral infections. Unlicensed live attenuated and inactivated vaccines are available against some alphaviruses, but they can have significant side effects. Several vaccines for NWAs are available; however, efficacy concerns limit their use principally to at-risk laboratory workers (2). The inactivated TSI-GSD-210 vaccine for WEEV has been evaluated in a study of several hundred volunteers. Fewer than half developed adequate antibody titers after initial immunization, but most nonresponders developed higher titers after a single booster immunization (3). Another study with the TSI-GSD-104 EEEV inactivated vaccine showed adequate titers in most volunteers after two doses (3, 4). The durability of these two vaccines is limited, perhaps as few as 2 years, although little is known about protection from disease. Moreover, same-day administration of WEEV/EEEV vaccines can cause immune interference as measured by reduced antibody titers (3); thus, the development of vaccines that confer protection without immune interference is a goal of pan-alphavirus vaccine strategies. VEEV TC-83 is an attenuated vaccine with a response rate of 82%, and a single boost with formalin-inactivated C-84 vaccine increases the response rate to over 90%; however, adverse events are reported in 23% of recipients (5). Thus, TC-83 is considered reactogenic and moderately effective as measured by neutralizing antibody. Clearly, better vaccines and/or adjuvants are needed for NWAs.
Alphaviruses have two envelope glycoproteins, E2 and E1, which function in viral adsorption and penetration, respectively (1). Subunit vaccines consisting of recombinant forms of WEEV E2 or E1 have been reported to induce significant protection in animal models (6–8). Although E2 is the major neutralizing antigen, E1 is highly conserved among alphaviruses (9, 10). Thus, E1 is an excellent vaccine candidate because it might offer broader (“pan-alphavirus”) protection against fatal encephalitis. While antibodies targeting E1 often fail to neutralize extracellular virus, nonneutralizing antibodies raised to the prototypic alphavirus (Sindbis virus) E1 glycoprotein are highly protective in an animal model of infection (11).
Cationic liposome-nucleic acid complexes (CLNCs) are potent activators of the innate immune system and are under investigation as vaccine adjuvants. The nucleic acid components of CLNCs, unmethylated CpG oligodeoxynucleotides (ODN) or the double-stranded RNA (dsRNA) analog polyinosinic-poly(C) (PIC), are agonists of Toll-like receptor 9 (TLR9) and Toll-like receptor 3 (TLR3), respectively (12–14). Treatment with ODN or PIC results in strong cytokine/chemokine induction, establishing an antiviral state within the host. Accordingly, ODN and PIC have been used as components of vaccine formulations to enhance the host's immune response (15–20), and further studies have shown that adjuvants containing both ODN and PIC can enhance the immunogenicity of vaccines (21, 22). Although ODN and PIC can each induce an antiviral immune response within the host, the responses differ in expression profile, cellular localization, and signaling pathways (23). Importantly, WEEV, EEEV, and VEEV are exquisitely sensitive to experimental immunomodulation with ODN or PIC (12–14).
In this study, we examined the protective potential of liposome-antigen-nucleic acid complexes (LANACs) consisting of CLNCs and the recombinant WEEV E1 ectodomain (E1ecto) produced using the baculovirus-insect cell system. We demonstrate that the CLNC component of the LANAC alone has a therapeutic impact on WEEV infection and that cationic liposomes with the ODN/PIC polyvalent-adjuvant formulation provide greater protection than cationic liposomes containing only a single agonist species (ODN or PIC). We further demonstrate that CLNCs mixed with the recombinant WEEV E1ecto provide effective prophylaxis against homologous and heterologous strains of WEEV (McMillan and Montana-64) as well as cross-protection against a neurovirulent strain of EEEV (Florida-93). This new vaccine formulation is protective against WEEV and EEEV transmitted by a variety of routes, including WEEV-infected mosquito vectors (Culex tarsalis), and induces a humoral response that does not include detectable levels of neutralizing antibodies. Taken together, these studies support the use of an adjuvant comprised of CLNCs mixed with recombinant WEEV E1ecto as a therapeutic and prophylactic vaccine capable of inducing rapid protection against multiple NWAs.
MATERIALS AND METHODS
Virus strains.
WEEV (McMillan and Montana-64 isolates) came from the Arbovirus Reference Collection at the Centers for Disease Control and Prevention, Fort Collins, CO, USA. The sources (host species) and passage histories of the viruses used in this study can be found in Table 1. Recombinant luciferase-expressing McMillan virus (WEEV.McM.FLUC) was generated as previously described (24). EEEV strain FL93-939 (Florida-93), a 1993 Florida mosquito isolate passed once in Vero cells (25), was obtained from Richard Bowen (Colorado State University). Viral stocks were produced by infecting Vero cells (ATCC) grown in minimal essential medium (MEM) with 10% fetal calf serum at a multiplicity of infection of ≤0.01 PFU/cell. Cell culture supernatants were collected at 48 h postinfection (hpi) and stored in aliquots at −80°C. Virus titers were determined by plaque assay on Vero cells as previously described (26).
TABLE 1.
Viruses
| Viral isolate | Location and yr of isolation | Host/passage historya |
|---|---|---|
| WEEV McMillan | Ontario, Canada, 1941 | Human brain/MP2, SMB1, V2 |
| WEEV Montana-64 | Montana, USA, 1967 | Horse brain/DE1 |
| EEEV Florida-1993 | Florida, USA, 1993 | Culiseta melanura mosquito pool/V1, SMB1 |
MP, mouse; SMB, suckling mouse brain; DE, duck embryo cells; V, Vero cells.
Mouse studies.
The animal use in this study was approved by the Institutional Animal Care and Use Committee at Colorado State University. Care and handling of the mice were consistent with the PHS Policy and Guide for the Care and Use of Laboratory Animals. Outbred, 4- to 6-week-old female CD-1 mice (Charles River, Willington, MA) were allowed to acclimate to the facility for 3 to 7 days. Subcutaneous (s.c.) and intranasal (i.n.) infections were performed with a dose of 1 × 104 to 5 × 104 PFU of virus diluted in phosphate-buffered saline (PBS). The s.c. inoculations were administered in the right footpads of the mice. The i.n. inoculations were performed by alternately dripping inocula onto the nostrils of lightly anesthetized mice until a volume of 20 μl was applied. The titers of the inocula were determined by plaque assay on Vero cells to confirm dosages. All mice were observed twice daily for signs of morbidity. Moribund mice were euthanized by CO2 inhalation, and the day of euthanization was taken as the day of death to calculate mean times to death (MTD). Survivorship was followed for a period of 28 days (initial studies).
Preparation and administration of CLNCs and LANACs.
Cationic liposomes (100 mM DOTIM lipid plus cholesterol) in 10% sucrose were provided by Steven Dow (Colorado State University). CLNCs were prepared essentially as described previously (13), with the only modification being the addition of PIC. Briefly, liposomes were diluted 1:5 in sterile Tris-buffered 5% dextrose water (pH 7.4). Poly (I·C), ODN 1826 CpG DNA (InvivoGen, San Diego, CA), or both nucleic acid species were then added to a final concentration of 0.1 mg/ml, causing spontaneous formation of liposome-nucleic acid complexes (Fig. 1A). For therapeutic studies (treatment after viral inoculation), a single dose of CLNCs was administered to mice (n = 10) at 24 h after s.c. inoculation with 104 PFU of WEEV Montana-64 or immediately after s.c. inoculation with 104 PFU of WEEV McMillan, a highly neurovirulent strain in mice (27).
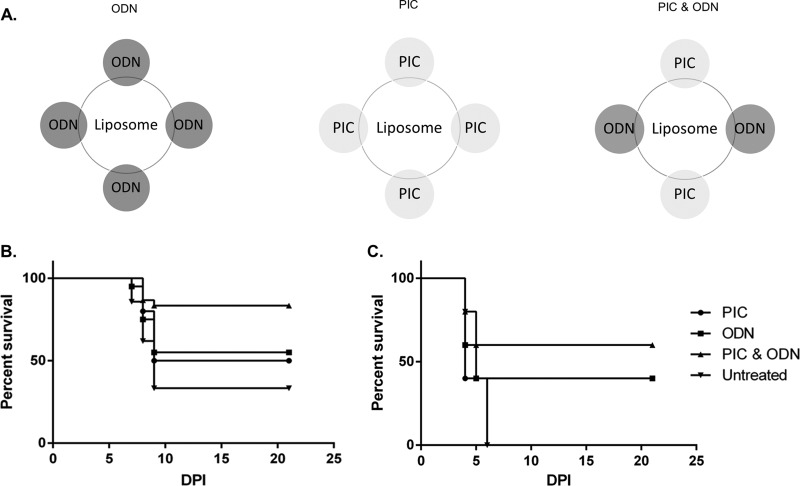
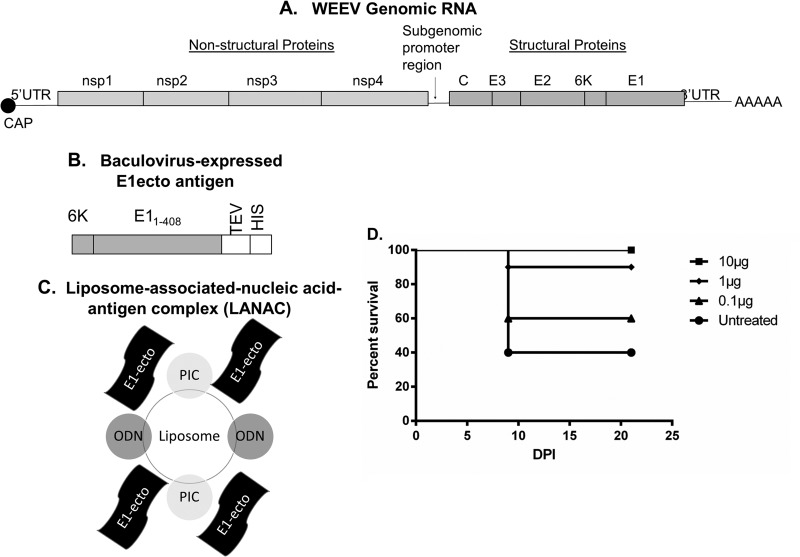
FIG 1.
Effect of nucleic acid species on therapeutic efficacy of CLNCs. (A) Schematic diagram of liposomes containing ODN, PIC, or both ODN and PIC. (B) Mice (n = 10/group) were infected by the s.c. route with 104 PFU of WEEV Montana-64 and then treated with liposomes containing ODN, PIC, or both ODN and PIC at 24 hpi (mice treated with liposome plus ODN and PIC versus untreated mice, P = 0.0154). (C) Same as panel B except that WEEV McMillan was used as the challenge virus and liposome treatments were performed immediately after infection (0 hpi; mice treated with liposome plus ODN and PIC versus untreated mice, P = 0.0488).
For vaccination studies, a recombinant His-tagged version of WEEV E1ecto was produced in the baculovirus-insect cell expression system and purified by immobilized metal affinity chromatography, as previously described (24, 28). Purified E1ecto was added to formed CLNCs at a final concentration of 50 μg/ml (10 μg/200-μl dose) unless otherwise noted (as during initial dosage evaluation studies). The antigen was allowed to associate with liposome complexes for 15 min with mixing by inversion, and the resulting liposome-antigen-nucleic-acid complexes (LANACs) were used to vaccinate mice (n = 10). Each dose of vaccine consisted of 200 μl of LANAC delivered via s.c. injection dorsal to the cervical spine. The priming dose was followed by a boost 2 weeks later. The immunized mice were challenged at 2, 3, 7, 9, or 11 weeks after the booster dose by i.n. or s.c. inoculation with WEEV McMillan, WEEV Montana-64, or EEEV Florida-93. Control animals received only CLNCs mixed with a sham antigen prepared by mock affinity purification of the cell-free medium from expresSF+ cells infected with an isogenic, empty baculovirus vector. Additional controls included mice that were neither treated nor virus inoculated to determine background luminescence during in vivo imaging studies.
In vivo imaging and quantification of luciferase activity.
Mice were vaccinated with E1ecto or sham LANACs using the prime-boost strategy described above. Two weeks after the booster, animals were challenged by i.n. infection with 104 PFU of WEEV.McM.FLUC and imaged at 24 and 48 hpi using IVIS 200, as previously described (24). Luciferase activity for each acquired image was quantified using Living Image 3.0 software (Caliper Life Science, CA, USA).
Virus titration of mouse brain tissue.
Whole brains from each treatment group were collected at 72 hpi (n = 4) from mice used for the imaging studies, as previously described (24). Samples were removed after a 5-min PBS perfusion by cardiac puncture to ensure that all systemic blood was removed. Brains were placed in preweighed 1-ml green bead tubes (Roche, Switzerland) containing 0.5 ml MEM and processed, as previously described (13).
Plaque reduction neutralization titer.
BHK-21 cells (ATCC) were maintained in MEM supplemented with 10% heat-inactivated fetal bovine serum, 1% glutamine, and 100 U/ml of penicillin-streptomycin and used to seed 24-well plates. Plaque reduction neutralization titers (PRNTs) were measured by incubating virus samples with serial dilutions of serum, inoculating samples into each well, and incubating the plates at 37°C in 5% CO2 for 1 h. The inocula were aspirated, and the cells were overlaid with nutrient agar. After being incubated at 37°C in 5% CO2 for 3 days, plaques were visualized using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT). Positive controls consisting of virus that was not pretreated with serum were included in each assay. Sera assayed in these PRNTs included sera collected 3 weeks after the booster dose from mice immunized with E1ecto LANACs, negative-control sera, and positive-control sera from mice that had survived footpad infection with WEEV.McM.FLUC. PRNT endpoints were calculated using probit analysis, as previously described (29). A 50% PRNT (PRNT50) was used as the neutralizing endpoint and the PRNT50 was expressed as the reciprocal of the lowest dilution of test sera able to neutralize 50% of the input virus.
Mouse serum antibody profile assay.
For isotype enzyme-linked immunosorbent assay (ELISA), polyvinylchloride plates were coated overnight at 4°C with 100 μl of E1ecto antigen diluted to 2 μg/ml in PBS (pH 7). Plates were washed twice with PBS containing 0.25% Tween 20 (PBS-TW) and twice more with PBS. Plates were blocked with 200 μl of SuperBlock T20 (Thermo Scientific) for 1 h at room temperature and then washed as described above. Serum samples were diluted 1:100 in PBS, and log2 serial dilutions were added to the plates, followed by 1 h of incubation at room temperature. Isotype-specific detection was performed by 1 h of incubation with monoclonal antibodies to IgG1 (clone X56, horseradish peroxidase [HRP] conjugate), IgG2a (R19-15, HRP), IgG2b (R12-3, biotinylated conjugate), IgG3 (R40-82, biotinylated), IgM (11/41, HRP), or IgA (C10-1, biotinylated) diluted 1:500 in PBS. Wells with biotinylated antibodies were incubated for an additional hour with HRP-streptavidin (Rockland) at 1:1,000 in PBS. ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] substrate (KPL) was added and incubated for 15 min, and absorbance at 405 nm was recorded. Endpoint titers were calculated as the reciprocal of the greatest dilution that was 0.200 optical density (OD) unit greater than that for the negative control.
Cell-based assay for viral replication inhibition by antibody activity.
WEEV.McM.FLUC (24) was used to infect SY5Y neuroblastoma cells (ATCC CRL-2266) for 1 h in a 24-well plate at a multiplicity of infection (MOI) of 0.01, and the infected cells were washed 3 times with cold PBS. Sera from immunized mice, negative controls, or positive-control mice that had survived previous challenge were diluted 1:200 in growth medium and added to each well, and images were acquired at 24 and 48 hpi. Supernatants were collected and used to quantify infectious virus by plaque assay.
Passive transfer of immune serum.
Sera were obtained from seven mice at 9 weeks following a prime-boost immunization with LANAC E1ecto. Sera were pooled to normalize the dose that each nonimmunized mouse would receive in the passive transfer experiment. The antibody titer of pooled immune sera was determined by ELISA using E1ecto antigen as described previously. Mice received 0.5 ml of pooled immune serum by the intraperitoneal (i.p.) route to determine whether immune sera conferred prophylactic protection. Four hours after passive transfer, mice were challenged by the i.n. route with 104 PFU of WEEV.McM.FLUC. Mice were imaged at 10 min after challenge and then daily to monitor infection. Control mice received 0.5 ml of pooled sera from nonimmunized mice. Bioluminescent imaging and quantitation of luciferase activity were performed as previously described (24).
Mosquito studies.
Female Culex tarsalis (Bakersfield strain) mosquitoes were reared at the insectary facility of the Arthropod-Borne and Infectious Disease Laboratory at Colorado State University and moved into a biosafety level 3 (BSL3) insectary as adults. At 1 week postemergence, mosquitoes were intrathoracically inoculated with 102 PFU of WEEV McMillan in a total volume of 69 nl. Mosquitoes were held for 7 days at 28°C and 75% humidity before being used for challenge. WEE E1ecto LANAC (n = 5) or sham LANAC (n = 5)-vaccinated mice were exposed to 6 to 12 infected mosquitoes per mouse at 2 weeks after the booster. A representative sample of blood-engorged mosquitoes was collected from each treatment group (E1ecto LANAC, n = 9; sham LANAC, n = 13), and then individual whole mosquitoes were homogenized and viral titrations performed.
Statistical analyses.
All titration data were log10 transformed and compared using an unpaired Student t test. Analysis was conducted using statistical analysis software (SAS) version 9.2. Survival curves were subjected to Kaplan-Meier (log rank test) analysis using Prism version 6.00 for Windows (GraphPad). Quantitative analysis of bioluminescence in the assessment of vaccine efficacy was conducted using a two-tailed t test.
RESULTS
Therapeutic efficacy of CLNCs.
The therapeutic efficacy of liposomes containing PIC and/or ODN (Fig. 1A) was assessed in mice that had been infected with either the Montana-64 or the McMillan strain of WEEV. Montana-64 has a longer MTD in CD-1 mice and is more suitable for modeling human disease following epizootic outbreaks than the mouse-adapted, highly virulent McMillan strain (26). Montana-64 is also more sensitive to therapeutic intervention (A. T. Phillips, unpublished data). We examined the effect of CLNCs containing ODN, PIC, or both at 24 hpi (Montana-64) or 0 hpi (McMillan) to determine which CLNC formulation provided the best protection in our mouse model. CLNC formulations containing both PIC and ODN, but not those containing only one or the other, significantly increased survival relative to that for untreated controls following infection with either Montana-64 or McMillan (Fig. 1B and C). CLNC-ODN-PIC treatments were statistically significant in protecting CD-1 mice from virus challenge (Montana-64, P = 0.0154 [Fig. 1B]; McMillan, P = 0.0488 [Fig. 1C]). However, CLNC-ODN-PIC administered at 48 (Montana-64) or 24 (McMillan) hpi had no statistically significant impact on survival relative to that for untreated controls (data not shown).
Protective efficacy of LANACs containing WEEV E1ecto.
We subsequently assessed the ability of LANACs comprised of CLNC-ODN-PIC and recombinant WEEV McMillan E1ecto to protect mice against WEEV Montana-64 or McMillan. (The WEEV genome, E1ecto expression cassette, and LANAC schematic are shown in Fig. 2A to C.) The protective efficacy of this formulation was first examined by immunizing mice with LANACs containing various amounts of E1ecto (10, 1, or 0.1 μg) followed by s.c. challenge with Montana-64 (the least virulent strain used in these studies). LANACs containing 10 μg of E1ecto provided 100% protection against challenge with WEEV Montana-64 at 2 weeks after the booster (Fig. 2D).
FIG 2.
Diagram of the WEEV genome, E1ecto construct, and LANAC formulation. (A) The WEEV genome contains a 5′ untranslated region (5′UTR), nonstructural polyprotein genes (nsp1-nsp2-nsp3-nsp4), subgenomic promoter sequence, structural polyprotein genes (capsid-E3-E2-6K-E1), and 3′ untranslated region (3′UTR). (B) The E1ecto construct encoded full-length WEEV (McMillan) 6K, the first 408 amino acids of WEEV (McMillan) E1, a tobacco etch virus protease cleavage site (TEV), and an 8× histidine purification tag. E1ecto was produced using the baculovirus-insect cell system. (C) E1ecto or a sham preparation (see Materials and Methods) was mixed with PIC- and ODN-containing liposomes to form E1ecto or sham LANACs that were used for vaccination experiments. (D) Optimal E1ecto antigen dose in LANAC. Mice (n = 10/group) were vaccinated with LANACs containing 0.1, 1.0, or 10 μg of E1ecto using a prime-boost protocol and then challenged by s.c. inoculation with 104 PFU of WEEV Montana-64. Complete protection was observed using the 10-μg antigen dose, and this dosage was used for the remainder of the vaccination experiments.
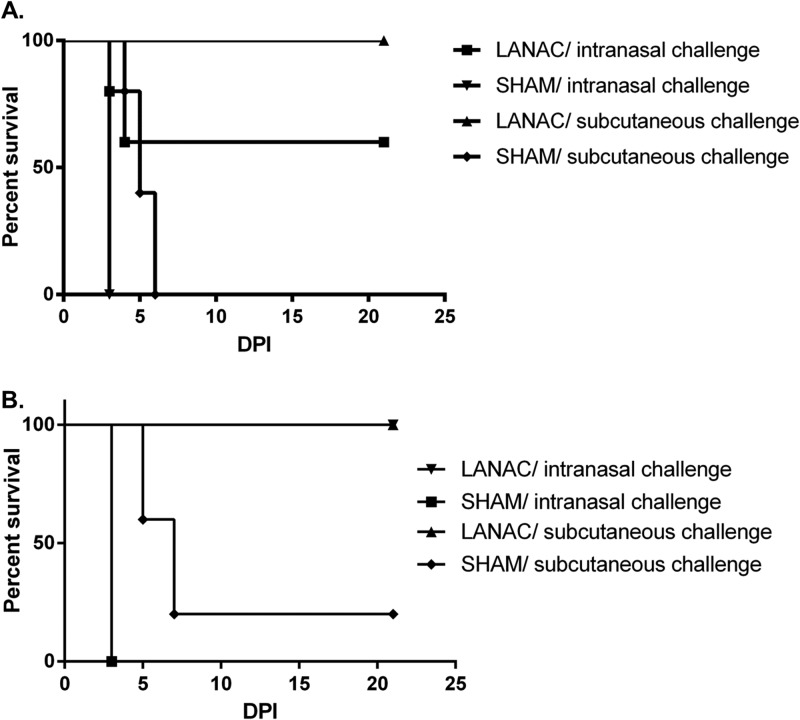
Next, we determined the protective efficacy of LANACs containing the optimal 10 μg of E1ecto against the more virulent WEEV McMillan strain. The effects of infection route and longevity of the immune response to the E1ecto LANACs were examined by challenging immunized mice by the s.c. or i.n. route at two different times after the booster. Immunization with the E1ecto LANACs provided 100% protection against s.c. infection and 60% protection against i.n. infection at 2 weeks (Fig. 3A) and 100% protection against virus administered by either route at 11 weeks (Fig. 3B) after the booster dose.
FIG 3.
LANAC protection against intranasal or subcutaneous challenge with WEEV. (A) Mice (n = 10/group) were prime-boost immunized with E1ecto LANACs (LANAC) or sham LANACs (SHAM) and challenged with 104 PFU of WEEV McMillan 2 weeks after the booster. The differences in survival among mice immunized with E1ecto or sham LANACs were statistically significant (P < 0.0001 for s.c. route; P = 0.0359 for i.n. route). (B) Same as panel A except the mice were challenged 11 weeks after the booster dose. Again, the differences in survival among mice immunized with E1ecto or sham LANACs were statistically significant (P < 0.001).
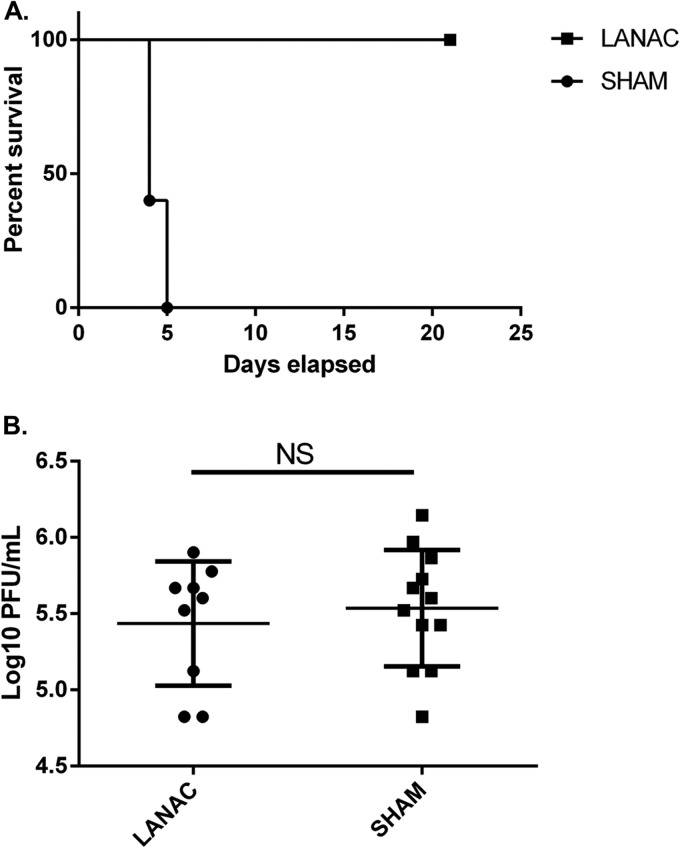
Finally, we examined the protective efficacy of LANACs in mice challenged with WEEV via exposure to infected Culex tarsalis mosquitoes. The results showed that the E1ecto LANACs provided complete protection (Fig. 4A). Plaque assays confirmed that the viral titers of blood-engorged mosquitoes for infecting E1ecto LANAC- or sham LANAC-immunized mice were not significantly different (Fig. 4B).
FIG 4.
LANAC protection against mosquito-delivered WEEV challenge. (A) Mice (n = 5/group) were prime-boost vaccinated with E1ecto LANACs (LANAC) or sham LANACs (SHAM) and exposed to WEEV McMillan-infected Culex tarsalis mosquitoes. Survivorship was monitored for 21 days postinfection. Differences in the survival curves of mice immunized with E1ecto or sham LANACs were statistically significant (P = 0.0295). (B) Infectious virus titers in individual mosquitoes used to infect mice from each treatment group in panel A. Differences were not statistically significant.
Bioluminescence imaging for visualizing effects of LANACs on virus replication.
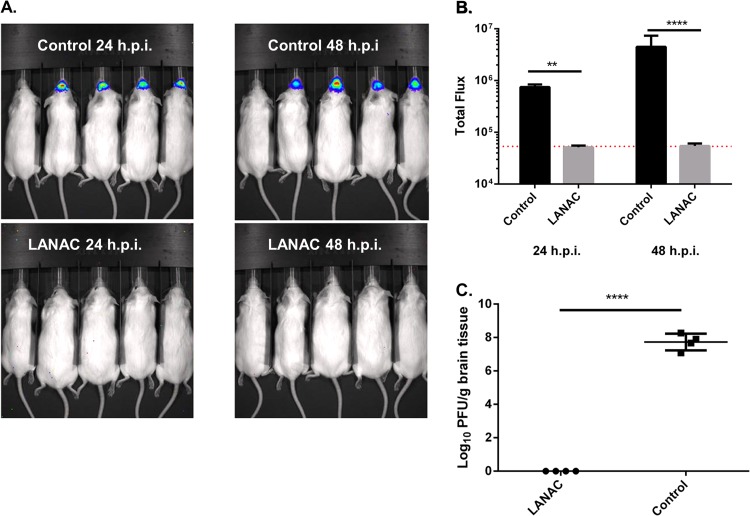
We also used imaging to examine nonimmunized or E1ecto LANAC-immunized mice 2 weeks after the booster dose with an i.n. inoculation of WEEV.McM.FLUC. The results revealed a significant reduction in the bioluminescence signal observed in the E1ecto LANACs-immunized animals compared to nonimmunized controls at both 24 and 48 hpi (Fig. 5A and B). In fact, there was no statistical difference in the levels of bioluminescence observed in the immunized animals and uninfected controls (Fig. 5B; the red line indicates the level of uninfected animal background luminescence). These findings were extended by measuring viral infectivity in homogenates of brains removed from euthanized animals at 72 hpi (Fig. 5C). The immunized mouse brain homogenates produced no plaques, indicating a viral titer lower than the detection limit of our plaque assay (6.6 PFU/ml). In contrast, we observed titers of ∼107 to 108 PFU/g tissue in the nonimmunized mouse brain homogenates, which was consistent with our previous results (24).
FIG 5.
In vivo bioluminescence imaging of the protective effects of LANACs. (A) Nonimmunized and E1ecto LANAC-immunized mice (n = 4/group) were challenged by i.n. inoculation with 104 PFU of WEEV.McM.FLUC virus and then imaged at 24 and 48 hpi. In each image, the first mouse on the left is an uninfected control to establish background bioluminescence. (B) Bioluminescence quantification. Each bar represents the average bioluminescence signal ± standard deviation for each treatment group. Differences between the two treatment groups were significant (P < 0.005). (C) Infectious virus titers in homogenates of brains isolated from animals used for panel A at 72 hpi. Differences between the two treatment groups were statistically significant (P < 0.005).
Neutralizing and antiviral activities of sera from LANAC-immunized mice.
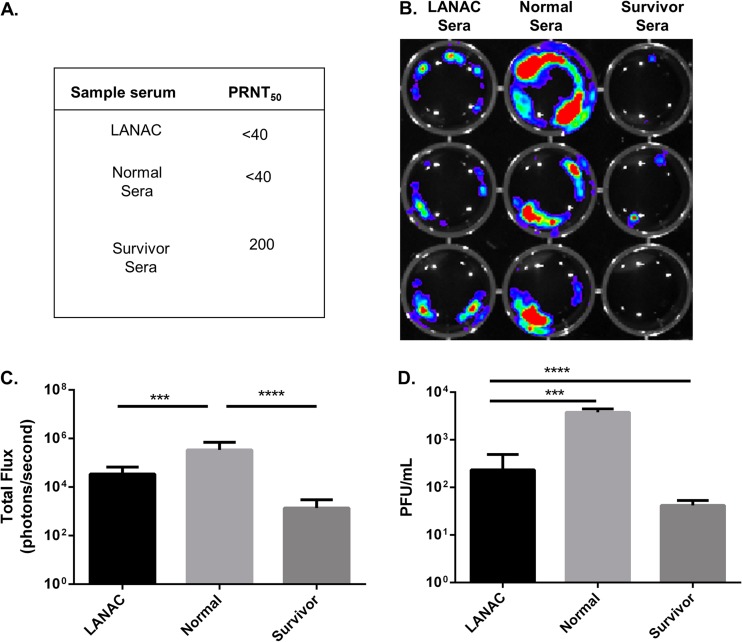
The viral neutralization titers of sera from E1ecto LANAC-immunized mice, nonimmunized (normal) mice, and untreated mice that had survived experimental s.c. challenge with recombinant McMillan (survivors) were measured using standard PRNT assays. Like the normal sera, the LANAC-immunized mouse sera had no neutralizing activity (PRNT50 of <40), while the survivor sera had a PRNT50 of 200 (Fig. 6A). We extended these results by imaging Vero cells 24 h after being infected with WEEV.McM.FLUC and treated (1 hpi) with a 1:200 dilution of sera. The results of this assay showed that sera from the LANAC-immunized mice significantly reduced the bioluminescence signal compared to the negative-control serum (Fig. 6B and C). The LANAC-immunized mouse sera also significantly reduced the amount of infectious virus produced by the infected Vero cells (Fig. 6D), compared to normal sera. Therefore, although the E1ecto LANAC-immunized mouse sera had no detectable virus neutralizing activity, the sera nevertheless had significant antiviral activity.
FIG 6.
Neutralizing and in vitro antiviral activities of sera from LANAC-immunized mice. (A) PRNTs of sera from E1ecto LANAC-vaccinated mice (LANAC), naive mouse sera (normal), or sera from mice which had survived s.c. challenge with WEEV (survivor). (B) SY5Y cells were infected with WEEV.McM.FLUC and then treated with the sera described for panel A. Cells were imaged at 24 (shown) and 48 hpi. (C) Quantitation of bioluminescence shown in panel B. Each bar represents the average ± standard deviation for the indicated treatment group. Sera from E1ecto LANAC-vaccinated mice significantly reduced luciferase activity (P value = 0.007) compared to normal (naive) control sera. (D) Infectious virus titers present in cell culture supernatants of each treatment group shown in panel B. Sera from LANAC-vaccinated mice significantly reduced virus titers compared to normal (naive) control sera (P = 0.007).
Antibody profiling.
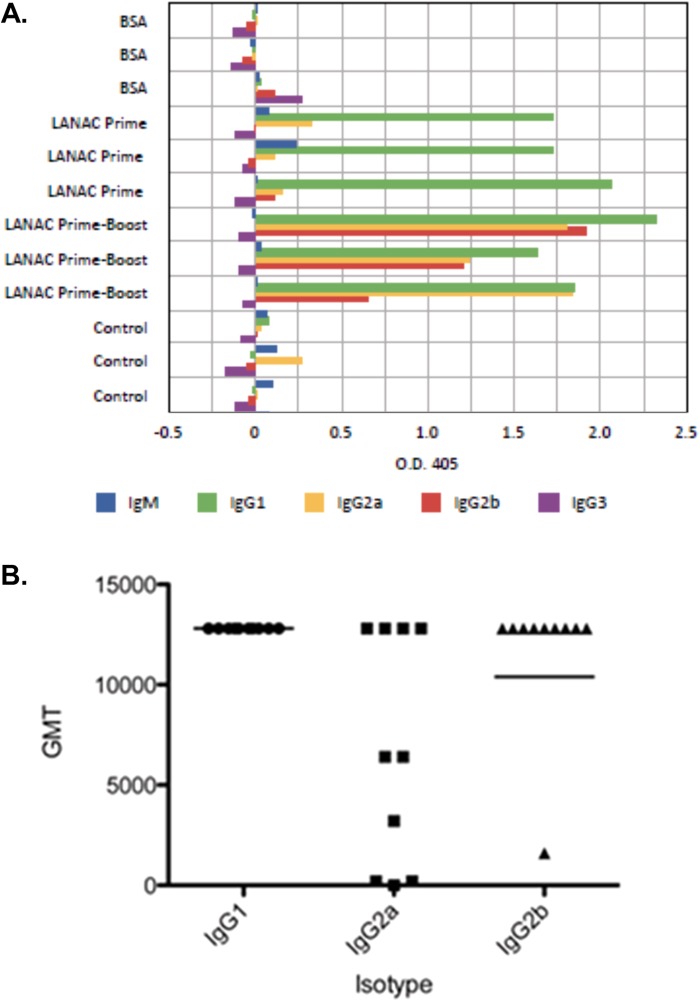
To further characterize antigen-specific humoral responses induced by immunization, sera collected at 2 weeks following the first (prime) and second (prime-boost) doses of LANACs or treatment with bovine serum albumin (BSA) were was assayed for relative isotype abundance by ELISAs (Fig. 7A). Sera from naive, unvaccinated animals were used as controls. The results showed that animals that were immunized with prime or prime-boost doses of LANACs produced E1ecto-specific IgG, whereas unimmunized and BSA-immunized animals did not. In addition, primed animals produced primarily IgG1, whereas prime-boosted animals produced IgG2a and IgG2b, indicating strong class switching. Immunization did not induce appreciable levels of IgG3. The geometric mean titers (GMTs) of both IgG1 and IgG2b in LANAC-immunized (prime-boost) mouse sera were >10,000 (Fig. 7B). There was more animal-to-animal variability in the IgG2a titers, with 4/10 animals failing to produce a GMT of >250.
FIG 7.
Humoral immune response to LANAC immunization. (A) Antigen-specific E1ecto ELISA. Serum samples from each of four groups were assayed for relative antibody isotype abundance. The groups were as follows: mice immunized with LANACs containing bovine serum albumin (LANAC BSA), mice primed with LANACs containing E1ecto (LANAC Prime), mice primed and boosted with LANACs containing E1ecto (LANAC Prime-Boost), and preimmune serum (control). (B) Geometric mean titers of antibody isotypes from E1ecto LANAC prime-boost serum.
Passive transfer of antibodies to confer protection against WEEV.
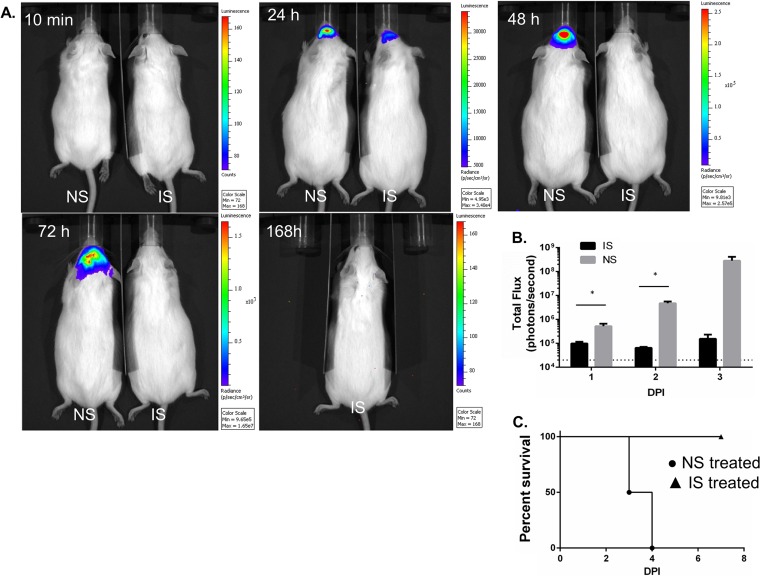
Mice were immunized with LANAC E1ecto, and sera were collected 9 weeks later. Pooled sera from immunized mice had an E1ecto antibody titer of 12,800. Four CD-1 mice/group were i.p. inoculated with either immune or nonimmune sera as described above and infected with recombinant WEEV.McM.FLUC virus by the i.n. route. No bioluminescence was detected in either group at 10 min after infection. All mice receiving nonimmune sera showed progressively intensifying bioluminescence in the head and had pronounced neurological deficits by 96 h postinfection when euthanized. Two of four mice injected with E1ecto LANAC immune sera had limited bioluminescence at 24 h postinfection; however, all of four mice showed no observable signs of bioluminescence at 48 h, 72 h, and 168 h postinfection and no observable neurological deficits. An example of two mice, one treated with immune pooled sera and the other with nonimmune sera, is shown in Fig. 8A. The mouse treated with immune sera survived beyond 7 days after infection. Figure 8B shows a quantification of bioluminescence of the four animals in the experimental and control groups. All four mice receiving immune sera were alive at 7 days postinfection; all mice receiving nonimmune or normal sera were euthanized by day 4 postinfection due to neurological deficits (Fig. 8C). The passive transfer experiments strongly indicate that antibody is the mechanism of protection.
FIG 8.
Passive transfer of pooled immune sera to nonimmunized mice. (A) Bioluminescence images of two mice, injected with either nonimmune (normal) mouse serum (NS) or 9-week immune serum (IS). Image acquisition occurred at specific times postinfection (104 PFU/dose of WEEV. McM.FLUC virus by the intranasal route). The mouse receiving NS was euthanized at 96 h postinfection. (B) Bioluminescence quantification (photons/second) of the four mice in each group treated prior to infection. Asterisks indicate statistically significant differences between treatment groups. (C) Survival curve for mice (n = 4/group) receiving IS or NS treatment prior to infection.
Cross-protection against EEEV.
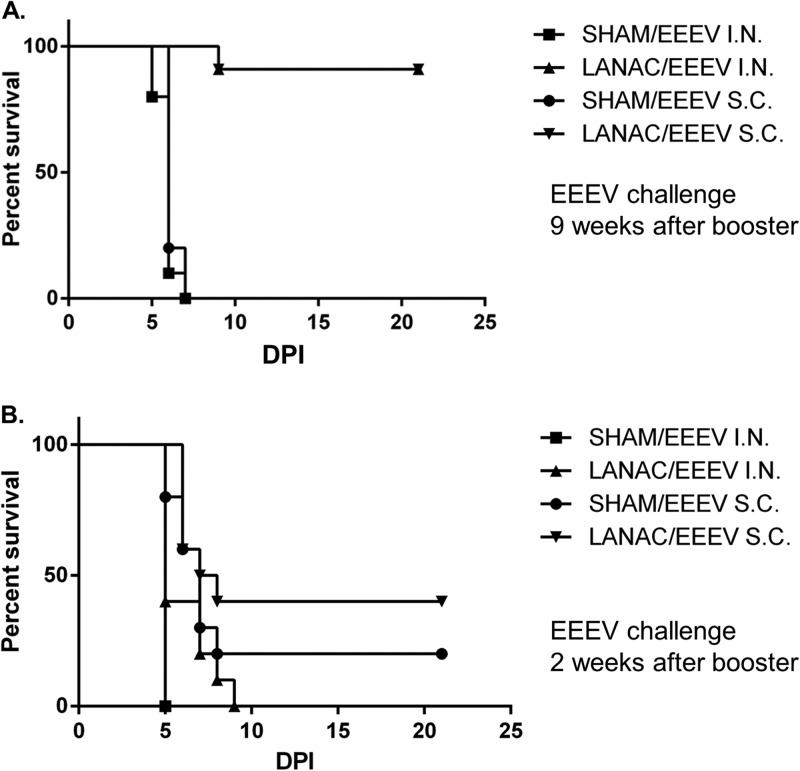
Finally, we examined the ability of WEEV E1ecto LANACs to cross-protect mice against EEEV. Mice were prime-boost immunized with either sham LANACs or E1ecto LANACs and challenged at 9 weeks after the booster dose by s.c. or i.n. inoculation with EEEV (Florida-93) (Fig. 9A). Mice immunized with the E1ecto LANACs survived the virus challenge via either route at significantly higher levels (P < 0.0001) than the sham LANAC controls. In fact, 9/10 CD-1 mice from each group immunized with the E1ecto LANACs survived EEEV (Florida-93) challenge, with only one from each group euthanized at 9 days postchallenge due to neurological deficits, while all 10 mice immunized with the sham LANACs were euthanized at day 6 or 7 due to obvious neurological deficits. In contrast, mice immunized with E1ecto LANACs and challenged at 2 weeks after the booster dose failed to show any significant difference in protection compared with the sham LANAC group (Fig. 9B). These results show that the LANAC formulation containing the recombinant WEEV E1ecto provided strong cross-protection after 9 weeks against the heterologous alphavirus, EEEV.
FIG 9.
LANAC cross-protection against EEEV. (A) CD-1 mice were immunized with E1ecto LANACs (LANAC) or sham LANACs (SHAM) and then challenged 9 weeks after the booster with 104 PFU of EEEV (Florida-93) by either the s.c. or i.n. route. The survival curve for each treatment was analyzed and found to be significantly different from that for the sham-immunized control (P < 0.0001 for both s.c. and i.n. routes). (B) CD-1 mice were immunized with E1ecto LANACs (LANAC) or sham LANACs (SHAM) and then challenged 2 weeks after the booster with 104 PFU of EEEV (Florida-93) by either the s.c. or i.n. route. There was no significant difference between the E1ecto LANACs (LANAC) and sham LANAC immunization groups.
DISCUSSION
We describe a new LANAC formulation that is effective against both WEEV and EEEV, two distinct alphaviruses that cause serious human disease. The CLNC component of the LANACs works therapeutically, most likely through innate immune system stimulation, with induction of antiviral activity occurring even when the CLNCs are administered after infection. In contrast, the recombinant E1ecto antigen component of the LANACs induces a humoral immune response providing specific and long-term protection against alphaviral disease.
It is not surprising that the CLNC component of our LANACs worked therapeutically against WEEV; CLDCs can rapidly induce potent antiviral activity across a wide range of viral infections (13, 30, 31). We previously reported that CLDCs (liposome-ODN complexes) protect against s.c. challenge with WEEV in the CD-1 mouse model when administered 24 h before infection (13). The findings presented here extend our previous results and indicate that cationic liposome-ODN-PIC complexes can be used therapeutically against WEEV when administered within the first day of infection. CLNC-ODN-PIC may result in safety issues due to massive cytokine releases and activation of multiple TLR pathways. This will be a topic of future studies in several animal models, including marmosets.
Our results indicated that CLNCs containing both PIC and ODN had stronger antiviral effects than CLNCs containing only PIC or only ODN. This additive effect might be due to the stimulation of separate innate immune pathways by these molecules. PIC binds TLR3, which can activate MyD88-dependent and -independent pathways, whereas ODN binds TLR9, which uses a MyD88-dependent pathway. Furthermore, PIC can activate the cytosolic sensors (MDA-5 and RIG-I) (32, 33) triggering IPS-1-dependent pathways (34).
Of particular importance was the cross-protection of the WEEV McMillan E1ecto component of LANACs. We purposefully chose this protein antigen for our LANAC formulation for several reasons. First, although E2 protein is the major neutralizing antigen, E1 is highly conserved among distinct alphaviral species (9, 10), and antibody to E1 is protective (11). Second, the E1 of WEEV is a recent ancestor of the E1 from a Sindbis-like virus, which has been shown to induce cross-reactive and cross-protective antibodies (11, 35). Given the WEEV evolutionary history, WEEV E1ecto may be an efficient antigen for developing cross-protective antibodies among NWA and OWA species. Finally, monoclonal antibodies against E1 (McMillan strain) are alphavirus group reactive (36–38). The insect cell-derived McMillan E1ecto antigen presented to mice in the LANAC context (CLNC-ODN-PIC) may explain why our study differs from a previous study that immunized mice with recombinant bacterium-derived WEEV E1 antigen and reported minimal protection against two WEEV strains (3). Gauchi et al. used a DNA vaccine approach to immunize mice with WEEV E1 and demonstrated protection against challenge with a low-virulence WEEV strain but not with a high-virulence WEEV strain (6, 7). In both studies, WEEV challenge occurred at 2 to 3 weeks after boost. Our studies showed that maximal efficiency of cross-protection occurred at 9 to 11 weeks after boost, suggesting that cross-protection to highly virulent WEEV and EEEV strains requires time to develop. In support of this observation, mice that were prime-boost immunized with LANACs containing WEEV E1 antigen and challenged 2 weeks after boost with EEEV (Florida-93) were poorly protected (Fig. 9).
Although LANAC E1ecto induced a strong humoral immune response, no detectable neutralizing activity was found in sera from immunized animals. The passive transfer of immune sera showed that antibody was sufficient for protection in mice. How, then, might the antibodies protect against fatal CNS infection? One possibility is that the E1 antibodies bind to the surface of infected cells and prevent disruption of host cell membranes, leading to the restoration of membrane potential, host protein synthesis, and interferon responsiveness (39, 40).
Metcalf et al. reported that viral antigen-specific antibody-secreting cells (ASCs) become enriched within the brain following infection with Sindbis virus (41) and that these Sindbis-specific ASCs are maximally enriched (as a percentage of total ASCs within the brain) at 8 to 9 weeks after inoculation. Such a timeline corresponds well with our observation that 9 weeks is required after boost before vaccine efficacy is realized in cross-protection experiments. It could be that E1ecto-specific ASCs require 9 weeks in order to attain effective enrichment within the CNS. In the study by Metcalf et al., production of IgG2a and IgG2b, but not IgG1, occurred, suggesting a role for Th1/gamma interferon (IFN-γ)-mediated class switching. Protective, nonneutralizing monoclonal antibodies developed to Sindbis virus were predominantly IgG2a; the one IgG1 isotype generated was not protective (11). In both of these studies, infectious virus was used, and such infections may naturally lead to IgG2a/IgG2b responses. In the LANAC work presented here, we found abundant E1-specific IgG1, but not IgG2a, IgG2b, IgG3, or IgM, at 2 weeks after priming. However, 2 weeks after boosting, levels of IgG2a and IgG2b were also elevated, suggesting that the LANAC method of immunization may initially induce a Th2/interleukin-4 (IL-4)-mediated IgG1 response, but boosting induces a Th1-mediated class switch in some responding B cells (42). Additional work is required to further characterize the responding T cells during LANAC prime-boost and how those T cells influence B cell maturation, class switching, and affinity maturation.
In summary, our data support the use of CLNCs as alphavirus therapeutics and highlight the utility of LANACs consisting of CLNCs and WEEV E1ecto as a vaccine against WEEV and EEEV. Studies are under way to more precisely determine the mechanism of protection. We are currently investigating the effectiveness of our WEEV E1ecto LANAC formulation to protect mice against other alphaviruses, including VEEV and Chikungunya virus, and we remain optimistic that this approach has potential as a broad-spectrum alphavirus vaccine. Finally, the LANAC E1ecto immunization strategy will require efficacy studies in additional animal models, including nonhuman primates.
ACKNOWLEDGMENTS
We thank Steve Dow (Colorado State University) for providing the liposomes, Richard Bowen (Colorado State University) for performing the EEEV challenge experiments, Lab Animal Resources (Colorado State University) for outstanding care of the animals used in these studies, Gerald Callahan (Colorado State University) for assistance in writing the report, Monica Heersink for care and maintenance of mosquitoes, and Tach Costello and Susan Rogers for superior administrative support.
This work is supported by the National Institutes of Health (NIH) (R01 AI46435) and the Rocky Mountain Regional Center of Excellence for Biodefense and Emerging Infectious Diseases (AI065357).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 20 November 2013
REFERENCES
- 1.Ryman KD, Klimstra WB. 2008. Host responses to alphavirus infection. Immunol. Rev. 225:27–45. 10.1111/j.1600-065X.2008.00670.x [DOI] [PubMed] [Google Scholar]
- 2.Pittman PR. 1999. Immunization of persons at risk of exposure to eastern equine encephalitis virus: continued assessment of the safety and effectiveness of eastern equine encephalitis vaccine, inactivated, TSI-GSD 104 (protocol no. IND 266:FY99-11). U.S. Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD [Google Scholar]
- 3.Reisler RB, Gibbs PH, Danner DK, Boudreau EF. 2012. Immune interference in the setting of same-day administration of two similar inactivated alphavirus vaccines: eastern equine and western equine encephalitis. Vaccine 30:7271–7277. 10.1016/j.vaccine.2012.09.049 [DOI] [PubMed] [Google Scholar]
- 4.Strizki JM, Repik PM. 1995. Differential reactivity of immune sera from human vaccinees with field strains of eastern equine encephalitis virus. Am. J. Trop. Med. Hyg. 53:564–570 [DOI] [PubMed] [Google Scholar]
- 5.Engler RJ, Mangiafico JA, Jahrling P, Ksiazek TG, Pedrotti-Krueger M, Peters CJ. 1992. Venezuelan equine encephalitis-specific immunoglobulin responses: live attenuated TC-83 versus inactivated C-84 vaccine. J. medical virology 38:305–310. 10.1002/jmv.1890380414 [DOI] [PubMed] [Google Scholar]
- 6.Das D, Gares SL, Nagata LP, Suresh MR. 2004. Evaluation of a western equine encephalitis recombinant E1 protein for protective immunity and diagnostics. Antiviral Res. 64:85–92. 10.1016/S0166-3542(04)00126-3 [DOI] [PubMed] [Google Scholar]
- 7.Gauci PJ, Wu JQ, Rayner GA, Barabe ND, Nagata LP, Proll DF. 2010. Identification of Western equine encephalitis virus structural proteins that confer protection after DNA vaccination. Clin. Vaccine Immunol. 17:176–179. 10.1128/CVI.00377-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu JQ, Barabe ND, Chau D, Wong C, Rayner GR, Hu WG, Nagata LP. 2007. Complete protection of mice against a lethal dose challenge of western equine encephalitis virus after immunization with an adenovirus-vectored vaccine. Vaccine 25:4368–4375. 10.1016/j.vaccine.2007.03.042 [DOI] [PubMed] [Google Scholar]
- 9.Hahn CS, Lustig S, Strauss EG, Strauss JH. 1988. Western equine encephalitis virus is a recombinant virus. Proc. Natl. Acad. Sci. U. S. A. 85:5997–6001. 10.1073/pnas.85.16.5997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Netolitzky DJ, Schmaltz FL, Parker MD, Rayner GA, Fisher GR, Trent DW, Bader DE, Nagata LP. 2000. Complete genomic RNA sequence of western equine encephalitis virus and expression of the structural genes. J. Gen. Virol. 81:151–159 [DOI] [PubMed] [Google Scholar]
- 11.Schmaljohn AL, Johnson ED, Dalrymple JM, Cole GA. 1982. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature 297:70–72. 10.1038/297070a0 [DOI] [PubMed] [Google Scholar]
- 12.Julander JG, Siddharthan V, Blatt LM, Schafer K, Sidwell RW, Morrey JD. 2007. Effect of exogenous interferon and an interferon inducer on western equine encephalitis virus disease in a hamster model. Virology 360:454–460. 10.1016/j.virol.2006.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logue CH, Phillips AT, Mossel EC, Ledermann JP, Welte T, Dow SW, Olson KE, Powers AM. 2010. Treatment with cationic liposome-DNA complexes (CLDCs) protects mice from lethal Western equine encephalitis virus (WEEV) challenge. Antiviral Res. 87:195–203. 10.1016/j.antiviral.2010.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson M, Poussard A, Taylor K, Seregin A, Smith J, Peng BH, Walker A, Linde J, Smith J, Salazar M, Paessler S. 2011. Rapid, non-invasive imaging of alphaviral brain infection: reducing animal numbers and morbidity to identify efficacy of potential vaccines and antivirals. Vaccine 29:9345–9351. 10.1016/j.vaccine.2011.09.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tewari K, Flynn BJ, Boscardin SB, Kastenmueller K, Salazar AM, Anderson CA, Soundarapandian V, Ahumada A, Keler T, Hoffman SL, Nussenzweig MC, Steinman RM, Seder RA. 2010. Poly(I:C) is an effective adjuvant for antibody and multi-functional CD4+ T cell responses to Plasmodium falciparum circumsporozoite protein (CSP) and alphaDEC-CSP in non human primates. Vaccine 28:7256–7266. 10.1016/j.vaccine.2010.08.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar H, Koyama S, Ishii KJ, Kawai T, Akira S. 2008. Cutting edge: cooperation of IPS-1- and TRIF-dependent pathways in poly IC-enhanced antibody production and cytotoxic T cell responses. J. Immunol. 180:683–687 [DOI] [PubMed] [Google Scholar]
- 17.Sloat BR, Cui Z. 2006. Nasal immunization with anthrax protective antigen protein adjuvanted with polyriboinosinic-polyribocytidylic acid induced strong mucosal and systemic immunities. Pharm. Res. 23:1217–1226. 10.1007/s11095-006-0206-9 [DOI] [PubMed] [Google Scholar]
- 18.von Hunolstein C, Mariotti S, Teloni R, Alfarone G, Romagnoli G, Orefici G, Nisini R. 2001. The adjuvant effect of synthetic oligodeoxynucleotide containing CpG motif converts the anti-Haemophilus influenzae type b glycoconjugates into efficient anti-polysaccharide and anti-carrier polyvalent vaccines. Vaccine 19:3058–3066. 10.1016/S0264-410X(01)00048-2 [DOI] [PubMed] [Google Scholar]
- 19.Xie H, Gursel I, Ivins BE, Singh M, O'Hagan DT, Ulmer JB, Klinman DM. 2005. CpG oligodeoxynucleotides adsorbed onto polylactide-coglycolide microparticles improve the immunogenicity and protective activity of the licensed anthrax vaccine. Infect. Immun. 73:828–833. 10.1128/IAI.73.2.828-833.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fogg CN, Americo JL, Lustig S, Huggins JW, Smith SK, Damon I, Resch W, Earl PL, Klinman DM, Moss B. 2007. Adjuvant-enhanced antibody responses to recombinant proteins correlates with protection of mice and monkeys to orthopoxvirus challenges. Vaccine 25:2787–2799. 10.1016/j.vaccine.2006.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kidner TB, Morton DL, Lee DJ, Hoban M, Foshag LJ, Turner RR, Faries MB. 2012. Combined intralesional Bacille Calmette-Guerin (BCG) and topical imiquimod for in-transit melanoma. J. Immunother. 35:716–720. 10.1097/CJI.0b013e31827457bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B. 2011. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470:543–547. 10.1038/nature09737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawai T, Akira S. 2007. Antiviral signaling through pattern recognition receptors. J. Biochem. 141:137–145. 10.1093/jb/mvm032 [DOI] [PubMed] [Google Scholar]
- 24.Phillips AT, Stauft CB, Aboellail TA, Toth AM, Jarvis DL, Powers AM, Olson KE. 2013. Bioluminescent imaging and histopathologic characterization of WEEV neuroinvasion in outbred CD-1 mice. PLoS One 8:2. 10.1371/journal.pone.0053462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang E, Petrakova O, Adams AP, Aguilar PV, Kang W, Paessler S, Volk SM, Frolov I, Weaver SC. 2007. Chimeric Sindbis/eastern equine encephalitis vaccine candidates are highly attenuated and immunogenic in mice. Vaccine 25:7573–7581. 10.1016/j.vaccine.2007.07.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logue CH, Bosio CF, Welte T, Keene KM, Ledermann JP, Phillips A, Sheahan BJ, Pierro DJ, Marlenee N, Brault AC, Bosio CM, Singh AJ, Powers AM, Olson KE. 2009. Virulence variation among isolates of western equine encephalitis virus in an outbred mouse model. J. Gen. Virol. 90:1848–1858. 10.1099/vir.0.008656-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mossel EC, Ledermann JP, Phillips AT, Borland EM, Powers AM, Olson KE. 2013. Molecular determinants of mouse neurovirulence and mosquito infection for Western equine encephalitis virus. PLoS One 8:e60427. 10.1371/journal.pone.0060427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toth AM, Geisler C, Aumiller JJ, Jarvis DL. 2011. Factors affecting recombinant Western equine encephalitis virus glycoprotein production in the baculovirus system. Protein Expr. Purif. 80:274–282. 10.1016/j.pep.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutchins E, Warren J, Jones WP. 1960. The antibody response to smallpox vaccination as measured by a tissue culture plaque method. J. Immunol. 85:275–283 [PubMed] [Google Scholar]
- 30.Gowen BB, Fairman J, Smee DF, Wong MH, Jung KH, Pace AM, Heiner ML, Bailey KW, Dow SW, Sidwell RW. 2006. Protective immunity against acute phleboviral infection elicited through immunostimulatory cationic liposome-DNA complexes. Antiviral Res. 69:165–172. 10.1016/j.antiviral.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 31.Morrey JD, Motter NE, Taro B, Lay M, Fairman J. 2008. Efficacy of cationic lipid-DNA complexes (CLDC) on hepatitis B virus in transgenic mice. Antiviral Res. 79:71–79. 10.1016/j.antiviral.2008.01.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M. 2006. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. U. S. A. 103:8459–8464. 10.1073/pnas.0603082103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O, Akira S. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23:19–28. 10.1016/j.immuni.2005.04.010 [DOI] [PubMed] [Google Scholar]
- 34.Zou J, Kawai T, Tsuchida T, Kozaki T, Tanaka H, Shin KS, Kumar H, Akira S. 2013. Poly IC triggers a cathepsin D- and IPS-1-dependent pathway to enhance cytokine production and mediate dendritic cell necroptosis. Immunity 38:717–728. 10.1016/j.immuni.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 35.Stec DS, Waddell A, Schmaljohn CS, Cole GA, Schmaljohn AL. 1986. Antibody-selected variation and reversion in Sindbis virus neutralization epitopes. J. Virol. 57:715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunt AR, Roehrig JT. 1985. Biochemical and biological characteristics of epitopes on the E1 glycoprotein of western equine encephalitis virus. Virology 142:334–346. 10.1016/0042-6822(85)90342-3 [DOI] [PubMed] [Google Scholar]
- 37.Roehrig JT. 1993. Immunogens of encephalitis viruses. Vet. Microbiol. 37:273–284. 10.1016/0378-1135(93)90029-7 [DOI] [PubMed] [Google Scholar]
- 38.Roehrig JT, Bolin RA. 1997. Monoclonal antibodies capable of distinguishing epizootic from enzootic varieties of subtype 1 Venezuelan equine encephalitis viruses in a rapid indirect immunofluorescence assay. J. Clin. Microbiol. 35:1887–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burdeinick-Kerr R, Wind J, Griffin DE. 2007. Synergistic roles of antibody and interferon in noncytolytic clearance of Sindbis virus from different regions of the central nervous system. J. Virol. 81:5628–5636. 10.1128/JVI.01152-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griffin D, Levine B, Tyor W, Ubol S, Despres P. 1997. The role of antibody in recovery from alphavirus encephalitis. Immunol. Rev. 159:155–161. 10.1111/j.1600-065X.1997.tb01013.x [DOI] [PubMed] [Google Scholar]
- 41.Metcalf TU, Baxter VK, Nilaratanakul V, Griffin DE. 2013. Recruitment and retention of B cells in the central nervous system in response to alphavirus encephalomyelitis. J. Virol. 87:2420–2429. 10.1128/JVI.01769-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snapper CM, Paul WE. 1987. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236:944–947. 10.1126/science.3107127 [DOI] [PubMed] [Google Scholar]