LETTER
Friend retrovirus complex (FV) induces acute erythroid cell hyperplasia and massive splenomegaly followed by the emergence of fatal erythroleukemia upon inoculation into adult mice of susceptible strains (1–3). Because the disease can progress in the presence of host immune responses, FV has served as a useful model to study how retroviruses evade immune control (1, 3, 4). Depending on genotypes at several host loci, some strains of mice can eliminate virus-producing cells and recover from splenomegaly, while others progress rapidly to fatal pathology (1, 3, 5). Results from several research groups largely agree on the role of virus-neutralizing antibodies and CD4+ T cells in immune control of FV infection (6–15). Natural killer cells also contribute to FV elimination and are essential for vaccine-induced protection of highly susceptible mice (8, 16). However, there are conflicting views on the role of CD8+ T cells in FV control.
Earlier studies associated major histocompatibility complex class I (MHC-I) alleles with spontaneous recovery from FV-induced splenomegaly, and FV-specific, CD8+ cytotoxic T cells were detected (1, 5). Further, the recovery in H2b mice was abrogated when CD8+ T cells were depleted (6). On the other hand, by using FV-encoded epitopes recognized by CD4+ T cells as peptide vaccines, we have shown that highly susceptible (BALB/c × C57BL/6)F1 mice can still be protected from FV challenge and eliminate virus-producing cells in the absence of CD8+ T cells (9). Interestingly, MHC-I genotypes influenced cytokine production from CD4+ T cells upon FV infection (17, 18), indicating the possible indirect role of CD8+ T cells.
C57BL/6 (B6) mice lack the expression of a short form of hematopoietic cell-specific receptor tyrosine kinase, Stk, and do not develop FV-induced erythroid cell proliferation (19). Some reports have indicated that CD8+ T cells are essential in controlling FV infection in B6 mice, as infectious centers at an early time point after FV infection increased upon depletion of CD8+ T cells (20–22). However, infectious centers were detected in the above-described reports with monoclonal antibody 720 (23) that reacts only with the helper component of FV, Friend murine leukemia virus (F-MuLV), but not with the pathogenic component, the spleen focus-forming virus (SFFV). In our recent work (24), SFFV was eliminated from B6 mice by 2 weeks after infection, and CD8+ T cell-deficient B6 mice remained resistant to FV-induced disease development. Thus, the increase of F-MuLV infectious centers after CD8+ T cell depletion, albeit statistically significant, may not reflect pathologically significant changes in SFFV load.
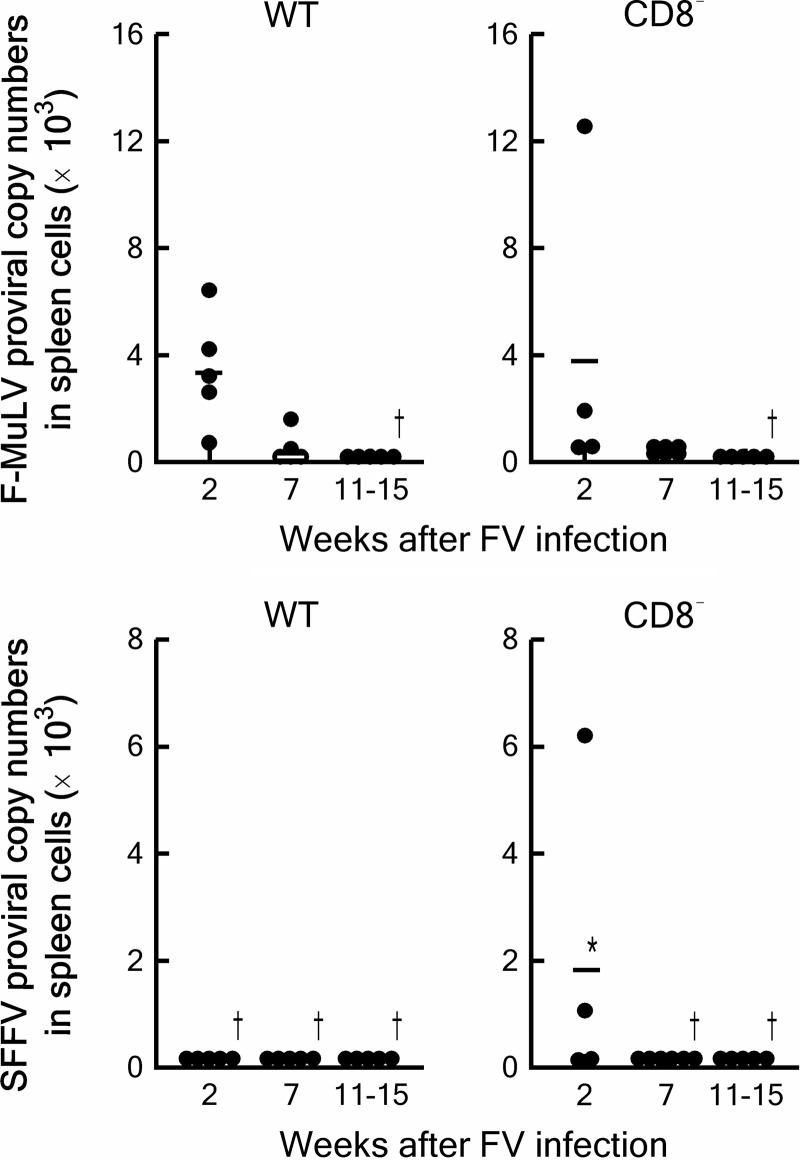
Here, we examined changes in SFFV copy numbers in CD8+ T cell-deficient B6 mice after FV infection. CD8+ T cell-deficient B6 mice nevertheless eliminated both F-MuLV and SFFV proviruses, though more slowly than the wild-type B6 mice did, as shown in Fig. 1. Thus, while CD8+ T cells do contribute to control FV infection, they are not essential for the elimination of FV in B6 mice.
FIG 1.
Changes in proviral copy numbers in the spleens of wild-type (WT) or CD8+ T cell-deficient (CD8−) B6 mice after inoculation of 5,000 spleen focus-forming units of FV. Wild-type B6 and CD8+ T cell-deficient B6.129P2-β2 mtm1Unc/J mice carrying homozygous disruption of the β2 microglobulin gene are those described in reference 24. Genomic DNA extraction and real-time PCR quantification of F-MuLV and SFFV proviruses were performed as described previously (24). Each closed circle represents an absolute copy number of F-MuLV or SFFV provirus in 100 ng of genomic DNA (equal to about 1.7 × 104 cells) detected from an individual mouse. Bars indicate averages for each genetic group and time point. *, significantly higher copy numbers than those in the WT animals [P = 0.0159 < α3(0.05) = 0.0170 by Mann-Whitney test for non-Gaussian distributions with Bonferroni's post hoc test for multiple comparisons]. †, undetectable in all animals examined.
Footnotes
Published ahead of print 13 November 2013
REFERENCES
- 1.Chesebro B, Miyazawa M, Britt WJ. 1990. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu. Rev. Immunol. 8:477–499. 10.1146/annurev.iy.08.040190.002401 [DOI] [PubMed] [Google Scholar]
- 2.Kabat D. 1989. Molecular biology of Friend viral erythroleukemia. Curr. Top. Microbiol. Immunol. 148:1–42. 10.1007/978-3-642-74700-7_1 [DOI] [PubMed] [Google Scholar]
- 3.Miyazawa M, Tsuji-Kawahara S, Kanari Y. 2008. Host genetic factors that control immune responses to retrovirus infections. Vaccine 26:2981–2996. 10.1016/j.vaccine.2008.01.004 [DOI] [PubMed] [Google Scholar]
- 4.Hasenkrug KJ, Dittmer U. 2007. Immune control and prevention of chronic Friend retrovirus infection. Front. Biosci. 12:1544–1551. 10.2741/2167 [DOI] [PubMed] [Google Scholar]
- 5.Miyazawa M, Nishio J, Wehrly K, Chesebro B. 1992. Influence of MHC genes on spontaneous recovery from Friend retrovirus-induced leukemia. J. Immunol. 148:644–647 [PubMed] [Google Scholar]
- 6.Robertson MN, Spangrude GJ, Hasenkrug K, Perry L, Nishio J, Wehrly K, Chesebro B. 1992. Role and specificity of T-cell subsets in spontaneous recovery from Friend virus-induced leukemia in mice. J. Virol. 66:3271–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyazawa M, Fujisawa R, Ishihara C, Takei YA, Shimizu T, Uenishi H, Yamagishi H, Kuribayashi K. 1995. Immunization with a single T helper cell epitope abrogates Friend virus-induced early erythroid proliferation and prevents late leukemia development. J. Immunol. 155:748–758 [PubMed] [Google Scholar]
- 8.Iwanami N, Niwa A, Yasutomi Y, Tabata N, Miyazawa M. 2001. Role of natural killer cells in resistance against Friend retrovirus-induced leukemia. J. Virol. 75:3152–3163. 10.1128/JVI.75.7.3152-3163.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawabata H, Niwa A, Tsuji-Kawahara S, Uenishi H, Iwanami N, Matsukuma H, Abe H, Tabata N, Matsumura H, Miyazawa M. 2006. Peptide-induced immune protection of CD8+ T cell-deficient mice against Friend retrovirus-induced disease. Int. Immunol. 18:183–198. 10.1093/intimm/dxh361 [DOI] [PubMed] [Google Scholar]
- 10.Nair SR, Zelinskyy G, Schimmer S, Gerlach N, Kassiotis G, Dittmer U. 2010. Mechanisms of control of acute Friend virus infection by CD4+ T helper cells and their functional impairment by regulatory T cells. J. Gen. Virol. 91:440–451. 10.1099/vir.0.015834-0 [DOI] [PubMed] [Google Scholar]
- 11.Pike R, Filby A, Ploquin MJ-Y, Eksmond U, Marques R, Antunes I, Hasenkrug K, Kassiotis G. 2009. Race between retroviral spread and CD4+ T-cell response determines the outcome of acute Friend virus infection. J. Virol. 83:11211–11222. 10.1128/JVI.01225-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messer RJ, Dittmer U, Peterson KE, Hasenkrug KJ. 2004. Essential role for virus-neutralizing antibodies in sterilizing immunity against Friend retrovirus infection. Proc. Natl. Acad. Sci. U. S. A. 101:12260–12265. 10.1073/pnas.0404769101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Browne EP. 2011. Toll-like receptor 7 controls the anti-retroviral germinal center response. PLoS Pathog. 7:e1002293. 10.1371/journal.ppat.1002293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Browne EP. 2013. Toll-like receptor 7 inhibits early acute retroviral infection through rapid lymphocyte responses. J. Virol. 87:7357–7366. 10.1128/JVI.00788-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kane M, Case LK, Wang C, Yurkovetskiy L, Dikiy S, Golovkina T. 2011. Innate immune sensing of retroviral infection via Toll-like receptor 7 occurs upon viral entry. Immunity 35:135–145. 10.1016/j.immuni.2011.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa T, Tsuji-Kawahara S, Yuasa T, Kinoshita S, Chikaishi T, Takamura S, Matsumura H, Seya T, Saga T, Miyazawa M. 2011. Natural killer cells recognize Friend retrovirus-infected erythroid progenitor cells through NKG2D-RAE-1 interactions in vivo. J. Virol. 85:5423–5435. 10.1128/JVI.02146-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson KE, Iwashiro M, Hasenkrug KJ, Chesebro B. 2000. Major histocompatibility complex class I gene controls the generation of gamma interferon-producing CD4+ and CD8+ T cells important for recovery from Friend retrovirus-induced leukemia. J. Virol. 74:5363–5367. 10.1128/JVI.74.11.5363-5367.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson KE, Stromnes I, Messer R, Hasenkrug K, Chesebro B. 2002. Novel role of CD8+ T cells and major histocompatibility complex class I genes in the generation of protective CD4+ Th1 responses during retrovirus infection in mice. J. Virol. 76:7942–7948. 10.1128/JVI.76.16.7942-7948.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Persons DA, Paulson RF, Loyd MR, Herley MT, Bodner SM, Bernstein A, Correll PH, Ney PA. 1999. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat. Genet. 23:159–165. 10.1038/13787 [DOI] [PubMed] [Google Scholar]
- 20.Zelinskyy G, Balkow S, Schimmer S, Schepers K, Simon MM, Dittmer U. 2004. Independent roles of perforin, granzymes, and Fas in the control of Friend retrovirus infection. Virology 330:365–374. 10.1016/j.virol.2004.08.040 [DOI] [PubMed] [Google Scholar]
- 21.Zelinskyy G, Balkow S, Schimmer S, Werner T, Simon MM, Dittmer U. 2007. The level of Friend retrovirus replication determines the cytolytic pathway of CD8+ T-cell-mediated pathogen control. J. Virol. 81:11881–11890. 10.1128/JVI.01554-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manzke N, Akhmetzyanova I, Hasenkrug KJ, Trilling M, Zelinskyy G, Dittmer U. 2013. CD4+ T cells develop antiretroviral cytotoxic activity in the absence of regulatory T cells and CD8+ T cells. J. Virol. 87:6306–6313. 10.1128/JVI.00432-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson MN, Miyazawa M, Mori S, Caughey B, Evans LH, Hayes SF, Chesebro B. 1991. Production of monoclonal antibodies reactive with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy, and Western blotting. J. Virol. Methods 34:255–271. 10.1016/0166-0934(91)90105-9 [DOI] [PubMed] [Google Scholar]
- 24.Tsuji-Kawahara S, Kawabata H, Matsukuma H, Kinoshita S, Chikaishi T, Sakamoto M, Kawasaki Y, Miyazawa M. 2013. Differential requirements of cellular and humoral immune responses for Fv2-associated resistance to erythroleukemia and for the regulation of retrovirus-induced myeloid leukemia development. J. Virol. 87:13760–13774. 10.1128/JVI.02506-13 [DOI] [PMC free article] [PubMed] [Google Scholar]