Abstract
Middle East respiratory syndrome coronavirus (MERS-CoV) replicates in cells of different species using dipeptidyl peptidase 4 (DPP4) as a functional receptor. Here we show the resistance of ferrets to MERS-CoV infection and inability of ferret DDP4 to bind MERS-CoV. Site-directed mutagenesis of amino acids variable in ferret DPP4 thus revealed the functional human DPP4 virus binding site. Adenosine deaminase (ADA), a DPP4 binding protein, competed for virus binding, acting as a natural antagonist for MERS-CoV infection.
TEXT
In 2012, a previously unknown human coronavirus (CoV), now named Middle East respiratory syndrome CoV (MERS-CoV), was isolated from the sputum of a 60-year-old man in Saudi Arabia who presented with acute pneumonia with a fatal outcome (1, 2). To date, several infection clusters have been reported over a 1-year period, with around 50% of the reported human cases being fatal (3). MERS-CoV represents a novel betacoronavirus species, with the closest known relatives being clade 2c bat CoVs detected in bats (4, 5). Although MERS-CoV replicates in cells of bats, pigs, and (non-)human primates (6), its ability to infect some animal species may be restricted given the fact that hamsters were shown to resist MERS-CoV infection (7). However, these host factors have not been well characterized.
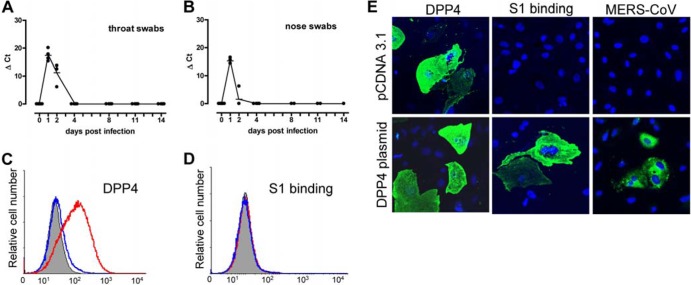
We recently identified dipeptidyl peptidase 4 (DPP4) as a functional MERS-CoV receptor in human and bat cells (8). To further analyze DPP4 usage by MERS-CoV in vivo, ferrets (Mustela putorius furo; n = 4), known to be susceptible to several respiratory viruses, including severe acute respiratory syndrome CoV (SARS-CoV) and influenza virus (9, 10), were inoculated intranasally and intratracheally with a 1 × 106 tissue culture infectious dose (TCID50) of MERS-CoV. Approval for animal experiments was obtained from the Institutional Animal Welfare Committee (no. EMC 2808). After MERS-CoV infection, the animals did not seroconvert and relatively low levels of input viral RNA were detected by reverse transcriptase quantitative PCR (RT-qPCR) (8) in respiratory swabs only at 1 to 2 days postinfection (dpi) (Fig. 1A and B), whereas no infectious virus was detected. In vitro, ferret primary kidney cells did not bind recombinant spike protein S1 (as described in reference 8) and could not be infected with MERS-CoV, despite DPP4 surface expression (Fig. 1C and D). Transfection of primary ferret kidney cells with human DPP4 (hDPP4), however, rendered the cells capable of binding S1 and susceptible to MERS-COV infection (Fig. 1E), suggesting that ferret DPP4 (fDPP4) does not efficiently bind MERS-CoV.
FIG 1.
Ferrets resist MERS-CoV infection. (A and B) MERS-CoV in throat (A) and nose (B) swabs of ferrets (n = 4) inoculated with MERS-CoV was tested for the presence of human CoV (HCoV-EMC) RNA using a TaqMan assay. Ct, threshold cycle. (C and D) Fluorescence-activated cell sorter (FACS) analyses of DPP4 staining or S1-Fc binding on ferret kidney cells incubated with either goat anti-DPP4 polyclonal serum or S1-Fc (5 μg/ml) followed by incubation with fluorescein isothiocyanate (FITC)-labeled rabbit anti-goat IgG antibody or FITC-labeled goat anti-human IgG, respectively (red lines). Normal goat serum, feline CoV S1-Fc protein (blue lines), and mock-incubated cells (gray shading) were used as controls. (E) MERS-CoV infection of primary ferret kidney cells transfected with a control plasmid or with a plasmid encoding hDPP4, stained for DPP4, S1 binding, and MERS-CoV as described previously (13).
Next, we isolated total RNA from ferret primary kidney cells using an RNeasy minikit (Qiagen) and cDNA was synthesized by using Superscript reverse transcriptase (Life Technologies). Complete fDPP4 was amplified with specific primers based on the available GenBank sequence (accession number DQ266376) using Pfu Ultra II fusion HS DNA polymerase (Stratagene) and cloned into the pcDNA 3.1 expression vector (Life Technologies). Plasmids were transfected into MDCK cells in triplicate, and after 24 h of incubation, individual wells were split to determine DPP4 cell surface expression, S1 binding, and susceptibility to MERS-CoV infection on the same transfected cell culture. S1 binding and infection were corrected for DPP4 cell surface expression as determined by the goat polyclonal antiserum against DPP4 (R&D Systems). As shown in Fig. 2, fDPP4 expressed in MDCK cells did not bind recombinant MERS-CoV spike protein (Fig. 2A and B) and did not support MERS-CoV infection (Fig. 2C).
FIG 2.
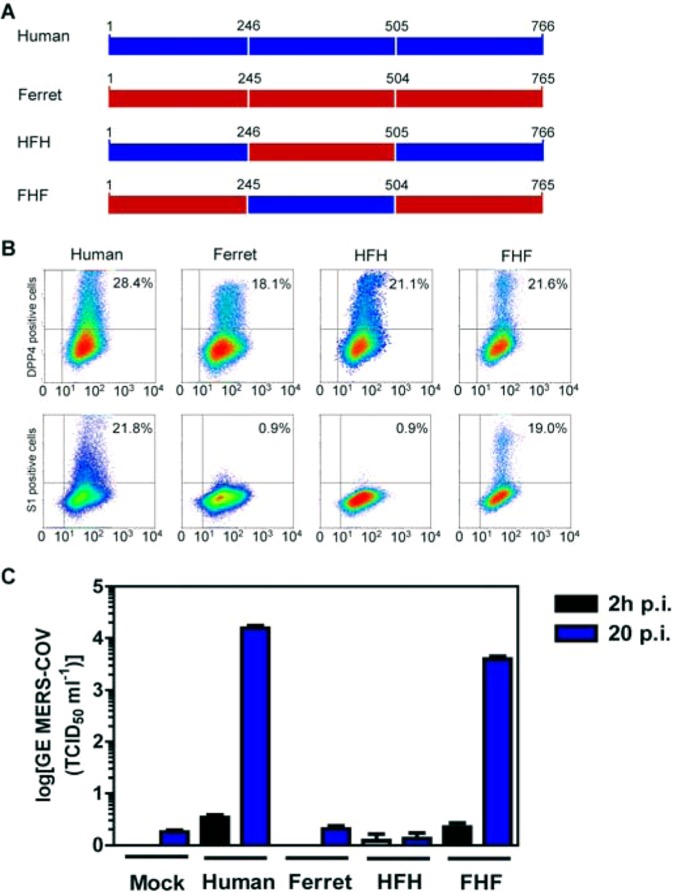
Ferret DPP4 does not bind MERS-CoV spike protein. (A) Different plasmids encoding full-length human DPP4, ferret DPP4, or human-ferret DPP4 chimeras (human-ferret-human and ferret-human-ferret [HFH and FHF, respectively]) were constructed. (B) DPP4 expression and S1 binding to cells transfected with different DPP4 constructs as determined by FACS analysis. Each experiment was conducted in triplicate, and the results shown are representative of two different experiments. (C) MERS-CoV RNA levels in supernatants of DPP4-transfected cells infected with MERS-CoV at 2 and 20 h after infection using a TaqMan assay. Results representative of three different experiments are shown and are expressed as genome equivalents (GE; half-maximal tissue culture infectious dose [TCID50] per ml).
DPP4 is an ectoenzyme that cleaves dipeptides from hormones, chemokines, and cytokines by the conserved C-terminal α/β-hydrolase domain of the protein (11), while its N-terminal eight-blade β-propeller domain contains more sequence variability (Fig. 3A). We made DPP4 chimeras utilizing unique restriction enzyme sites shared by human and ferret DPP4; PstI can cut human and ferret DPP4 into three fragments (for human, amino acids 1 to 246, 247 to 504, and 505 to 766; for ferret, amino acids 1 to 245, 246 to 503, and 504 to 765). Subsequently, the middle fragments of human DPP4 and ferret DPP4 were exchanged, producing human-ferret-human (HFH) or ferret-human-ferret (FHF) expression plasmids (Fig. 2A). Blades 4 and 5 containing hDPP4 domain (residues 246 to 505) could confer to ferret DPP4 the ability to bind to S1 and to mediate MERS-CoV infection when expressed in nonsusceptible cells (Fig. 2B and C). Subsequently, we made point mutations on selected solvent-exposed residues present in blades 4 and 5 of hDPP4 by those occurring at these positions in fDPP4 using a Quick Change site-directed mutagenesis kit (Stratagene). The presence of the correct mutations and the absence of undesired mutations were confirmed by sequencing analysis. Several mutations abrogated DPP4's capacity to bind to S1 and to mediate MERS-CoV cell susceptibility upon transfection (Fig. 3B and C), showing that these residues are involved in MERS-CoV binding and entry. Reciprocal substitutions of these amino acids in fDPP4 on their own, however, did not confer S1 binding, demonstrating the complexity of the interaction in the face of the highly polymorphic nature of these two blades (12). Recent data obtained by cocrystallization of the receptor binding domains of S1 and DPP4 revealed that the 11 amino acid residues K267, Q286, T288, A291, L294, I295, R317, Y322, R366, Q344, and I346 present in blades 4 and 5 of the DPP4 β-propeller domain are critically involved in the interaction with the receptor binding domain of MERS-CoV (13, 14). Our data indicate that at least 6 of 7 residues that are different in fDPP4 may functionally impact the binding of S1. The results of characterization of the DPP4 virus binding site using monoclonal antibodies are in line with our data (15).
FIG 3.
Characterization of the functional MERS-CoV DPP4 receptor S1 binding site. (A) Amino acid sequence alignment of DPP4 loops 4 and 5 from different species. (B) S1 binding assy. Different hDPP4 mutant-transfected cells were harvested and incubated with 5 μg of MERS-CoV-S1-Fc protein, a second step was performed with FITC-labeled anti-human IgG, and cells were analyzed by FACS. The error bars indicate standard errors of the means. (C) MERS-CoV infection of cells transfected with different hDPP4 constructs. Data in panel B and C were corrected for DPP4 expression of the different constructs (one-way analysis of variance [ANOVA] test; *, P < 0.05; **, P < 0.01; n = 3 per group). (D and E) Phylogenetic tree based on the complete DPP4 (D) and a DPP4 fragment containing the S1 binding region (residues 246 to 504) (E) of 13 different species. Sequence alignment was performed by using ClustalW in the MEGA5.0 software package (www.megasoftware.net), and the trees were constructed by using the neighbor-joining method with P distances (gap/missing data treatment; complete deletion) and 1,000 bootstrap replicates as in MEGA version 5.0.
Phylogenetic analysis of the complete DPP4 showed that bat DPP4, shown to act as a functional MERS-CoV receptor (8), is highly divergent from human DPP4 (Fig. 3D). However, phylogenetic analysis of the virus binding region of DPP4 indicated that human, macaque, horse, and rabbit DPP4 now clustered together with cattle, pig, and bat DPP4, which are somewhat more distantly related (Fig. 3E). Small animals, including ferret and mice, are more divergent with respect to the DPP4 virus binding region, which, at least in the case of ferrets, has consequences for MERS-CoV binding.
Some of the identified residues (especially Q286 and L294) have been shown to be crucial in binding human enzyme adenosine deaminase (ADA) (16), a natural DPP4 ligand (17). Recombinant ADA (5 μg/ml) competed with S1 (5 μg/ml) for binding to DPP4 when tested on MDCK cells transfected with hDPP4 (Fig. 4A). In line with these observations, MERS-CoV infection of hDPP4-transfected cells was inhibited by ADA (Fig. 4A). Limited infection was also observed in Huh7 cells preincubated with different concentrations of recombinant ADA but not in cells preincubated with a control protein (Fig. 4B). In this study, we did not compare different concentrations of S1 and ADA that block infection. However, compared to previous results obtained (18), at 40 μg/ml ADA seems to block MERS-CoV infection of Huh7 cells more efficiently. These results reveal that ADA may act as a naturally occurring antagonist able to block binding and infection with MERS-CoV.
FIG 4.
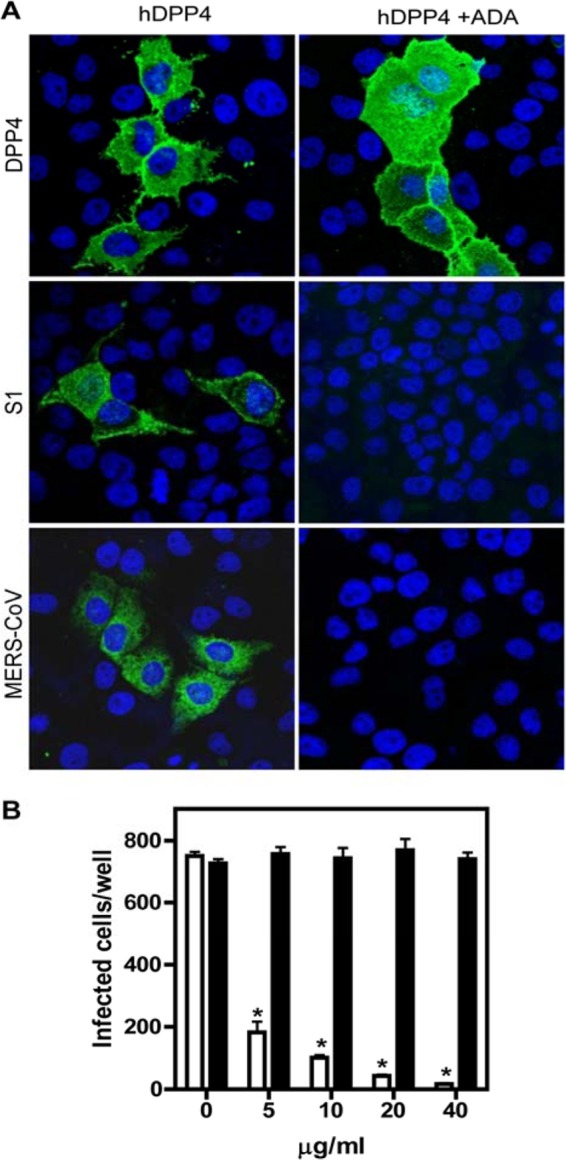
Natural antagonist ADA blocks MERS-CoV binding and infection. (A) hDPP4 plasmid-transfected MDCK cells were preincubated with 5 μg of recombinant ADA for 1 h, after which the cells were analyzed for S1 binding, DPP4 staining, and MERS-CoV infection. On hDPP4-transfected cells, ADA blocks S1 binding and MERS-CoV infection despite the expression of DPP4, visualized by fluorescent staining for DPP4, S1, and MERS-CoV, respectively. (B) Recombinant human ADA (R&D Systems)-preincubated Huh7 cells were infected with MERS-CoV for 8 h and processed. ADA (open bars) but not recombinant human angiotensin-converting enzyme 2 (rACE2) (closed bars) dose-dependently blocked MERS-CoV infection of Huh7 cells. Results representative of three different experiments are shown (one-way ANOVA test; *, P < 0.05; n = 3 per group).
Our findings demonstrate that the host range potential of the emerging novel human MERS-CoV is primarily determined by the MERS-CoV binding to DPP4. The inability to infect ferrets with a nonfunctional DPP4 further supports the crucial role of DPP4 in MERS-CoV entry. Both the site-directed mutagenesis and ADA experiments point to the crucial role of a few amino acids in blades 4 and 5 of the β-propellor region of DPP4 for binding MERS-CoV (12–14). ADA plays a central role in the differentiation and maturation of the lymphoid cells (16, 17, 19). Colocalization with adenosine receptors found on dendritic cells causes increased production of proinflammatory cytokines and T-helper cell costimulation (20). Although ADA is present in different human tissues, in serum, and in some body fluids, paracrine production by lymphoid cells may result in relatively high ADA concentrations sufficient to locally block MERS-CoV. The fact that ADA may limit infection of cells by binding to the virus binding site may be of importance in the pathogenesis of MERS-CoV but may also provide clues to help develop other antagonists for the DPP4-mediated entry of MERS-CoV.
ACKNOWLEDGMENTS
We thank Eric Snijder (LUMC, Leiden, the Netherlands) for providing the anti-SARS-CoV nsp4 antibody.
The study was financed by CEIRS (NIAID/NIH contract HHSN266200700010C) and European Union FP7 projects EMPERIE (contract number 223498) and ANTIGONE (contract number 278976).
Footnotes
Published ahead of print 20 November 2013
REFERENCES
- 1.van Boheemen S, de Graaf M, Lauber C, Bestebroer TM, Raj VS, Zaki AM, Osterhaus AD, Haagmans BL, Gorbalenya AE, Snijder EJ, Fouchier RA. 2012. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio 3:e00473–12. 10.1128/mBio.00473-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. 2012. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 367:1814–1820. 10.1056/NEJMoa1211721 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization 2013. Middle East respiratory syndrome coronavirus (MERS-CoV)—update 2013/10/04. World Health Organization, Geneva, Switzerland [Google Scholar]
- 4.Ithete NL, Stoffberg S, Corman Cottontail VMVM, Richards LR, Schoeman MC, Drosten C, Drexler JF, Preiser W. 2013. Close relative of human middle East respiratory syndrome coronavirus in bat, South Africa. Emerg. Infect. Dis. 19:1697–1699. 10.3201/eid1910.130946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Memish ZA, Mishra N, Olival KJ, Fagbo SF, Kapoor V, Epstein JH, AlHakeem R, Durosinloun A, Al Asmari M, Islam A, Kapoor A, Briese T, Daszak P, Al Rabeeah AA, Lipkin WI. November 2013. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg. Infect. Dis. 10.3201/eid1911.131172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan JF, Chan KH, Choi GK, To KK, Tse H, Cai JP, Yeung ML, Cheng VC, Chen H, Che XY, Lau SK, Woo PC, Yuen KY. 2013. Differential cell line susceptibility to the emerging novel human betacoronavirus 2c EMC/2012: implications for disease pathogenesis and clinical manifestation. J. Infect. Dis. 207:1743–1752. 10.1093/infdis/jit123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Wit E, Prescott J, Baseler L, Bushmaker T, Thomas T, Lackemeyer MG, Martellaro C, Milne-Price S, Haddock E, Haagmans BL, Feldmann H, Munster VJ. 2013. The Middle East respiratory syndrome coronavirus (MERS-CoV) does not replicate in Syrian hamsters. PLoS One 8:e69127. 10.1371/journal.pone.0069127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, Haagmans BL. 2013. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495:251–254. 10.1038/nature12005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belser JA, Katz JM, Tumpey TM. 2011. The ferret as a model organism to study influenza A virus infection. Dis. Model. Mech. 4:575–579. 10.1242/dmm.007823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martina BE, Haagmans BL, Kuiken T, Fouchier RA, Rimmelzwaan GF, Van Amerongen G, Peiris JS, Lim W, Osterhaus AD. 2003. Virology: SARS virus infection of cats and ferrets. Nature 425:915. 10.1038/425915a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boonacker E, Van Noorden CJ. 2003. The multifunctional or moonlighting protein CD26/DPPIV. Eur. J. Cell Biol. 82:53–73. 10.1078/0171-9335-00302 [DOI] [PubMed] [Google Scholar]
- 12.Bosch BJ, Raj VS, Haagmans BL. 2013. Spiking the MERS-coronavirus receptor. Cell Res. 23:1069–1070. 10.1038/cr.2013.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu G, Hu YQ, Wang J, Qi F, Gao Y, Li Y, Zhang W, Zhang Y, Yuan J, Bao B, Zhang Y, Shi J, Yan G, Gao F. 2013. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature 500:227–231. 10.1038/nature12328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang N, Shi X, Jiang L, Zhang S, Wang D, Tong P, Guo D, Fu L, Cui Y, Liu X, Arledge KC, Chen YH, Zhang LH, Wang X. 2013. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 23:986–993. 10.1038/cr.2013.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohnuma K, Haagmans BL, Hatano R, Raj VS, Mou H, Iwata S, Dang NH, Bosch BJ, Morimoto C. 25 September 2013. Inhibition of Middle East respiratory syndrome coronavirus (MERS-CoV) infection by anti-CD26 monoclonal antibody. J. Virol. 10.1128/JVI02448-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weihofen WA, Liu J, Reutter W, Saenger W, Fan H. 2004. Crystal structure of CD26/dipeptidyl-peptidase IV in complex with adenosine deaminase reveals a highly amphiphilic interface. J. Biol. Chem. 279:43330–43335. 10.1074/jbc.M405001200 [DOI] [PubMed] [Google Scholar]
- 17.Cristalli G, Costanzi S, Lambertucci C, Lupidi G, Vittori S, Volpini R, Camaioni E. 2001. Adenosine deaminase: functional implications and different classes of inhibitors. Med. Res. Rev. 21:105–128. [DOI] [PubMed] [Google Scholar]
- 18.Mou H, Raj VS, van Kuppeveld FJ, Rottier PJ, Haagmans BL, Bosch BJ. 2013. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J. Virol. 87:9379–9383. 10.1128/JVI.01277-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van der Weyden MB, Kelley WN. 1976. Human adenosine deaminase. Distribution and properties. J. Biol. Chem. 251:5448–5456 [PubMed] [Google Scholar]
- 20.Pacheco R, Martinez-Navio JM, Lejeune M, Climent N, Oliva H, Gatell JM, Gallart T, Mallol J, Lluis C, Franco R. 2005. CD26, adenosine deaminase, and adenosine receptors mediate costimulatory signals in the immunological synapse. Proc. Natl. Acad. Sci. U. S. A. 102:9583–9588. 10.1073/pnas.0501050102 [DOI] [PMC free article] [PubMed] [Google Scholar]