Abstract
Severe fever with thrombocytopenia syndrome virus (SFTSV) is a newly discovered Phlebovirus causing an emerging hemorrhagic fever in East Asia, with reported case fatality rates up to 30%. Despite the high case fatality rate and large number of persons at risk of infection, the pathobiology of the disease is unknown, and no effective animal model has been available for investigating its pathogenesis. We have studied mice and hamsters as potential small-animal models of SFTSV infection following subcutaneous, intraperitoneal, or intracerebral inoculation. Animal tissues were processed for viral load determination, histopathology, immunohistochemistry, and confocal microscopic studies. We found that immunocompetent adult mice and hamsters did not become ill after SFTSV infection. However, alpha/beta interferon receptor knockout (IFNAR−/−) mice were highly susceptible to SFTSV infection, and all mice died within 3 to 4 days after subcutaneous inoculation of 106 focus-forming units of SFTSV. Histologic examination of tissues of IFNAR−/− mice infected with SFTSV showed no detectable lesions. In contrast, by immunohistochemistry virus antigen was found in liver, intestine, kidney, spleen, lymphoid tissue, and brain, but not in the lungs. Mesenteric lymph nodes and spleen were the most heavily infected tissues. Quantitative reverse transcription-PCR (RT-PCR) confirmed the presence of virus in these tissues. Confocal microscopy showed that SFTSV colocalized with reticular cells but did not colocalize with dendritic cells, monocytes/macrophages, neutrophils, or endothelium. Our results indicate that SFTSV multiplied in all organs except for lungs and that mesenteric lymph nodes and spleen were the most heavily infected tissues. The major target cells of SFTSV appear to be reticular cells in lymphoid tissues of intestine and spleen.
INTRODUCTION
Severe fever with thrombocytopenia syndrome (SFTS) is a newly recognized viral hemorrhagic fever that has emerged in rural areas of China and more recently in Japan and Korea (1–3). SFTS is caused by SFTS virus (SFTSV), a new phlebovirus of the family Bunyaviridae. A virus genetically similar to SFTSV and causing similar human illness (Heartland virus) was also recently discovered in the United States (4). SFTSV has caused infection in thousands of people a year in China, with a reported case fatality rate among hospitalized patients of up to 30%. The major clinical manifestations and laboratory abnormalities of SFTS are fever, gastrointestinal symptoms, thrombocytopenia, leukopenia, and elevated serum hepatic enzymes. Patients with severe cases of SFTSV hemorrhagic fever usually die of multiorgan failure (1). The disease was first recognized in cases of nosocomial transmission, which were caused by contact with infectious blood of patients (5, 6). However, humans probably play little or no role in maintenance of SFTSV in nature. SFTSV is believed to be transmitted by ticks, because the virus has been detected in Haemaphysalis longicornis ticks collected from domestic animals (1). Domestic animals such as goats, dogs, and cattle within the area of endemicity have a high seroprevalence of SFTSV antibodies (7, 8). However, the animal host(s) of SFTSV remains elusive. The pathology of SFTSV infection and its target cells are unknown, since autopsies have not been performed on humans with fatal cases because of cultural resistance to autopsies and lack of motivation of physicians. SFTSV infection has been studied in animals, including mice and hamsters, but adult animals of these species are not susceptible to SFTSV (9, 10). Newborn mice and rats are susceptible to SFTSV infection when they are inoculated intracerebrally. The lack of a realistic animal model hampers understanding of the pathogenesis of SFTSV and the testing of potential therapeutic and preventive approaches, such as vaccines and drugs. In this communication, we describe a mouse model for SFTSV infection and the multiplication sites and target cells of SFTSV in an infected vertebrate host.
MATERIALS AND METHODS
Animals.
Pregnant and nonpregnant adult CD-1 female mice (ICR strain) and golden hamsters (Mesocricetus auratus) were purchased from Harlan Sprague-Dawley (Indianapolis, IN). Six- to 10-week-old mice deficient for alpha/beta interferon receptor (IFNAR−/−) on the 129/Sv background (originally provided by Herbert Virgin, Washington University School of Medicine, St. Louis, MO) (11) were obtained from the laboratory colony of one of the investigators.
Virus.
The YL-1 strain of SFTSV was used in these experiments. This virus was originally isolated in 2011 from the acute-phase serum of a Chinese patient with SFTS. The virus used to infect the immunocompetent mice (CD-1) and hamsters had been passaged twice in Vero cells. The SFTSV strain used to infect the immunodeficient mice (IFNAR−/−) had been passaged twice in Vero cells (2×Vero) and two additional times by intracranial (IC) inoculation of newborn suckling mice (2×SM).
Focus-forming assay.
SFTSV stock was diluted in 10-fold increments, and each dilution (250 μl) was inoculated onto a monolayer of Vero cells in 6-well plates. After rocking for 1 h at 37°C, the suspension was removed from each well, and each well was overlaid with 2 ml of 0.8% methyl cellulose solution. The cells were cultivated for 7 days in a 37°C incubator with 5%CO2. The methyl cellulose overlay was removed from each well, and the monolayers were fixed with methanol-acetone (1:1) (1 ml) for 30 min. After three 15-min washes with phosphate-buffered saline (PBS), the monolayers were blocked with 3% fetal bovine serum in PBS for 15 min and were incubated for 18 h with rabbit antibody against SFTSV. The monolayers were incubated for 1 h with 1:2,000 goat anti-rabbit IgG conjugated to horseradish peroxidase (HRP) in blocking solution, and the color was developed with aminoethylcarbazole (Sigma).
Infection of animals.
Newborn (1- to 2-day-old) CD-1 mice and hamsters were inoculated by the IC route; each animal received approximately 15 μl of virus suspension (∼104.2 focus-forming units [FFU] of SFTSV). The adult CD-1 mice and hamsters were inoculated intraperitoneally (IP) with 100 μl (∼105.0 FFU of SFTSV) of the 2×Vero-passaged virus suspension. The adult IFNAR−/− mice were inoculated subcutaneously (SC) with 100 μl of the 2×Vero-, 2×SM-passaged virus stock, containing 106 FFU of SFTSV/ml.
All animal procedures complied with USDA guidelines and were carried out under a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Medical Branch (UTMB). All infected animals were housed in an animal biosafety level 3 (ABSL3) animal facility.
Quantitative real-time PCR.
Viral loads were determined by quantitative real-time PCR (qPCR) on samples of blood, brain, lung, heart, liver, spleen, intestine, and mesenteric lymph nodes collected from IFNAR−/− adult mice daily for four consecutive days after infection. EDTA-anticoagulated blood or homogenized tissue (140 μl) was mixed with 560 μl of AVL buffer (QIAamp viral RNA minikit; Qiagen). RNA was isolated following the manufacturer's instructions. To determine viral titers in tissues, a 3-mm3 piece of tissue was removed during necropsy and homogenized using metal beads (Tissue Lyser; Qiagen) in RLT buffer (Qiagen). The RNA was extracted using the RNeasy kit (Qiagen), according to the manufacturer's instructions. SFTSV-specific qPCR was performed as previously described (12). Viral titers were reported as genome equivalents (GEQ). To normalize viral content in tissues to the total amount of RNA, a housekeeping gene, encoding glyceraldehyde 3-phosphate dehydrogenase (GAPDH), was measured by reverse transcription-PCR (RT-PCR). Genome equivalents were normalized to 1 μg of RNA for each tissue. Predeveloped mouse GAPDH hydrolysis probes and TaqMan gene expression assays (both from Applied Biosystems, Foster City, CA) were used. Assays were performed on a StepOnePlus thermal cycler (Applied Biosystems) and analyzed with StepOne software, version 2.1.
Immunohistochemistry (IHC).
Samples of SFTSV-infected IFNAR−/− mouse tissues were collected daily for four consecutive days postinoculation (p.i.). The tissues from infected mice were fixed in 10% neutral buffered formalin for 36 h and then transferred to 70% ethanol before being processed for routine paraffin embedding. Several 4- to 5-μm sections of each organ were prepared and were stained by the hematoxylin-and-eosin (H&E) method. Immunohistochemical (IHC) staining procedures were also performed to detect the presence of SFTSV antigens in the tissues as previously described (13, 14). An SFTSV-specific rabbit immune serum, prepared in our laboratories, was used for detecting SFTSV antigens by IHC.
Confocal microscopy.
Thin sections of SFTSV-infected IFNAR−/− mouse tissues were deparaffinized with xylene and ethanol. The slides were blocked with normal goat IgG. Each slide was then incubated with the rabbit anti-SFTSV polyclonal serum and one of the following antibodies: hamster anti-mouse CD11c monoclonal antibody, mouse monoclonal antibody against CD62P (Thermo Scientific), rat monoclonal antibody against mouse CD31, mouse monoclonal antibody against CD68, rat monoclonal antibody (NIMP-R14) to murine neutrophil marker Ly-6 antigen (Abcom), or rat monoclonal antibody against the murine macrophage marker F4/80. The slides were further incubated with Alexa Fluor 594 goat anti-rabbit IgG (heavy plus light chain [H+L]), Alexa Fluor 488 goat anti-rat IgG (H+L), Alexa Fluor 594 goat and rabbit IgG(H+L), Alexa Fluor 488 goat and hamster IgG (H+L), or Alexa Fluor 488 F(ab′)2 fragment of goat anti-mouse IgG (H+L) (Invitrogen). The slides were finally stained with DAPI (4′,6-diamidino-2-phenylindole) and then examined under an Olympus confocal microscope.
RESULTS
Development of a small-animal model of SFTS.
In our initial attempts to identify a small-animal model for SFTS, newborn and adult CD-1 mice and newborn and adult hamsters were inoculated with the 2×Vero-passaged SFTSV. None of the adult CD-1 mice nor the hamsters showed signs of illness, and all survived. However, some of the newborn CD-1 mice developed signs of illness (lethargy, tremors, and loss of balance) beginning 7 to 8 days p.i. Brains from some of the sick pups were homogenized in PBS with 20% fetal bovine serum to prepare a 10% brain suspension. After centrifugation and filtration, the supernatant was inoculated IC into other groups of newborn CD-1 mice. On the second passage, the virus consistently produced illness in the newborn mice within 5 to 7 days and death by day 9 p.i. A 10% brain homogenate of the second mouse-passaged virus (2×Vero, 2×SM) was prepared and served as the virus stock for subsequent animal experiments. This virus stock still did not produce illness in newborn hamsters or in adult CD-1 mice or hamsters.
At this point, it was decided to test the susceptibility of the IFNAR−/− mice. Accordingly, 21 adult (both male and female) IFNAR−/− mice were inoculated subcutaneously (SC) with 100 μl of the 2×Vero-, 2×SM-passaged SFTSV strain. All IFNAR−/− mice were inoculated with 106 SFTSVs. Fresh blood and tissue samples were collected for assay by sacrificing five mice on days 1 and 2 and four mice on days 3 and 4 after inoculation of SFTSV. The knockout mice proved to be highly susceptible. The animals appeared ill by day 3, and all mice were dead (3 mice) or moribund (4 mice) on day 4.
Viral load.
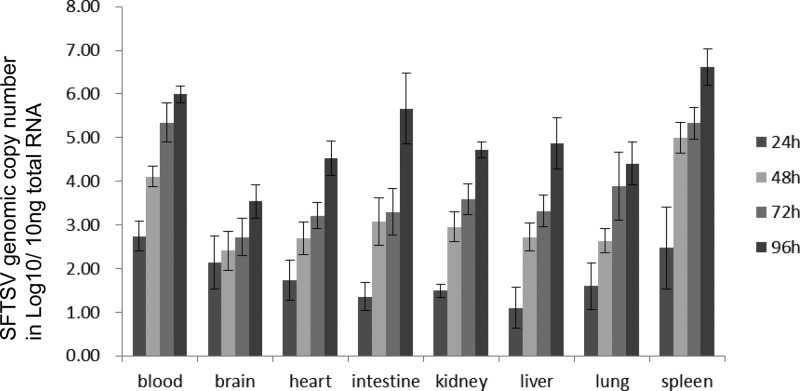
Viral loads in the blood and tissues of the IFNAR−/− mice collected at various times after infection were determined by qPCR as described above. SFTSV RNA was detected at all time points in all organs and blood. The amount of viral RNA increased daily, and the highest titers were observed on day 4 (Fig. 1). Spleen had the highest titers of SFTSV at all time points, and blood contained the next highest viral load. The viral loads in blood and spleen reached 106 and 106.6, respectively, on day 4 after inoculation. Intestine had a relatively low viral load initially but rapidly reached a high titer, with the peak on day 4. Brain had the lowest viral load among the organs. Lung, kidney, liver, and heart were intermediate.
FIG 1.
Concentrations of SFTSV in organs of IFNAR−/− mice at various time points after infection, as determined by qPCR.
Detection of SFTSV antigens in tissues.
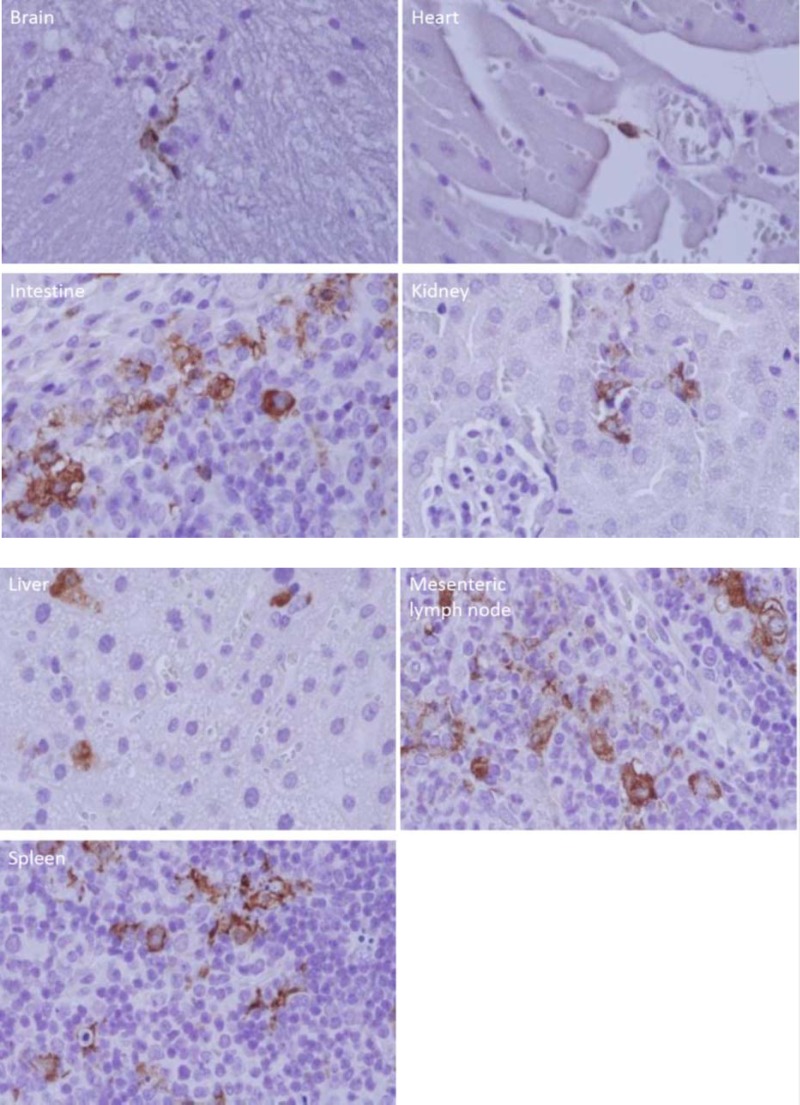
H&E staining of IFNAR−/− mouse tissues revealed no identifiable histological differences between SFTSV-infected mice and noninfected mice. However, IHC staining revealed viral antigen on day 4 in the brain, heart, intestine, mesenteric lymph node, liver, kidney, and spleen. Viral antigen was detected in liver and spleen as early as day 2 after inoculation. SFTSV antigen was most abundant in intestinal lymphoid follicles, mesenteric lymph nodes, and spleen (Fig. 2). Viral antigen was observed to be most abundant in mononuclear-type cells. Viral antigen was not detected in the lung by IHC (data not shown). The absence of viral antigen in lung tissue differed from the qPCR result, as the latter detected a small amount of SFTSV in lungs. The discrepancy between qPCR and IHC viral content in the lungs was most likely due to qPCR detection of SFTSV in residual blood in lung tissue. In uninfected controls, no IHC reaction was detected in the mouse tissues (data not shown), indicating the specificity of our immunohistochemical method for SFTSV antigens in infected mouse tissues. The results suggested that lymphoid tissues, including spleen, intestinal lymphoid follicles, and mesenteric lymph nodes, were the primary replication sites of SFTSV.
FIG 2.
Immunohistochemistry-stained tissues of a mouse infected with SFTSV. The anti-SFTSV antibody-stained cells are shown in each organ: an unidentified perivascular cell in the cerebrum; an unidentified perivascular, spindle-shaped cell in the myocardium; mononuclear cells of an intestinal submucosal lymphoid follicle; unidentified cells in the location of intertubular capillaries in the renal cortex; hepatocytes and sinusoidal lining cells; unidentified mononuclear cells in a mesenteric lymph node; and unidentified mononuclear cells in the spleen. The experiments were repeated 3 times, and the results were consistent.
Target cells of SFTSV.
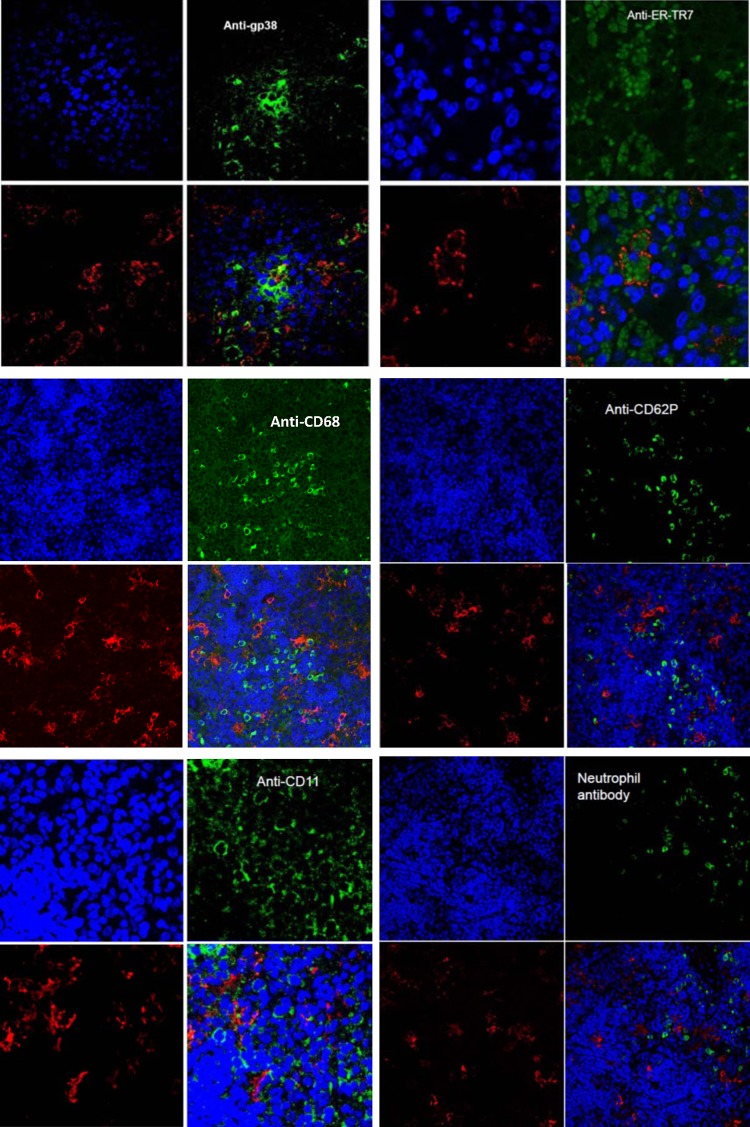
To determine the specific SFTSV-infected cells in mouse tissues, we first tested colocalization of SFTSV with hematopoietic cells, using confocal microscopy on splenic tissues of SFTSV-infected IFNAR−/− mice. Spleen was examined because it had the heaviest SFTSV load among the mouse tissues. Deparaffinized spleen tissues in thin sections were double stained with antibodies to SFTSV and antibodies to monocyte/macrophages (anti-CD68 and anti-F4/80), dendritic cells (anti-CD11c), endothelium (anti-CD31 and -CD34), megakaryocyte/platelets (anti-CD62P), or neutrophils (anti-Ly-6). Confocal microscopy showed that SFTSV did not colocalize with CD11c, CD31, CD34, CD62P, CD68, F4/80, or anti-neutrophil antibody (Fig. 3) or CD31 and CD34 (data not shown). The results suggested that SFTSV did not infect dendritic cells, megakaryocytes, monocytes/macrophages, neutrophils, or endothelium.
FIG 3.
Confocal microscopic images of thin sections of SFTSV-infected mouse spleen stained with antibody to gp38 and antibody to ER-TR7 recognizing reticular cells, antibody to CD68 recognizing macrophages, antibody to CD62P recognizing megakaryocytes/platelets, antibody to Ly-6 recognizing neutrophils, and antibody to CD11c (dendritic cells). Each panel shows the following: top left, DAPI-stained cell nuclei (blue); top right, specific antibody-stained cells (green); bottom left, rabbit anti-SFTSV antibody-labeled SFTSV (red); bottom right, merged images. SFTSV colocalized with gp38-stained host cells (yellow dots) and with antibody to ER-TR7-stained cells (SFTSV surrounding ER-TR7 markers inside cells). No colocalization of SFTSV with other cells was observed. The confocal microscopy for each antibody was repeated at least five times, and the results were consistent.
Then we analyzed the colocalization of SFTSV with reticular cells in spleen, because the SFTSV-infected cells were similar in shape and distribution to reticular cells. Confocal results showed that most SFTSV-infected host cells were also recognized by antibody to ER-TR7b, which reacted with an antigen that was located in the cytoplasm of reticular fibroblasts, and antibody to gp38, which recognizes fibroblast cell podoplanin antigen (Fig. 3). These results suggested that SFTSV primarily infected reticular cells in mouse spleen.
DISCUSSION
A previous study showed that all newborn mice of the Kungming (KM), BALB/c, and C57/BL6 strains died after intracerebral inoculation of SFTSV, while 35 to 50% of newborn mice died after intraperitoneal inoculation of SFTSV (10). However, we demonstrated that newborn CD-1 mice developed signs of illness (lethargy, tremors, and loss of balance) beginning 7 to 8 days p.i., but no mouse died of SFTSV infection. The difference in the susceptibility of the mice to SFTSV may be caused by genetic differences in the mice. Previous studies demonstrated that adult mice of the KM, BALB/c, and C57/BL6 strains are not susceptible to SFTSV regardless of the inoculation route (9, 10). The only signs of SFTSV infection in C57/BL6 mice are slightly low white blood cell and platelet counts at one time point (7). These studies indicated that immunocompetent adult mice are not susceptible to SFTSV.
In the present study using IFNAR−/− mice, we developed an animal model that closely mimics SFTSV infection in humans. The fact that the virus causes severe disease in alpha/beta interferon receptor knockout mice suggests that type I interferon may be essential for resistance to SFTSV infection. SFTSV causes severe illness mainly in elderly people. The age of reported SFTS patients in China ranges from 1 to 90 years, but people over 50 years of age comprise 75% of the cases. This age group constitutes only 26% of the human population under surveillance (P < 0.001) (15). The observed susceptibility of elderly people to severe SFTSV infection may result from a decreased level of host immunity that occurs in aged persons.
We have detected viral antigens in the spleen, mesenteric lymph nodes, intestinal lymphoid nodules, liver, kidney, and heart, but not the lung. Our results indicated that SFTSV mainly infects lymphoid tissues. Chen et al. demonstrated that the mouse brain contained the largest amount of SFTSV RNA, followed by the lung, spleen, kidney, and heart (10). The highest level of viral RNA in the brain might have been caused by intracerebral inoculation. Jin et al. demonstrated that SFTSV RNA was detected only in the spleen, liver, and kidney in C57/BL6 mice (9), which may be caused by low viral load in the organs, because C57/BL6 mice are resistant to SFTSV.
Our finding of SFTSV infection of intestinal lymphoid nodules may explain the frequent clinical manifestations observed in SFTSV-infected patients, namely, gastrointestinal symptoms. The gastrointestinal symptoms may be caused by multiplication of SFTSV in the patient's intestine, mesenteric lymph nodes, and other abdominal sites. SFTSV-infected patients often have multiple organ failure before death; this may be caused by viral multiplication in all organs except for lungs, as observed in our animal model.
Chen et al. observed necrosis and mononuclear cell infiltrations in the liver of mice that died of SFTSV infection, but no obvious pathological changes were observed in other organs (10). Jin et al. demonstrated that lymphocyte cellularity of the red pulp was decreased in spleens of SFTSV-infected mice during the first week after inoculation (9). In addition, at an early stage of SFTSV infection, a marked increase in megakaryocytes was observed in the spleen and bone marrow. During the late phase of SFTSV infection, pathological changes were noted in liver and kidney. The primary lesions in liver consisted of ballooning degeneration of hepatocytes and scattered necrosis, the latter indicated by multifocal pyknosis, karyorrhexis, and karyolysis. The kidney showed glomerular hypercellularity, mesangial thickening, and congestion in Bowman's space, but infiltration of inflammatory cells was absent. In contrast, we did not detect any pathological lesions in the mouse tissues.
SFTSV was first isolated using DH82 cells (1), a monocyte cell line, and its target cell was suspected to be the monocyte/macrophage. A further study proposed the macrophage as the target cell of SFTSV (9). However, we were unable to colocalize SFTSV in macrophages/monocytes in the mouse tissues, suggesting that macrophages are not the target cells of SFTSV. We used ER-TR7 and GP38 antibodies against fibroblast reticular cell markers to demonstrate that SFTSV infects fibroblast reticular cells (FRCs). However, determining the target cells of SFTSV in humans will need further histological studies of SFTS patients.
FRCs are central to the highly ordered microarchitecture of secondary lymphoid organs. There are four FRC subsets within the outer lymph node, where they provide a three-dimensional supporting scaffold, define the T- and B-lymphocyte compartments, direct the movement of fluid and cellular constituents, provide homeostatic factors for T lymphocytes, and, finally, interact directly with T and B lymphocytes, natural killer (NK) cells, and dendritic cells (16). FRCs may thus play pivotal roles in the host immune response to some viral pathogens, as shown recently for lymphocytic choriomeningitis virus (LCMV) and Ebola virus (16). It is possible that the FRC conduit was destroyed by SFTSV infection, altering the host immune response to SFTSV. This hypothesis needs to be further investigated.
FRCs express a number of cytokine and chemokine molecules, including interleukin 6 (IL-6), IL-7, CCL19, CCL21, CCL2/MCP-1, CXCL16, cell adhesion VCAM-1, ICAM-1, BP-3, alpha-type and beta-type platelet-derived growth factor receptors (PDGF-RA and PDGF-RB), the lymphotoxin-beta receptor (LT[beta]-R), tumor necrosis factor receptor 1, tissue transglutaminase (TTG), fibronectin, Meca-79, vimentin, smooth-muscle actin, desmin, and gp38 (16). The molecules expressed by FRCs in SFTS patients are largely unknown, except for IL-6, which is increased (17).
ACKNOWLEDGMENTS
This study was funded by a Pilot Grant from the Institute of Human Infection and Immunity, University of Texas Medical Branch, and by contract HHSN27220100004OI/HHSN27200004/O4 from the National Institutes of Health.
Footnotes
Published ahead of print 20 November 2013
REFERENCES
- 1.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 364:1523–1532. 10.1056/NEJMoa1010095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.AProMED-mail post 21 May 2013. Severe fever with thrombocytopenia syndrome—South Korea: suspected. Archive no. 20130521.1729124 http://www.promedmail.org [Google Scholar]
- 3.Avian Flu Diary 2013. SFTS fatality reported in Japan. http://afludiary.blogspot.com/2013/01/sfts-fatality-reported-in-japan.html [Google Scholar]
- 4.McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, Metcalfe MG, Batten BC, Albarino CG, Zaki SR, Rollin PE, Nicholson WL, Nichol ST. 2012. A new phlebovirus associated with severe febrile illness in Missouri. N. Engl. J. Med. 367:834–841. 10.1056/NEJMoa1203378 [DOI] [PubMed] [Google Scholar]
- 5.Bao CJ, Qi X, Wang H. 2011. A novel bunyavirus in China. N. Engl. J. Med. 365:862–863. 10.1056/NEJMc1106000 [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Li Q, Hu W, Wu J, Wang Y, Mei L, Walker DH, Ren J, Wang Y, Yu XJ. 2012. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis. 12:156–160. 10.1089/vbz.2011.0758 [DOI] [PubMed] [Google Scholar]
- 7.Zhao L, Zhai S, Wen H, Cui F, Chi Y, Wang L, Xue F, Wang Q, Wang Z, Zhang S, Song Y, Du J, Yu XJ. 2012. Severe fever with thrombocytopenia syndrome virus, Shandong Province, China. Emerg. Infect. Dis. 18:963–965. 10.3201/eid1806.111345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiao Y, Zeng X, Guo X, Qi X, Zhang X, Shi Z, Zhou M, Bao C, Zhang W, Xu Y, Wang H. 2012. Preparation and evaluation of recombinant severe fever with thrombocytopenia syndrome virus nucleocapsid protein for detection of total antibodies in human and animal sera by double-antigen sandwich enzyme-linked immunosorbent assay. J. Clin. Microbiol. 50:372–377. 10.1128/JCM.01319-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin C, Liang M, Ning J, Gu W, Jiang H, Wu W, Zhang F, Li C, Zhang Q, Zhu H, Chen T, Han Y, Zhang W, Zhang S, Wang Q, Sun L, Liu Q, Li J, Wang T, Wei Q, Wang S, Deng Y, Qin C, Li D. 2012. Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse model. Proc. Natl. Acad. Sci. U. S. A. 109:10053–10058. 10.1073/pnas.1120246109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen XP, Cong ML, Li MH, Kang YJ, Feng YM, Plyusnin A, Xu J, Zhang YZ. 2012. Infection and pathogenesis of Huaiyangshan virus (a novel tick-borne bunyavirus) in laboratory rodents. J. Gen. Virol. 93:1288–1293. 10.1099/vir.0.041053-0 [DOI] [PubMed] [Google Scholar]
- 11.Yun NE, Poussard AL, Seregin AV, Walker AG, Smith JK, Aronson JF, Smith JN, Soong L, Paessler S. 2012. Functional interferon system is required for clearance of Lassa virus. J. Virol. 86:3389–3392. 10.1128/JVI.06284-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, Liang M, Qu J, Jin C, Zhang Q, Li J, Jiang X, Wang Q, Lu J, Gu W, Zhang S, Li C, Wang X, Zhan F, Yao W, Bi Z, Wang S, Li D. 2012. Early diagnosis of novel SFTS bunyavirus infection by quantitative real-time RT-PCR assay. J. Clin. Virol. 53:48–53. 10.1016/j.jcv.2011.09.031 [DOI] [PubMed] [Google Scholar]
- 13.Xiao SY, Guzman H, Zhang H, Travassos da Rosa AP, Tesh RB. 2001. West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg. Infect. Dis. 7:714–721. 10.3201/eid0704.017420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao SY, Zhang H, Guzman H, Tesh RB. 2001. Experimental yellow fever virus infection in the golden hamster (Mesocricetus auratus). II. Pathology J. Infect. Dis. 183:1437–1444. 10.1086/320200 [DOI] [PubMed] [Google Scholar]
- 15.Xiong WY, Feng ZJ, Matsuib T, Foxwell A. 2012. Risk assessment of human infection with a novel bunyavirus in China. Western Pac. Surveill. Response J. 3:61–66. 10.5365/wpsar.2012.3.3.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steele KE, Anderson AO, Mohamadzadeh M. 2009. Fibroblastic reticular cell infection by hemorrhagic fever viruses. Immunotherapy 1:187–197. 10.2217/1750743X.1.2.187 [DOI] [PubMed] [Google Scholar]
- 17.Deng B, Zhang S, Geng Y, Zhang Y, Wang Y, Yao W, Wen Y, Cui W, Zhou Y, Gu Q, Wang W, Wang Y, Shao Z, Wang Y, Li C, Wang D, Zhao Y, Liu P. 2012. Cytokine and chemokine levels in patients with severe fever with thrombocytopenia syndrome virus. PLoS One 7:e41365. 10.1371/journal.pone.0041365 [DOI] [PMC free article] [PubMed] [Google Scholar]