Abstract
The woodchuck model is an informative model for studies on hepadnaviral infection. In this study, woodchuck hepatitis virus (WHV) transgenic (Tg) mouse models based on C57BL/6 mice were established to study the pathogenesis associated with hepadnaviral infection. Two lineages of WHV Tg mice, harboring the WHV wild-type genome (lineage 1217) and a mutated WHV genome lacking surface antigen (lineage 1281), were generated. WHV replication intermediates were detected by Southern blotting. DNA vaccines against WHV proteins were applied by intramuscular injection. WHV-specific immune responses were analyzed by flow cytometry and enzyme-linked immunosorbent assays (ELISAs). The presence of WHV transgenes resulted in liver-specific but sex- and age-dependent WHV replication in Tg mice. Pathological changes in the liver, including hepatocellular dysplasia, were observed in aged Tg mice, suggesting that the presence of WHV transgenes may lead to liver diseases. Interestingly, Tg mice of lineage 1281 spontaneously developed T- and B-cell responses to WHV core protein (WHcAg). DNA vaccination induced specific immune responses to WHV proteins in WHV Tg mice, indicating a tolerance break. The magnitude of the induced WHcAg-specific immune responses was dependent on the effectiveness of different DNA vaccines and was associated with a decrease in WHV loads in mice. In conclusion, sex- and age-dependent viral replication, development of autoimmune responses to viral antigens, pathological changes in the liver in WHV Tg mice, and the possibility of breaking immune tolerance to WHV transgenes will allow future studies on pathogenesis related to hepadnaviral infection and therapeutic vaccines.
INTRODUCTION
Hepatitis B virus (HBV) is a major cause of acute and chronic hepatitis in humans. The currently available treatments for hepatitis B, such as alpha interferon (IFN-α) or nucleoside/nucleotide analogues, are costly and have limited long-term efficacy (1, 2).
HBV has a very narrow host range and can infect only humans and higher primates such as chimpanzees (3, 4). However, experiments using chimpanzees are costly and require both scientific and ethical justification. HBV transgenic (Tg) mice have allowed examination of the influence of viral and host factors on HBV pathogenesis and replication and assessment of the antiviral potential of pharmacological agents (5). For example, the transfer of HBsAg-specific CD8+ T cells into these mice led to the inhibition of HBV replication in the liver by a noncytolytic mechanism (6), leading to the important hypothesis that the antiviral cytokines IFN-γ and tumor necrosis factor alpha are major mediators of noncytolytic inhibition of HBV replication (7). Furthermore, activation of innate immune responses in HBV Tg mice by Toll-like receptor (TLR) ligands also inhibits HBV replication (8). Thus, the HBV Tg mouse model was and remains a useful research tool to study HBV infection. However, as HBV Tg mice are immunologically tolerant to HBV proteins (5, 9), obvious disease-related phenotypes have not been observed. On the other hand, HBsAg is supposed to play an important role in HBV persistence and hepatocarcinogenesis (10). The presence of HBsAg may inhibit host immune responses and facilitate HBV persistence (11, 12). Therefore, transgenic mice replicating HBV but lacking small HBs expression represent an interesting model for studies of the role of HBsAg in HBV persistence, hepatocarcinogenesis, and antiviral immune responses (13).
Woodchuck hepatitis virus (WHV) is a member of the Hepadnaviridae family and was discovered in 1978 (14). WHV causes acute and chronic infections in woodchucks (Marmota monax) similar to HBV infections in humans. The woodchuck model has been proven to be an informative model for studies of hepadnaviral infection and pathogenesis and for the screening and development of antiviral drugs (15). Despite the establishment of immunological assays and tools for the woodchuck model during the last years, immunological studies in outbred and immunogenetically uncharacterized woodchucks are still difficult (16, 17). Therefore, it was desirable to have a WHV Tg mouse model that would allow the determination of immunological parameters and complement infection experiments in woodchucks.
In the present study, we report the establishment of two WHV Tg mouse strains, one with a wild-type WHV genome and the other with a WHV genome harboring artificially introduced stop codons in the coding region of WHV surface antigen (WHsAg). The presence of WHV transgenes resulted in liver-specific but sex- and age-dependent WHV replication in Tg mice. One particularly interesting feature of WHV Tg mice was the development of autoimmune T-cell responses to WHV core antigen and pathological changes in aged Tg mice, allowing the study of liver diseases related to hepadnaviral infection. In addition, immunizations using different DNA vaccines induced specific immune responses to WHV proteins that were associated with a decrease in WHV loads. WHV Tg mouse strains appear to be excellent tools for testing vaccines for therapy of chronic hepadnavirus infections.
MATERIALS AND METHODS
Molecular cloning of the transgene construct for Tg mouse generation.
To construct a 1.3-fold-overlength WHV genome in which precore and core transcription would be under the control of their endogenous promoters and enhancers, plasmid puc119WHV-1.3 containing 1.3-fold WHV DNA (WHV nucleotide [nt] 1050-nt 3320-nt 2190) was constructed based on plasmid puc119CMVWHV82 (18). In addition, a WHsAg-minus variant of the WHV genome was constructed by using a recombinant PCR mutagenesis method. Three stop codons were introduced into the WHsAg open reading frame (ORF) at amino acid positions 12, 18, and 19, downstream of the WHsAg initiation codon, leading to the truncation of large-, middle-, and small-WHsAg. The amino acid sequence of the WHV polymerase remained intact. The wild-type and WHsAg-minus WHV constructs were sequenced to exclude the presence of undesired mutations. The WHV constructs were linearized by KpnI and PvuII digestion and then purified by gel electrophoresis prior to microinjection. C57BL/6 × C3H mice were used to make Tg mice, which were then backcrossed >10 times with C57BL/6. All mice were kept in the Central Animal Laboratory of the University of Essen. Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (19) and were reviewed and approved by the local Animal Care and Use Committee of the district government (Düsseldorf, Germany) and the Fox Chase Cancer Center.
Genotyping of WHV Tg mice.
Genomic DNA was extracted from the tail tissue of mice. One microgram of DNA from the samples was subjected to PCR to analyze the presence of the WHV transgene. Primer pairs used for amplification of the WHV core region were wc1 (5′-TGG GGC ATG GAC ATA GAT CCC TA-3′) (nt 2015) and wc-149s (5′-AAG ATC TCT AAA TGA CTG TAT GTT CCG-3′) (nt 2467), with numbering according to the reference sequence under GenBank accession number M19183. PCR was carried out as described previously (15, 20).
Analysis of transgenic copy number and integration sites.
The transgene copy number and number of integration sites in WHV Tg mice were determined according to the protocol of Thom Saunders (http://www.med.umich.edu/tamc/std.pdf). Briefly, 10 μg of hepatic genomic DNA was digested by EcoRI, phenol extracted, precipitated, and then analyzed by Southern blotting with copy standards.
Detection of WHV DNA in serum.
Serum WHV DNA was extracted by using the QIAamp DNA Minikit and detected by real-time PCR, as described previously (15), with primers wc1 and wc-149s.
Isolation and analysis of viral RNA and WHV replicative intermediates from liver tissue.
Total RNA was extracted from liver tissue samples with TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Real-time reverse transcription (RT)-PCR was carried out according to previously reported protocols (21). Encapsidated WHV DNA was analyzed as described previously (9). WHV DNAs were detected by hybridization with a 32P-labeled full-length WHV probe.
Histological examination of liver tissues in WHV Tg mice.
Liver tissue samples were fixed in 10% zinc-buffered formalin (Anatek, Battle Creek, MI) and embedded in paraffin, and sections were cut for histological and microscopic examination according to standard methods, as described previously (22).
ELISA for detection of antibodies to WHcAg (anti-WHc) and WHsAg (anti-WHs).
Anti-WHc IgGs were detected by an enzyme-linked immunosorbent assay (ELISA) as described previously (23). Total bound mouse IgG, IgG1, or IgG2a antibodies were detected with appropriate secondary antibodies labeled with horseradish peroxidase (DB Biosciences, CA) at a dilution of 1:1,000. This ELISA format did not strictly differentiate antibodies against WHcAg or WHeAg, as the preparation of recombinant WHcAg contained a fraction of polypeptides exposing various epitopes. However, the ELISA allowed the differentiation of subtypes of WHcAg/WHeAg-specific antibodies. Anti-WHs antibodies in sera were also detected by ELISA, as described previously (24). Normal C57BL/6 mouse serum samples were used as negative controls. The cutoff value of ELISAs was set as 2.1 times over the mean optical density (OD) of negative controls.
Immunization of mice by intramuscular injection of pWHcIm, pWHsIm, and pCGWHc.
Plasmids pWHsIm and pWHcIm containing the WHV preS2-S or core gene, respectively, under the control of the cytomegalovirus (CMV) promoter, were described previously (24). Plasmid pCGWHc containing the WHV core gene downstream of the β-globin intron sequence, under the control of the cytomegalovirus (CMV) promoter, was described previously by Kosinska et al. (25). Immunization of mice was performed according to a procedure described previously by Schirmbeck et al. (26). Plasmid injections were repeated twice at 2-week intervals. Mice were sacrificed 2 weeks after the last immunization.
Detection of cytotoxic T lymphocytes by flow cytometry.
WHcAg-specific cytotoxic T lymphocytes (CTLs) were detected by flow cytometric analysis (16). Briefly, a set of 36 synthetic peptides covering the complete WHcAg sequence, with a length of 188 amino acid (aa) residues, was purchased from EMC Microcollections (Tübingen, Germany). The sequences of the peptides used for the immunological assays matched the sequence of the WHV transgene. The 15-mer peptides overlapped by 10 aa and were combined in six pools (16). Three peptides containing CD8 dominant epitopes of WHcAg identified in C57BL/6 mice were used to detect specific CTLs postimmunization, at aa 13 to 21 (YQLLNFLPL), aa 6 to 20 (YKEFGSSYQLLNFLP), and aa 11 to 25 (SSYQLLNFLPLDFFP). An unrelated CMV-derived peptide, YILEETSVM, served as an unrelated control. For dimer staining of WHc-specific CTLs, recombinant soluble dimeric mouse H-2D[b]:Ig (DimerX; BD Bioscience) was loaded by WHc peptide aa 13 to 21 overnight and then used to stain the splenocytes according to the manufacturer's instructions. For the determination of the frequencies of specific CD8+ IFN-γ+ T cells, the cutoff value was set as 2 times over the value obtained by stimulation with the CMV peptide.
Up to 1 × 106 splenocytes per well were plated into 96-well plates (Greiner Bio-One, Frickenhausen, Germany) in a total of 200 μl AIM-V medium (Gibco Invitrogen) supplemented with 10% fetal calf serum and 10 U/ml penicillin-streptomycin (PAA Laboratories, Pasching, Austria). For stimulation, peptide pools or individual peptides were added to a final concentration of 2 μg/ml. After 3 days, a final concentration of 10 U/ml recombinant human interleukin 2 (Roche Diagnostics, Mannheim, Germany) was added. Flow cytometric analysis was carried out with a FACSCalibur flow cytometer (Becton, Dickinson, Heidelberg, Germany). In all cases, at least 100,000 events were collected for each sample. Data files were analyzed with FlowJo software (Tree Star, Ashland, OR).
RESULTS
Genomic features of Tg mice.
WHV Tg mice with the wild-type WHV genome and the mutated version unable to express WHsAg were generated (Fig. 1A) and designated lineages 1217 and 1281, respectively. Integrated WHV DNA and the flanking nucleotide sequence were analyzed by inverse PCR. Sequence analysis of WHV transgenes in the mouse genome excluded unexpected mutations in the integrated WHV sequences (data not shown). IPCRs generated a single PCR product each. Direct sequencing of PCR products suggested only a single integration site of the WHV genome in the mouse genome in both WHV Tg mouse strains (data not shown). The integration sites of the transgenes were mapped, showing that WHV transgenes were integrated into chromosome 10 of lineage 1217 mice and into chromosome 5 of lineage 1281 mice (data not shown). Southern blot analysis of EcoRI-restricted genomic DNA of WHV Tg mice detected only 2 bands when a complete WHV genome was used as a probe. The signals were comparable to that of a single-copy WHV standard, indicating that a single copy of the WHV genome was integrated at a single site in the mouse genomes of both lineages 1217 and 1281 (Fig. 1B), consistent with the cloning results.
FIG 1.
Generation and identification of WHV Tg mice. (A) Structure of plasmid puc119WHV-1.3. Shown is a schematic representation of 1.3-fold-overlength WHV constructs for the generation of WHV Tg mice. En, enhancer; Poly A, polyadenylation signal. The black ovals indicate the promoter sequences of WHV ORFs. The three stop codons introduced into the S ORF are underlined. (B) Transgenic copy number and number of integration sites of WHV transgenes. A total of 10 μg of mouse genomic DNA was digested with EcoRI and then subjected to Southern blot analysis with linear full-length WHV copy standards.
WHV gene expression and replication in Tg mice are liver specific and age and sex dependent.
The levels of WHV replicative intermediates were significantly lower in the liver of mice of lineage 1217 than in the liver of mice of lineage 1281 (Fig. 2A). Encapsidated WHV replicative intermediates were detected only in the livers and not in kidney and spleen from both lineages of WHV Tg mice (Fig. 2B). These bands were comparable to the signals detected in liver tissues from WHV chronically infected woodchucks. Interestingly, WHV replication increased in an age- and sex-dependent manner. WHV replication intermediates were detected in mice at 2 weeks of age and increased as the mice reached maturity, with high levels being detected through 40 weeks of age (Fig. 2B and C). Compared with female mice, male mice had high levels of WHV replicative intermediates in the liver (Fig. 2C) and significantly high levels of serum WHV DNA loads (Fig. 2D).
FIG 2.
Organ-, age-, and sex-dependent WHV gene expression and replication in Tg mice. (A to C) WHV replicative intermediates in the liver, spleen, and kidney of mice of lineages 1281 (A to C) and 1217 (A) were analyzed by Southern blotting. (D) WHV viral loads in the serum of mice of lineage 1217 were determined by real-time PCR. (E) Intrahepatic WHV mRNAs in mice of lineage 1281 were determined by real-time RT-PCR. PWHs, primary woodchuck hepatocytes; M, male; F, female; L, liver; K, kidney; S, spleen; ET, exposure time; GE, genome equivalents.
The 3.7-kb pregenome and 2.1-kb mRNA transcripts were detectable in the liver, equally in males and females of both lineages (data not shown). The levels of WHV mRNA in mice of lineage 1281 were relatively stable and age and sex independent (Fig. 2E). The 0.7-kb viral mRNA corresponding to the WHV x gene transcript was not detected by Northern blotting in any sample from these animals (data not shown). This finding is consistent with the low level of expression of the WHV x gene in the woodchuck liver during natural infection (27). The detection of WHV proteins has failed so far, as no suitable antibodies were available (15, 25, 28, 29).
Serum WHV DNA was detected only in mice of lineage 1217 with the wild-type WHV transgene (Fig. 2D) but not in mice of lineage 1281 with a WHsAg stop codon mutation (data not shown). This result is consistent with the fact that the formation of secreted WHV virions requires intact WHsAg.
Age-dependent development of hepatocellular dysplasia in WHV Tg mice.
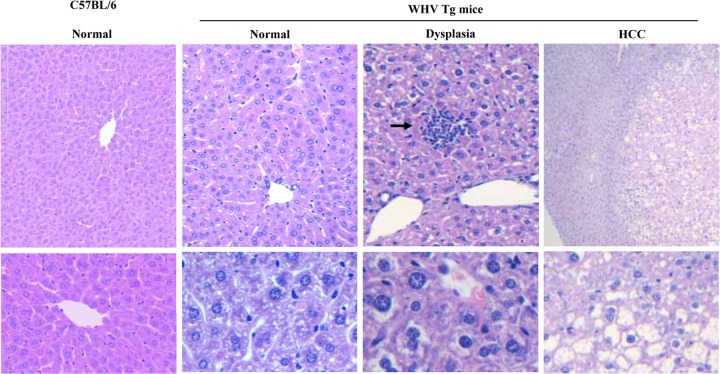
Livers from 99 WHV Tg mice were examined for histological changes. In both WHV Tg lineages, animals younger than 11 weeks of age (n = 80; 25 lineage 1217 mice [14 males and 11 females] and 55 lineage 1281 mice [31 males and 24 females]) showed normal or slight nonspecific hepatocellular damage. However, older animals, between 37 weeks and 41 weeks of age (n = 19; 10 lineage 1217 mice [4 males and 6 females] and 9 lineage 1281 mice [6 males and 3 female]), all exhibited dysplasia, with increased numbers of double-nucleated hepatocytes, variations in the size of hepatocellular nuclei, and variations in the cellular density of hepatocytes (Fig. 3). No differences between male and female Tg mice in the development of dysplastic changes were observed. Interestingly, 1 of the 10 aged lineage 1217 mice (male, aged 40 weeks) developed hepatocellular carcinoma (HCC).
FIG 3.
Histological investigation of liver tissues. Liver tissue samples were fixed in formalin and embedded in paraffin. Sections were cut and processed for histological and microscopic examination according to standard methods. Representative photographs are shown for the histological presentation of normal and dysplastic changes of liver sections. The characteristically histological presentation of HCC found in an aged mouse of lineage 1217 is also shown. The arrows show inflammatory cell infiltrates in the hematoxylin- and eosin-stained section.
Spontaneous development of humoral and cellular immune responses to WHV proteins.
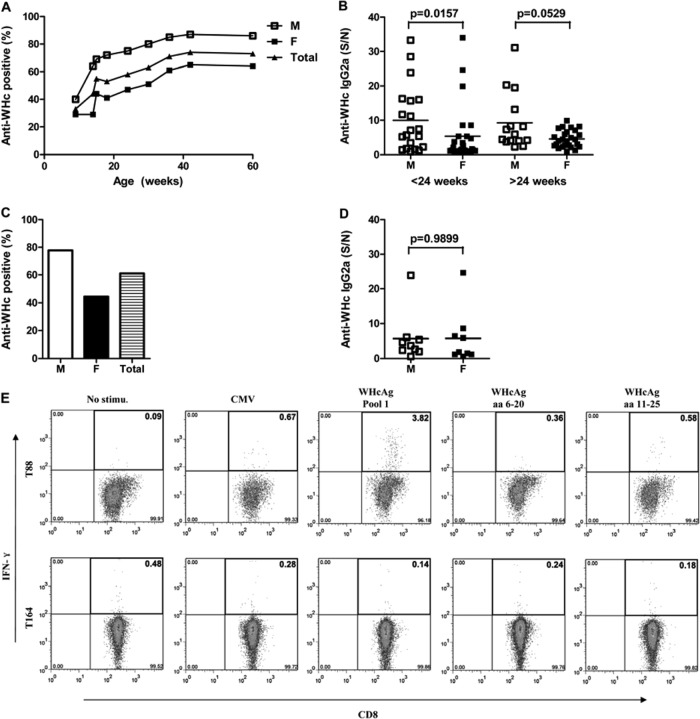
To investigate whether the WHV transgene products WHsAg and WHcAg are recognized by the host immune system, antibodies to WHsAg and WHcAg and CTLs to WHcAg epitopes were determined in WHV Tg mice. All WHV Tg mice were negative for anti-WHs antibodies. However, anti-WHc antibodies were detectable in mice of both lineages, starting at 9 weeks of age. Interestingly, the development of anti-WHc antibodies increased in an age-dependent manner in mice of lineage 1281 (Fig. 4A). More than 50% of the mice older than 16 weeks of age showed high titers of serum anti-WHc antibodies. Moreover, more male mice developed anti-WHc antibodies than females at any age (Fig. 4A and Table 1). In mice of lineage 1217, anti-WHc antibody was detectable in about 60% of mice (Fig. 4C and Table 1), and there was no significant difference in the titers of serum anti-WHc antibodies between male and female lineage 1217 mice (Fig. 4D).
FIG 4.
WHcAg-specific humoral and cellular immune responses in WHV Tg mice. (A to D) Anti-WHc antibodies in the serum of WHV Tg mice of lineages 1281 (A and B) and 1217 (C and D) were detected by ELISA. (E) WHcAg-specific CTLs in the splenocytes of Tg mice of lineage 1281 were analyzed by flow cytometry. S/N, sample/negative control ratio. Mice T88 and T164 of lineage 1281 are two representative examples positive and negative for WHcAg-specific CTL responses, respectively.
TABLE 1.
Occurrence of anti-WHc antibodies and WHcAg-specific CTLs in WHV Tg mice
| Lineage | Sex | Age (wk) | No. of mice |
|||
|---|---|---|---|---|---|---|
| Total | CTL+ | IgG2a+ | CTL+ IgG2a+ | |||
| 1281 | Female | <30 | 8 | 1 | 6 | 0 |
| >30 | 11 | 3 | 4 | 2 | ||
| Male | <30 | 6 | 2 | 5 | 1 | |
| >30 | 10 | 0 | 6 | 0 | ||
| 1217 | Female | <30 | 5 | 0 | 2 | 0 |
| >30 | 4 | 0 | 2 | 0 | ||
| Male | <30 | 6 | 1 | 2 | 1 | |
| >30 | 7 | 1 | 3 | 0 | ||
IFN-γ-secreting WHc-specific CTLs were detectable at very low levels in young mice of both WHV Tg lineages only when splenocytes were stimulated with WHcAg peptide pool 1 (Fig. 4E, top). Consistent with the levels of anti-WHc antibodies, WHcAg-specific CTLs were detected more frequently in mice of lineage 1281 than in mice of lineage 1217 (6/35 versus 2/22) (Table 1). WHcAg-specific CTLs detected in the Tg mice recognized the WHcAg-derived peptide SSYQLLNFLPLDFFP (WHcAg aa 11 to 25) but not the peptide YKEFGSSYQLLNFLP (WHcAg aa 6 to 20), which comprises the dominant CD8+ T-cell epitope in C57BL/6 mice. In the majority of Tg mice, no WHcAg-specific CTLs were detected, consistent with a T-cell tolerance to WHV proteins (Fig. 4E, bottom, and Table 1). WHcAg induced weak cellular immune responses in WHV Tg mice, which were not strong enough to suppress WHV replication but might result in infiltration of inflammatory cells into the liver, as shown in Fig. 3.
DNA vaccination induced WHV-specific immune responses and led to the suppression of WHV replication in WHV Tg mice.
WHV Tg mice at 8 to 10 weeks of age were tested for the presence of anti-WHc antibodies. Mice negative for anti-WHc were selected to test the immune response to DNA immunization. Immunization with pWHsIm induced anti-WHs antibodies in mice of lineage 1281 but not in mice of lineage 1217 (data not shown). This is consistent with the fact that the wild-type WHV transgene is able to produce WHsAg and may cause tolerance to WHsAg. Immunization with pWHcIm induced both anti-WHc antibodies and WHcAg-specific CTLs in mice of both lineages (Fig. 5A to C). However, the immune responses were significantly weaker than those in nontransgenic C57BL/6 mice that underwent the same immunization protocol (Fig. 5A and B). WHcAg-specific T-cell responses in Tg mice were weak and detectable only after the in vitro expansion of specific T cells with peptides for 7 days (Fig. 5B and C). In contrast to the spontaneous cellular response to WHcAg, WHcAg vaccination-induced CTLs in the Tg mice recognized dominant epitope WHcAg aa 6 to 20, as was also seen in C57BL/6 mice immunized with WHcAg (Fig. 5C). However, only part of the specific CD8 cells maintained the ability to produce IFN-γ or degranulate after stimulation with WHcAg peptide aa 6 to 20, which indicated that the WHcAg-specific CD8 cells were functionally impaired. Interestingly, pWHcIm immunization resulted in a significant decrease of the serum viral load (Fig. 5D), even though the vaccination-induced CD8 cell response was weak.
FIG 5.
Immunization-induced humoral and cellular responses to WHcAg in WHV Tg mice. WHV Tg mice of lineage 1217 (wild-type WHV) and normal C57BL/6 mice were immunized three times with pWHcIm (A to D) or pCGWHc (E to H). Serum anti-WHc antibodies (A and E), WHcAg-specific CTLs after in vitro expansion in the presence of specific peptides for 7 days (B and C) or ex vivo stimulation for 4 h (F and G), and serum WHV DNA loads (D and H) were determined by ELISA, flow cytometry, and real-time PCR, respectively. PBS, phosphate-buffered saline; n.s., not significant.
Furthermore, pCGWHc, a vector with a higher WHcAg expression level, was tested in WHV Tg mice. Interestingly, pCGWHc immunizations induced significantly higher levels of anti-WHc IgG2a antibodies and IFN-γ-producing WHcAg-specific CD8 cells in mice of lineage 1217 (Fig. 5E to G) and also led to a significant decrease of serum viral loads (Fig. 5H).
DISCUSSION
In this study, two Tg mouse strains with 1.3-fold-overlength WHV genomes were established. These WHV Tg mouse strains revealed liver-specific and age- and sex-dependent viral replication activity and developed spontaneous humoral and cellular immune responses to WHV antigens with a high frequency. In addition, aged mice of these strains showed pathological changes in the liver. Thus, these novel WHV Tg mice represent useful models to study clinically relevant features related to hepadnaviral infections.
The low levels of viral gene expression and replication in the liver of lineage 1217 mice with the wild-type WHV genome are consistent with results reported for HBV Tg mice (22, 30). Halverscheid et al. (13) observed high levels of viral gene expression and replication in the liver of HBV Tg mice lacking expression of small HBs. In the present study, significantly higher levels of viral replication were observed in the liver of mice of lineage 1281, which harbor the S-minus WHV genome construct, than in the liver of mice of lineage 1217. Accumulation of core antigen/particles in hepatocytes may contribute to high levels of viral replication in the liver of Tg mice lacking expression of envelope proteins. No viral particles were found in the serum of mice of lineage 1281, as the mutated WHV genome was not able to express WHsAg.
Levels of WHV replication appeared higher in males than in females of both lineages of WHV Tg mice. The same phenomenon was also observed for HBV Tg mice (13, 31), consistent with HBV infections in humans. The surrounding sequences of WHV transgene integration sites in the mouse genomes of lineages 1217 and 1281 are reported under GenBank accession numbers AC123865.5 and AC159977.4, respectively (data not shown), and contain repeated sequences with some undefined regions. No functional genes were identified in the direct neighborhood of WHV transgenes within the range of 3 to 10 kb, making it unlikely that sex- and tissue-specific WHV replication was determined by such specific host genes. Certainly, the insertion of WHV transgenes may potentially cause chromosome abnormality, and this possibility could not be completely ruled out. Higher levels of WHV replicative intermediates in male than in female animals suggest that the male androgen axis regulates HBV replication (32). It has been reported by different research groups that male patients with chronic HBV infections have higher viral loads and tend to develop HCC with a significantly higher frequency than female patients (5:1 to 10:1) (33, 34). The consistent male predominance in liver injury and incidence of HCC in both humans and rodents in the context of hepadnaviral infection supports the concept that sex is an important factor with respect to hepatocarcinogenesis in general.
One interesting feature of the WHV Tg mouse strains is the development of autoimmune CTL responses to WHV proteins with age. Higher levels of IgG2a antibodies against WHcAg were detectable in the sera of mice of lineage 1281 than in the sera of mice of lineage 1217. This could be explained by the fact that the intracellular retention of WHcAg in hepatocytes in mice of lineage 1281 leads to accumulation of WHcAg in situ, thus increasing the possibility of being presented to immune cells. It is also possible that WHcAg is released into peripheral blood by dead hepatocytes and induces specific antibody responses. WHcAg-specific CTLs were detected in 6 of 28 mice of lineage 1281, indicating that WHcAg induced weak cellular immune responses in WHV Tg mice. The different levels of T-cell exhaustion, circulating WHV DNA, and/or antigenemia may contribute to the difference in responses to the WHV transgenes. The cellular responses were not strong enough to suppress WHV replication but might contribute to the nonspecific infiltration of inflammatory cells into the liver. There was no obvious difference between mice with and those without detectable immune responses in terms of the levels of liver pathology, serum WHV DNA, and intrahepatic WHV replication intermediates.
There was no WHV-specific hepatocellular damage in the livers of young WHV Tg mice, consistent with the general notion that the pathogenesis of chronic HBV infection is immune mediated (35). The observed slight unspecific hepatocellular damage, lobular nodular infiltrates of inflammatory cells, might have been caused by the weak immune responses to the WHV Tg products. Interestingly, dysplasia developed in all aged Tg mice, and hepatocellular carcinoma was found in one WHV Tg mouse, providing more supporting evidence that hepadnaviruses, including WHV, are carcinogenic (36, 37). In addition, the development of immune responses may lead to liver injury over an extended period of time. This issue remains to be investigated in the future.
The induction of WHsAg-specific antibodies occurred in mice of lineage 1281 but not in mice of lineage 1217 following vaccination with pWHsIm, suggesting that mice of lineage 1217 are tolerant to WHsAg due to constitutive expression beginning early in life. Two plasmids expressing WHcAg, pWHcIm and pCGWHc, induced WHcAg-specific T-cell responses, indicating that it is possible to break WHV-specific immune tolerance. DNA vaccines induced CTLs and reduced serum WHV DNA loads to a low level of 103 to 104 copies/ml. The possibility of breaking immune tolerance to WHcAg in Tg mice allows the testing of different therapeutic vaccines and vaccination regimens. Recently, an immunization protocol including priming with DNA vaccines and boosts with adenoviral vectors was shown to elicit robust and effective humoral and cellular immune responses against WHV in woodchucks (25, 38). This protocol was also evaluated in Tg mice and was superior to DNA vaccination alone (A. D. Kosinska, M. Lu, and M. Roggendorf., unpublished data). Thus, the WHV Tg mouse strains may be valuable research tools for the development of therapeutic vaccines against chronic HBV infection. Taken together, WHV Tg mice represent new animal models for studies of hepadnaviral infection and pathogenesis related to viral hepatitis and will be especially useful in the development of new antiviral strategies.
ACKNOWLEDGMENTS
We thank Thekla Kemper, Barbara Bleekmann, Carol Aldrich (FCCC), and Jeffry Saputelli (FCCC) for excellent technical assistance. We are also thankful to William Mason for critical reading and helpful suggestions.
This study was supported by grants from the German Research Foundation (TRR60, GK1045, and GK1949) and the Chinese Research Foundation (Z20102101, 2011BCB030, and 2013ZX10002-001).
Footnotes
Published ahead of print 20 November 2013
REFERENCES
- 1.Locarnini S, Mason WS. 2006. Cellular and virological mechanisms of HBV drug resistance. J. Hepatol. 44:422–431. 10.1016/j.jhep.2005.11.036 [DOI] [PubMed] [Google Scholar]
- 2.Marcellin P. 2002. Advances in therapy for chronic hepatitis B. Semin. Liver Dis. 22(Suppl 1):33–36. 10.1055/s-2002-35698 [DOI] [PubMed] [Google Scholar]
- 3.Barker LF, Maynard JE, Purcell RH, Hoofnagle JH, Berquist KR, London WT. 1975. Viral hepatitis, type B, in experimental animals. Am. J. Med. Sci. 270:189–195. 10.1097/00000441-197507000-00026 [DOI] [PubMed] [Google Scholar]
- 4.Barrera A, Lanford RE. 2004. Infection of primary chimpanzee hepatocytes with recombinant hepatitis D virus particles: a surrogate model for hepatitis B virus. Methods Mol. Med. 96:131–142. 10.1385/1-59259-670-3:131 [DOI] [PubMed] [Google Scholar]
- 5.Morrey JD. 2004. Transgenic hepatitis B virus mouse model in the study of chemotherapy. Methods Mol. Med. 96:239–252. 10.1385/1-59259-670-3:239 [DOI] [PubMed] [Google Scholar]
- 6.Guidotti LG, Ando K, Hobbs MV, Ishikawa T, Runkel L, Schreiber RD, Chisari FV. 1994. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 91:3764–3768. 10.1073/pnas.91.9.3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidotti LG, Chisari FV. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65–91. 10.1146/annurev.immunol.19.1.65 [DOI] [PubMed] [Google Scholar]
- 8.Isogawa M, Robek MD, Furuichi Y, Chisari FV. 2005. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J. Virol. 79:7269–7272. 10.1128/JVI.79.11.7269-7272.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guidotti LG, Matzke B, Schaller H, Chisari FV. 1995. High-level hepatitis B virus replication in transgenic mice. J. Virol. 69:6158–6169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chisari FV, Isogawa M, Wieland SF. 2010. Pathogenesis of hepatitis B virus infection. Pathol. Biol. (Paris) 58:258–266. 10.1016/j.patbio.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Chen Z, Hu C, Qian F, Cheng Y, Wu M, Shi B, Chen J, Hu Y, Yuan Z. 2013. Hepatitis B virus surface antigen selectively inhibits TLR2 ligand-induced IL-12 production in monocytes/macrophages by interfering with JNK activation. J. Immunol. 190:5142–5151. 10.4049/jimmunol.1201625 [DOI] [PubMed] [Google Scholar]
- 12.Wu J, Meng Z, Jiang M, Pei R, Trippler M, Broering R, Bucchi A, Sowa JP, Dittmer U, Yang D, Roggendorf M, Gerken G, Lu M, Schlaak JF. 2009. Hepatitis B virus suppresses Toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology 49:1132–1140. 10.1002/hep.22751 [DOI] [PubMed] [Google Scholar]
- 13.Halverscheid L, Mannes NK, Weth R, Kleinschmidt M, Schultz U, Reifenberg K, Schirmbeck R, Nassal M, Blum HE, Reimann J, Geissler M. 2008. Transgenic mice replicating hepatitis B virus but lacking expression of the major HBsAg. J. Med. Virol. 80:583–590. 10.1002/jmv.21115 [DOI] [PubMed] [Google Scholar]
- 14.Summers J, Smolec JM, Snyder R. 1978. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc. Natl. Acad. Sci. U. S. A. 75:4533–4537. 10.1073/pnas.75.9.4533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu M, Yao X, Xu Y, Lorenz H, Dahmen U, Chi H, Dirsch O, Kemper T, He L, Glebe D, Gerlich WH, Wen Y, Roggendorf M. 2008. Combination of an antiviral drug and immunomodulation against hepadnaviral infection in the woodchuck model. J. Virol. 82:2598–2603. 10.1128/JVI.01613-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank I, Budde C, Fiedler M, Dahmen U, Viazov S, Lu M, Dittmer U, Roggendorf M. 2007. Acute resolving woodchuck hepatitis virus (WHV) infection is associated with a strong cytotoxic T-lymphocyte response to a single WHV core peptide. J. Virol. 81:7156–7163. 10.1128/JVI.02711-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menne S, Maschke J, Tolle TK, Lu M, Roggendorf M. 1997. Characterization of T-cell response to woodchuck hepatitis virus core protein and protection of woodchucks from infection by immunization with peptides containing a T-cell epitope. J. Virol. 71:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seeger C, Maragos J. 1989. Molecular analysis of the function of direct repeats and a polypurine tract for plus-strand DNA priming in woodchuck hepatitis virus. J. Virol. 63:1907–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Research Council 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC [Google Scholar]
- 20.Meng Z, Qiu S, Zhang X, Wu J, Schreiter T, Xu Y, Yang D, Roggendorf M, Schlaak J, Lu M. 2009. Inhibition of woodchuck hepatitis virus gene expression in primary hepatocytes by siRNA enhances the cellular gene expression. Virology 384:88–96. 10.1016/j.virol.2008.11.012 [DOI] [PubMed] [Google Scholar]
- 21.Meng Z, Zhang X, Wu J, Pei R, Xu Y, Yang D, Roggendorf M, Lu M. 2013. RNAi induces innate immunity through multiple cellular signaling pathways. PLoS One 8:e64708. 10.1371/journal.pone.0064708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Araki K, Miyazaki J, Hino O, Tomita N, Chisaka O, Matsubara K, Yamamura K. 1989. Expression and replication of hepatitis B virus genome in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 86:207–211. 10.1073/pnas.86.1.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z, Tian Y, Li L, Fiedler M, Schmid E, Roggendorf M, Xu Y, Lu M, Yang D. 2006. A conserved linear B-cell epitope at the N-terminal region of woodchuck hepatitis virus core protein (WHcAg). J. Virol. Methods 135:17–25. 10.1016/j.jviromet.2006.01.015 [DOI] [PubMed] [Google Scholar]
- 24.Lu M, Hilken G, Kruppenbacher J, Kemper T, Schirmbeck R, Reimann J, Roggendorf M. 1999. Immunization of woodchucks with plasmids expressing woodchuck hepatitis virus (WHV) core antigen and surface antigen suppresses WHV infection. J. Virol. 73:281–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosinska AD, Johrden L, Zhang E, Fiedler M, Mayer A, Wildner O, Lu M, Roggendorf M. 2012. DNA prime-adenovirus boost immunization induces a vigorous and multifunctional T-cell response against hepadnaviral proteins in the mouse and woodchuck model. J. Virol. 86:9297–9310. 10.1128/JVI.00506-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schirmbeck R, Bohm W, Ando K, Chisari FV, Reimann J. 1995. Nucleic acid vaccination primes hepatitis B virus surface antigen-specific cytotoxic T lymphocytes in nonresponder mice. J. Virol. 69:5929–5934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moroy T, Etiemble J, Trepo C, Tiollais P, Buendia MA. 1985. Transcription of woodchuck hepatitis virus in the chronically infected liver. EMBO J. 4:1507–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulkarni K, Jacobson IM, Tennant BC. 2007. The role of the woodchuck model in the treatment of hepatitis B virus infection. Clin. Liver Dis. 11:707–725. 10.1016/j.cld.2007.08.012 [DOI] [PubMed] [Google Scholar]
- 29.Lu M, Menne S, Yang D, Xu Y, Roggendorf M. 2007. Immunomodulation as an option for the treatment of chronic hepatitis B virus infection: preclinical studies in the woodchuck model. Expert Opin. Invest. Drugs 16:787–801. 10.1517/13543784.16.6.787 [DOI] [PubMed] [Google Scholar]
- 30.Farza H, Hadchouel M, Scotto J, Tiollais P, Babinet C, Pourcel C. 1988. Replication and gene expression of hepatitis B virus in a transgenic mouse that contains the complete viral genome. J. Virol. 62:4144–4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guidotti LG, Eggers CM, Raney AK, Chi SY, Peters JM, Gonzalez FJ, McLachlan A. 1999. In vivo regulation of hepatitis B virus replication by peroxisome proliferators. J. Virol. 73:10377–10386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang SH, Yeh SH, Lin WH, Wang HY, Chen DS, Chen PJ. 2009. Identification of androgen response elements in the enhancer I of hepatitis B virus: a mechanism for sex disparity in chronic hepatitis B. Hepatology 50:1392–1402. 10.1002/hep.23163 [DOI] [PubMed] [Google Scholar]
- 33.Lee CM, Lu SN, Changchien CS, Yeh CT, Hsu TT, Tang JH, Wang JH, Lin DY, Chen CL, Chen WJ. 1999. Age, gender, and local geographic variations of viral etiology of hepatocellular carcinoma in a hyperendemic area for hepatitis B virus infection. Cancer 86:1143–1150. [DOI] [PubMed] [Google Scholar]
- 34.Shiratori Y, Shiina S, Imamura M, Kato N, Kanai F, Okudaira T, Teratani T, Tohgo G, Toda N, Ohashi M, Ogura K, Niwa Y, Kawabe T, Omata M. 1995. Characteristic difference of hepatocellular carcinoma between hepatitis B- and C-viral infection in Japan. Hepatology 22:1027–1033. 10.1002/hep.1840220403 [DOI] [PubMed] [Google Scholar]
- 35.Feitelson MA. 1989. Hepatitis B virus gene products as immunological targets in chronic infection. Mol. Biol. Med. 6:367–393 [PubMed] [Google Scholar]
- 36.Tennant BC, Toshkov IA, Peek SF, Jacob JR, Menne S, Hornbuckle WE, Schinazi RD, Korba BE, Cote PJ, Gerin JL. 2004. Hepatocellular carcinoma in the woodchuck model of hepatitis B virus infection. Gastroenterology 127:S283–S293. 10.1053/j.gastro.2004.09.043 [DOI] [PubMed] [Google Scholar]
- 37.Tsai WL, Chung RT. 2010. Viral hepatocarcinogenesis. Oncogene 29:2309–2324. 10.1038/onc.2010.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kosinska AD, Zhang E, Johrden L, Liu J, Seiz PL, Zhang X, Ma Z, Kemper T, Fiedler M, Glebe D, Wildner O, Dittmer U, Lu M, Roggendorf M. 2013. Combination of DNA prime-adenovirus boost immunization with entecavir elicits sustained control of chronic hepatitis B in the woodchuck model. PLoS Pathog. 9:e1003391. 10.1371/journal.ppat.1003391 [DOI] [PMC free article] [PubMed] [Google Scholar]