Abstract
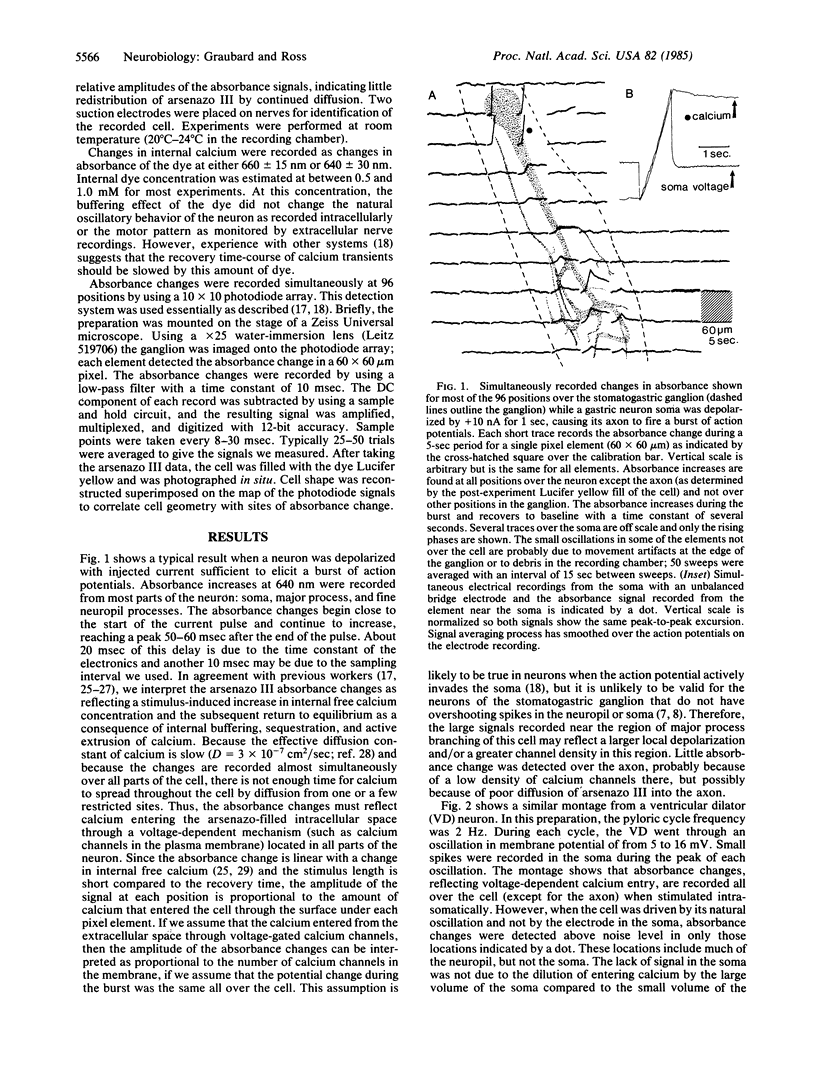
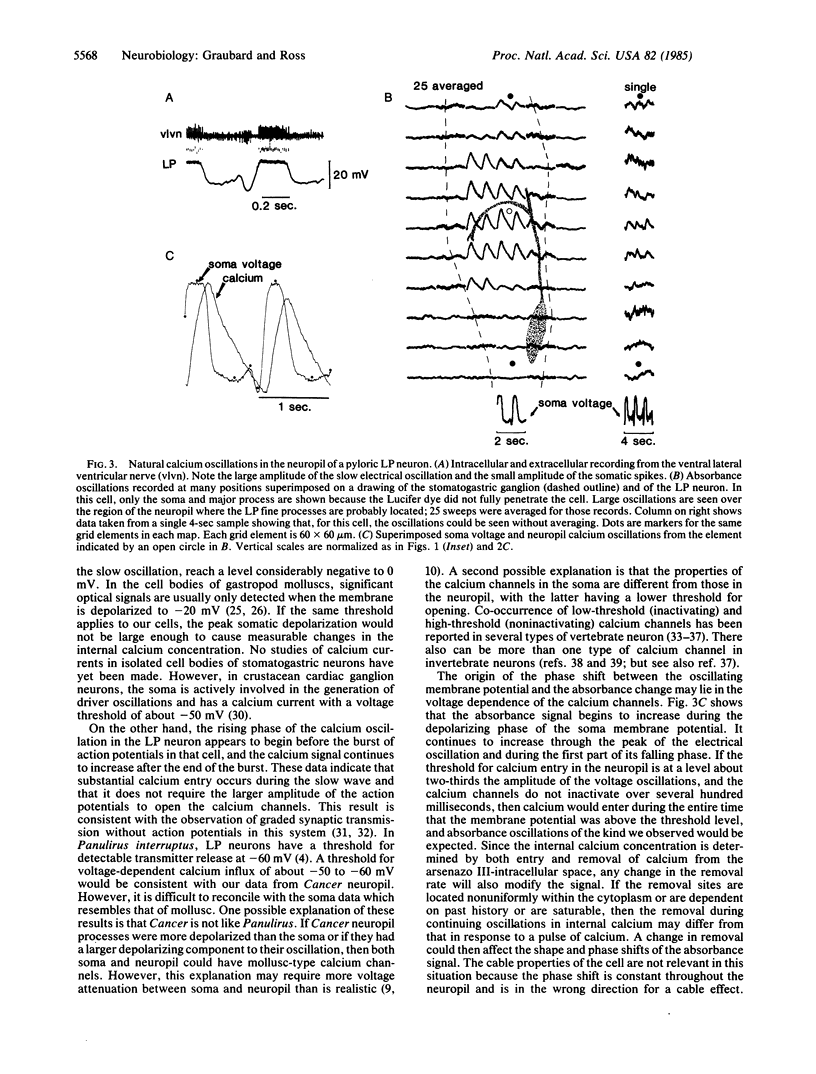
Absorbance changes of the metallochromic indicator arsenazo III were used in conjunction with an array of 100 photodiodes to measure changes in intracellular calcium concentration at many positions simultaneously in identified neurons of the crab stomatogastric ganglion. When stimulated with intrasomatically injected current, several of these neurons showed calcium changes all over the cell, indicating that calcium channels were distributed widely in the neuropil and on the soma. When the membrane potential was allowed to oscillate without stimulation, absorbance oscillations were detected all over the neuropil but not in the soma. A comparison between the membrane potential recorded in the soma and the calcium signal in the neuropil shows that calcium entry followed the slow voltage oscillation with the peak calcium signal detected 50-150 msec after the end of the voltage plateau.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z., Connor J. A. Measurement of calcium influx under voltage clamp in molluscan neurones using the metallochromic dye arsenazo III. J Physiol. 1979 Jan;286:61–82. doi: 10.1113/jphysiol.1979.sp012607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Eckert R., Lux H. D. A voltage-sensitive persistent calcium conductance in neuronal somata of Helix. J Physiol. 1976 Jan;254(1):129–151. doi: 10.1113/jphysiol.1976.sp011225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. H., Jr, Mulloney B. Compartmental models of electrotonic structure and synaptic integration in an identified neurone. J Physiol. 1984 Mar;348:89–113. doi: 10.1113/jphysiol.1984.sp015101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Changes in the intracellular concentration of free calcium ions in a pace-maker neurone, measured with the metallochromic indicator dye arsenazo III. J Physiol. 1978 Feb;275:357–376. doi: 10.1113/jphysiol.1978.sp012194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Intracellular calcium accumulation during depolarization in a molluscan neurone. J Physiol. 1980 Nov;308:259–285. doi: 10.1113/jphysiol.1980.sp013471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graubard K., Raper J. A., Hartline D. K. Graded synaptic transmission between identified spiking neurons. J Neurophysiol. 1983 Aug;50(2):508–521. doi: 10.1152/jn.1983.50.2.508. [DOI] [PubMed] [Google Scholar]
- Graubard K., Raper J. A., Hartline D. K. Graded synaptic transmission between spiking neurons. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3733–3735. doi: 10.1073/pnas.77.6.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graubard K. Synaptic transmission without action potentials: input-output properties of a nonspiking presynaptic neuron. J Neurophysiol. 1978 Jul;41(4):1014–1025. doi: 10.1152/jn.1978.41.4.1014. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Cohen L. B., Lesher S., Boyle M. B. Simultaneous optical monitoring of activity of many neurons in invertebrate ganglia using a 124-element photodiode array. J Neurophysiol. 1981 May;45(5):829–840. doi: 10.1152/jn.1981.45.5.829. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Ross W. N., Farber I. Simultaneous optical measurements of electrical activity from multiple sites on processes of cultured neurons. Proc Natl Acad Sci U S A. 1981 May;78(5):3245–3249. doi: 10.1073/pnas.78.5.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harary H. H., Brown J. E. Spatially nonuniform changes in intracellular calcium ion concentrations. Science. 1984 Apr 20;224(4646):292–294. doi: 10.1126/science.6710144. [DOI] [PubMed] [Google Scholar]
- Hooper S. L., Marder E. Modulation of a central pattern generator by two neuropeptides, proctolin and FMRFamide. Brain Res. 1984 Jul 2;305(1):186–191. doi: 10.1016/0006-8993(84)91138-7. [DOI] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984 Apr;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D. G. Organization of crustacean neuropil. I. Patterns of synaptic connections in lobster stomatogastric ganglion. J Neurocytol. 1976 Apr;5(2):207–237. doi: 10.1007/BF01181657. [DOI] [PubMed] [Google Scholar]
- King D. G. Organization of crustacean neuropil. II. Distribution of synaptic contacts on identified motor neurons in lobster stomatogastric ganglion. J Neurocytol. 1976 Apr;5(2):239–266. doi: 10.1007/BF01181658. [DOI] [PubMed] [Google Scholar]
- Krauthamer V., Ross W. N. Regional variations in excitability of barnacle neurons. J Neurosci. 1984 Mar;4(3):673–682. doi: 10.1523/JNEUROSCI.04-03-00673.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Greenfield S. A., Jahnsen H. Electrophysiology of pars compacta cells in the in vitro substantia nigra--a possible mechanism for dendritic release. Brain Res. 1984 Feb 27;294(1):127–132. doi: 10.1016/0006-8993(84)91316-7. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981 Jun;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard D. M. Simpler networks. Ann N Y Acad Sci. 1972 Aug 25;193:59–72. doi: 10.1111/j.1749-6632.1972.tb27823.x. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Calcium transients recorded with arsenazo III in the presynaptic terminal of the squid giant synapse. Proc R Soc Lond B Biol Sci. 1981 May 22;212(1187):197–211. doi: 10.1098/rspb.1981.0034. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Krauthamer V. Optical measurements of potential changes in axons and processes of neurons of a barnacle ganglion. J Neurosci. 1984 Mar;4(3):659–672. doi: 10.1523/JNEUROSCI.04-03-00659.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. F. CNS control of pattern generators in the lobster stomatogastric ganglion. Brain Res. 1979 Nov 30;177(3):598–602. doi: 10.1016/0006-8993(79)90480-3. [DOI] [PubMed] [Google Scholar]
- Russell D. F., Hartline D. K. Bursting neural networks: a reexamination. Science. 1978 Apr 28;200(4340):453–456. doi: 10.1126/science.644309. [DOI] [PubMed] [Google Scholar]
- Selverston A. I., Russell D. F., Miller J. P. The stomatogastric nervous system: structure and function of a small neural network. Prog Neurobiol. 1976;7(3):215–290. doi: 10.1016/0301-0082(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Stockbridge N., Ross W. N. Localized Ca2+ and calcium-activated potassium conductances in terminals of a barnacle photoreceptor. Nature. 1984 May 17;309(5965):266–268. doi: 10.1038/309266a0. [DOI] [PubMed] [Google Scholar]
- Tazaki K., Cooke I. M. Neuronal mechanisms underlying rhythmic bursts in crustacean cardiac ganglia. Symp Soc Exp Biol. 1983;37:129–157. [PubMed] [Google Scholar]
- Thomas M. V., Gorman A. L. Internal calcium changes in a bursting pacemaker neuron measured with arsenazo III. Science. 1977 Apr 29;196(4289):531–533. doi: 10.1126/science.850795. [DOI] [PubMed] [Google Scholar]


