Abstract
An assay to identify interactions between Citrus Dwarfing Viroid (CDVd) and Citrus Tristeza Virus (CTV) showed that viroid titer was enhanced by the coinfecting CTV in Mexican lime but not in etrog citron. Since CTV encodes three RNA silencing suppressors (RSSs), p23, p20 and p25, an assay using transgenic Mexican limes expressing each RSS revealed that p23 and, to a lesser extent, p25 recapitulated the effect observed with coinfections of CTV and CDVd.
TEXT
Viroids are infectious, unencapsidated, single-stranded, and highly structured noncoding circular RNAs of 250 to 400 nucleotides (nt). Despite their simplicity, viroids interact with cell factors, which redirect them to replicate and traffic throughout the plant while avoiding the RNA silencing machinery they trigger in their hosts (1).
Indexing tests have shown that viroids are widespread in commercial citrus orchards, wherein trees may be coinfected with other graft-transmissible pathogens. Data regarding virus-viroid interactions are very scarce, but the information available indicates that such interactions may alter symptom expression (2, 3). Since coinfection of citrus species with Citrus Tristeza Virus (CTV) and viroids is not rare, assays were conducted to establish whether interactions between these two types of pathogens actually exist.
The assays were performed by comparing single and double infections of the two pathogens in etrog citron (Citrus medica L.) (viroid bioindicator) and Mexican lime (Citrus aurantifolia [Christm.] Swingle) (CTV bioindicator). Citrus Dwarfing Viroid (CDVd) (GenBank accession number EU934004), inducing moderate but specific symptoms in etrog citron (4), and CTV (T-305 strain), inducing symptoms in Mexican lime (5), were chosen for this study. To synchronize infection, Volkamer lemon (Citrus volkameriana Ten. & Pasq.) seedlings were graft inoculated with CDVd and/or CTV before being used as rootstocks. Etrog citron and Mexican lime were graft propagated to produce groups of five plants for each of the following treatments: (i) plants singly infected with CDVd, (ii) plants singly infected with CTV, (iii) plants coinfected with CTV and CDVd, and (iv) noninfected controls. CTV and CDVd infection was assessed by immunoprinting enzyme-linked immunosorbent assay (ELISA) (6) and dot blot hybridization (7), respectively.
After an incubation period of 4 months at 25°C, plant height was measured (Fig. 1) and data were subjected to multifactor analysis of variance (ANOVA). The results indicated that etrog citron singly infected with CTV (P value, 0.00001) or CDVd (P value, 0.05) was stunted, as was Mexican lime singly infected with CTV (P value, 0.0013) but not that with CDVd (P value, 0.98). Height reduction in coinfected plants resulted from the added effect of each pathogen, indicating no interaction between CDVd and CTV in either host (interaction P value for etrog citron, 0.29; interaction P value for Mexican lime, 0.44). Similarly, symptom expression in stems and leaves resulted from the added effect of each pathogen, revealing again a lack of interactions between the two pathogens (Fig. 2).
FIG 1.
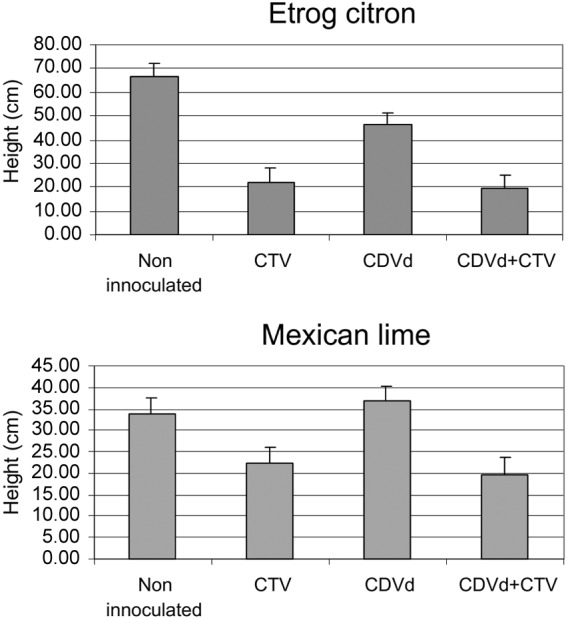
Plant height measured 4 months after graft propagation of the scion on previously infected rootstocks. Bars show the means and standard errors of the height measured from the grafting point to the shoot tip.
FIG 2.
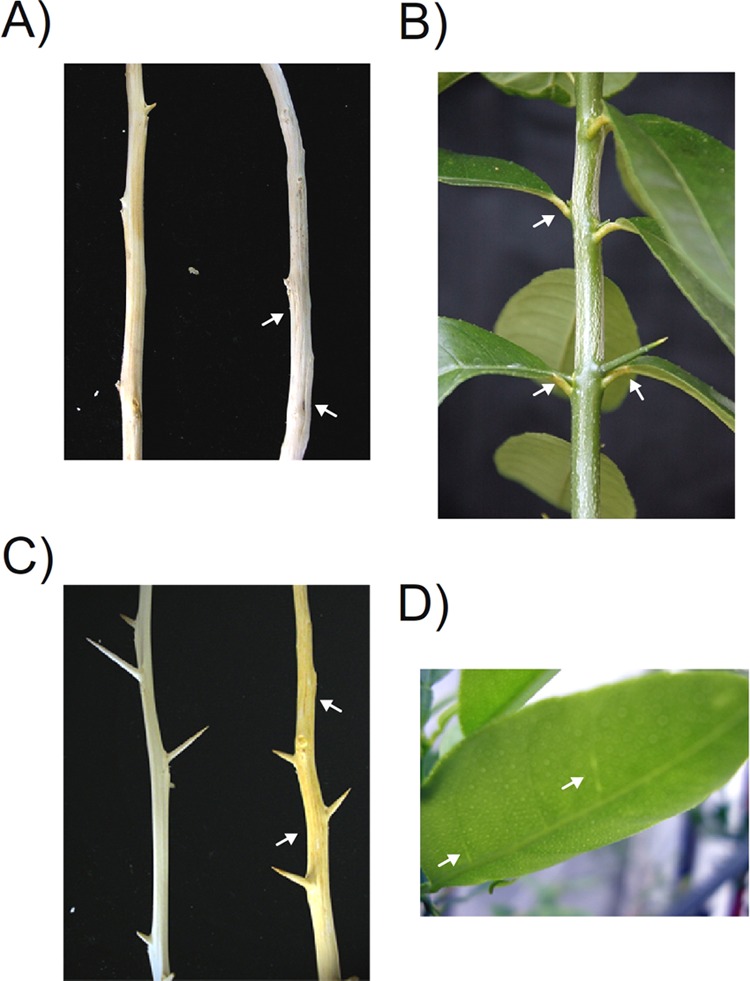
(A and B) Effects observed with etrog citron. Symptomless (A, left), CTV-induced stem pitting (A, right, arrows), CDVd-induced petiole necrosis (B, arrows). (C and D) Effects observed with Mexican lime. Symptomless (C, left), CTV-induced stem pitting (C, right, arrows), CTV-induced vein clearing (D, arrows).
To elucidate if coinfected plants presented any deviation of CDVd and/or CTV accumulation from their singly infected counterparts, the titer of each pathogen was analyzed. For viroid quantification, equalized preparations of total nucleic acids were subjected to PAGE in 5% gels containing 1× Tris-borate-EDTA (TBE) and 8 M urea, electrotransferred to positively charged nylon membranes (Roche Diagnostics), fixed by UV cross-linking, and hybridized with a digoxigenin (DIG)-labeled full-length CDVd DNA probe. Northern blot results showed that CDVd titers in etrog citron were not affected by CTV coinfection (Fig. 3A), whereas Mexican lime plants coinfected with CTV and CDVd had considerably higher viroid titers than plants singly infected with CDVd (Fig. 3B). In addition, nucleic acid preparations of Mexican lime were subjected to reverse transcription-PCR (RT-PCR) using a CDVd-specific primer pair (8). Amplicon sequencing revealed that the CDVd progeny of plants singly infected or coinfected with CTV were identical to the parental CDVd used as the inoculum, thus eliminating the possibility that the observed changes in CDVd titer were due to changes in the composition of the viroid population.
FIG 3.
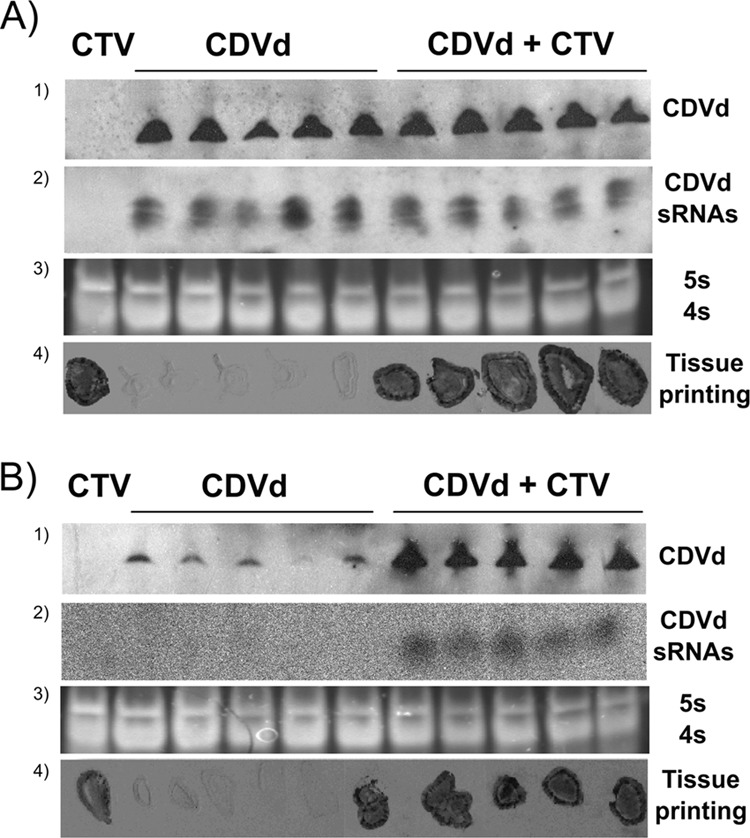
Molecular analysis of CDVd and CTV in (A) etrog citron and (B) Mexican lime. (Row 1) Circular forms of CDVd were tested by Northern blot hybridization using a DIG-labeled CDVd-specific probe in plants singly inoculated with CDVd and coinoculated with CDVd and CTV. (Row 2) CDVd-sRNAs were detected by Northern blot hybridization using a DIG-labeled CDVd-specific probe (etrog citron) or an α-UTP-radiolabeled riboprobe (Mexican lime). (Row 3) In all instances, RNA levels (5S and 4S RNAs) were comparable. (Row 4) CTV infection was confirmed by tissue-print ELISA using monoclonal antibodies 3DF1 and 3CA5.
For CTV quantification, total protein preparations were analyzed by double-antibody sandwich ELISA (DAS-ELISA) using the CTV-specific monoclonal antibodies 3DF1 and 3CA5 (9), and equalized preparations of total nucleic acids were examined by spot-print quantitative RT-PCR (qRT-PCR) using CTV-specific primers and TaqMan (10, 11). Numerical data were subjected to one-way ANOVA. Results showed a statistically significant reduction of CTV accumulation in coinfected Mexican limes compared to singly infected ones: by DAS-ELISA, the mean difference was 0.15 ± 0.05 optical density units (405 nm) (P value, 0.02), and by qRT-PCR, the mean difference was 18.53 × 104 ± 7.4 × 104 molecules/μl (P value, 0.04). No statistical differences were found for etrog citron. Therefore, interactions between the two pathogens are host dependent. In Mexican lime, but not in etrog citron, CTV enhances viroid accumulation while, concomitantly, CDVd limits the accumulation of CTV. The reduced CTV titer in the presence of CDVd suggests that these pathogens compete for a common biological factor. However, in spite of this apparent competition, the higher CDVd titer in the coinfected plants indicates that CTV provides a beneficial effect for viroid accumulation. Posttranscriptional gene silencing (PTGS) provides a feasible mechanistic explanation for the latter result.
PTGS involves 21- to 24-nt small RNAs (sRNAs) that guide the RNA-induced silencing complex (RISC) to complementary viral or endogenous mRNAs, mediating their degradation or repressing their translation (12). sRNAs are generated from double-stranded RNAs (dsRNAs) or structured single-stranded RNAs (ssRNAs) by DICER enzymes (DICER-like in plants) of the RNase III class (13). In infected tissues, dsRNAs and structured ssRNAs from viruses and viroids serve as precursors of the virus- and viroid-derived small RNAs, involved in host defense (14, 15). Similarly, there is evidence supporting the hypothesis that viroids are elicitors (16 to 19) and targets (20, 21) of the RNA silencing machinery. As a counterdefense strategy, viruses encode RNA silencing suppressor (RSS) proteins, which inhibit certain steps of this process (22). Since CTV codes for three RSSs (p23, p20, and p25) that have been characterized for Nicotiana benthamiana (23), their potential role in the accumulation of viroid sRNAs in plants coinfected with CTV and CDVd was addressed. Briefly, RNAs from equalized nucleic acid preparations were separated by PAGE in 15% gels containing 1× TBE and 8 M urea and examined by Northern blot hybridization with a full-length CDVd-specific RNA probe. In etrog citron, CDVd-sRNAs were detected at similar concentrations regardless of whether the plants were singly infected with CDVd or coinfected with CDVd and CTV, thus indicating that, as with the accumulation of genomic CDVd (Fig. 3A), CTV did not have any effect on the accumulation of CDVd-sRNAs. In Mexican lime, in which the accumulation of circular and linear CDVd forms was enhanced in the presence of CTV, CDVd-sRNAs were detected only in doubly infected plants (Fig. 3B). Therefore, a positive correlation between the titers of the viroid genomic RNA and sRNAs was consistently observed. A similar correlation has been found for other viroid-host combinations (20, 24).
To gain an insight into the possible implication of PTGS in the enhancement of viroid titer by CTV, an assay was performed using transgenic Mexican limes expressing p23, p20, or p25 (25, 26; L. Peña, unpublished data), propagated on CDVd-infected Volkamer lemon; nontransgenic Mexican limes propagated on the same rootstock infected with CDVd, or coinfected with CDVd and CTV, served as controls. As described previously, transgenic plants expressing p23 displayed vein clearing and epinasty, and transgenic plants expressing p20 exhibited aberrations in development; transgenic plants expressing p25 were symptomless and phenotypically identical to the nontransformed controls. Examination of equalized nucleic acid preparations by Northern blot hybridization (Fig. 4) showed that (i) CTV infection enhanced CDVd titer in nontransgenic controls, corroborating the results presented above, (ii) transgenic lines expressing p23 displayed an enhanced CDVd titer similar to that of nontransformed controls coinfected with CTV, (iii) transgenic lines expressing p20 had a viroid titer similar to that of nontransformed controls singly infected with CDVd, and (iv) transgenic plants expressing p25 had a CDVd titer slightly higher than that of the singly infected nontransformed controls. Sequencing of RT-PCR amplicons from CDVd-infected plants indicated that the CDVd used as inoculum remained stable in all the treatments. The presence of p23, p20, and p25 was confirmed in equalized protein extracts of the transgenic lines, as well as in singly infected and coinfected nontransformed controls, by SDS-PAGE and Western blot analysis with p23 and p20 antisera (27) (Twentyfirst Century Biochemicals, Inc.) and with a mixture of 3DF1 and 3CA5 antibodies for detecting p25 (data not shown). Northern blot hybridization only detected the CDVd-sRNAs in transgenic lines expressing p23 (Fig. 4), thus confirming the correlation between the accumulation of the viroid genomic RNA and sRNAs.
FIG 4.
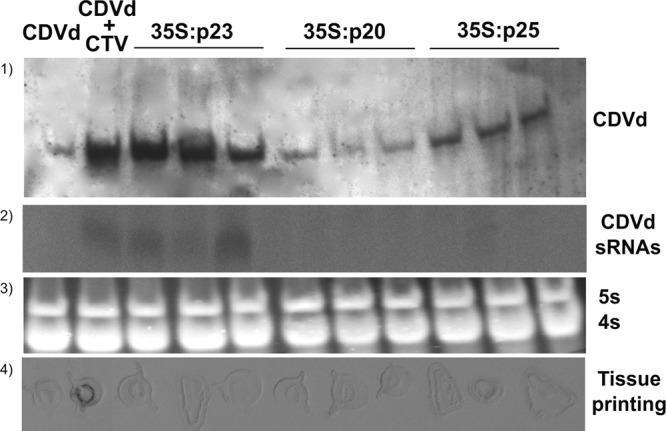
Molecular analysis of CDVd and CTV in wild-type Mexican lime singly infected with CDVd or coinfected with CTV and CDVd and transgenic Mexican lime lines expressing p23, p20, and p25 singly infected with CDVd. (Row 1) Circular forms of CDVd were tested by Northern blot hybridization using a DIG-labeled CDVd-specific probe. (Row 2) CDVd-sRNAs were detected by Northern blot hybridization using a α-UTP-radiolabeled riboprobe. (Row 3) In all instances, RNA levels (5S and 4S RNAs) were comparable. (Row 4) CTV infection was confirmed by tissue-print ELISA using monoclonal antibodies 3DF1 and 3CA5.
Therefore, p23 and, to a lesser extent, p25 appear responsible for the enhancement of CDVd accumulation, suggesting the involvement of an RNA silencing mechanism via sequestration of the viroid sRNAs or inactivation of some of the protein-mediated steps, including those involving members of the Argonaute family. In this context, it is worth pointing out that the ectopic expression of RSSs enhances the accumulation of some viruses (28–30), most likely by suppressing the host antiviral RNA silencing. Consistent with this view, CTV also accumulates when p23 is ectopically expressed in sour orange (27). In contrast, expression of some RSSs has no effect on viroid accumulation in PSTVd-infected N. benthamiana (17). However, our results show that the effect of p23 on viroid accumulation is host dependent, with the interaction between CTV and CDVd being perceptible in Mexican lime but not in etrog citron; this difference may result from the number of infected cells in each host or from distinct regulation of the replication/degradation pathways. Interestingly, the ectopic expression of p23 enhances CTV accumulation in sour orange (27) but not in Mexican lime (25), where the virus reaches low and high accumulation levels, respectively (31). Altogether, these data suggest that hosts in which the pathogen reaches high titers are not appropriate to study the effects of RSS. Finally, since p23 is an RNA-binding protein (32) with nucleolar (and plasmodesmatal) localization (33), which besides acting as an RSS also regulates viral plus-minus-strand accumulation (34) and movement (27), we cannot eliminate the possibility that some of these functions may also influence the observed effect on viroid accumulation. Intriguingly, Gambino et al. found that Grapevine yellow speckle viroid 1, another viroid of the same genus, also accumulates in the nucleus and possibly in the nucleolus (35), although the plants in their study were not directly examined for CDVd.
To summarize, the present study shows the existence of an interaction between CDVd and CTV that affects accumulation but not symptom expression. This interaction is discernible in Mexican lime, wherein CTV infection enhances CDVd accumulation through the expression of the RSSs p23 and, to a lesser extent, p25. In addition, CDVd infection causes a gentle but statistically significant drop in CTV titer.
ACKNOWLEDGMENTS
This research was supported by grant AGL2008-01491 from the Ministerio de Educación y Ciencia.
We acknowledge R. Flores, L. Peña, M. Cambra, and E. Carbonell for helpful comments and R. Carbó for technical assistance.
Footnotes
Published ahead of print 13 November 2013
REFERENCES
- 1.Flores R, Hernández C, Martínez de Alba AE, Darós JA, Di Serio F. 2005. Viroids and viroid-host interactions. Annu. Rev. Phytopathol. 43:117–139. 10.1146/annurev.phyto.43.040204.140243 [DOI] [PubMed] [Google Scholar]
- 2.Szychowski JA, McKenry MV, Walker A, Wolpert JA, Credi R, Semancik JS. 1995. The vein-banding disease syndrome: a synergistic reaction between grapevine viroids and fanleaf virus. Vitis 34:229–232 [Google Scholar]
- 3.Vidalakis G, Garnsey SM, Bash JA, Greer GD, Gumpf DJ. 2004. Efficacy of bioindexing for graft-transmissible citrus pathogens in mixed infections. Plant Dis. 88:1328–1334. 10.1094/PDIS.2004.88.12.1328 [DOI] [PubMed] [Google Scholar]
- 4.Murcia N, Bernad L, Serra P, Bani Hashemian SM, Duran-Vila N. 2009. Molecular and biological characterization of natural variants of citrus dwarfing viroid. Arch. Virol. 154:1329–1334. 10.1007/s00705-009-0430-9 [DOI] [PubMed] [Google Scholar]
- 5.Moreno P, Guerri J, Ballester-Olmos JF, Albiach R, Martínez ME. 1993. Separation and interference of strains from a citrus tristeza virus isolate evidenced by biological activity and double-stranded RNA (dsRNA) analysis. Plant Pathol. 42:35–41. 10.1111/j.1365-3059.1993.tb01469.x [DOI] [Google Scholar]
- 6.Cambra M, Gorris MT, Román MP, Terrada E, Garnsey SM, Camarasa E. 2000. Routine detection of citrus tristeza virus by direct immunoprinting-ELISA method using specific monoclonal and recombinant antibodies, p 34–41 In da Gra̧ca JV, Lee RF, Yokomi RK. (ed), Proc. 14th Conf. Intl. Org. Citrus Virologists. International Organization of Citrus Virologists, Riverside, CA [Google Scholar]
- 7.Romero-Durbán J, Cambra M, Duran-Vila N. 1995. A simple imprint hybridization method for detection of viroids. J. Virol. Methods 55:37–47. 10.1016/0166-0934(95)00043-T [DOI] [PubMed] [Google Scholar]
- 8.Bernad L, Duran-Vila N. 2006. A novel RT-PCR approach for detection and characterization of citrus viroids. Mol. Cell. Probes 20:105–113. 10.1016/j.mcp.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 9.Garnsey SM, Cambra M. 1991. Enzyme-linked immunosorbent assay (ELISA) for citrus pathogens. In Graft-transmissible diseases of citrus: handbook for detection and diagnosis, p 193–216 Food and Agriculture Organization of the United Nations, Rome, Italy [Google Scholar]
- 10.Bertolini E, Moreno A, Capote N, Olmos A, de Luis A, Vidal E, Pérez-Panadés J, Cambra M. 2008. Quantitative detection of citrus tristeza virus in plant tissues and single aphids by real-time RT-PCR. Eur. J. Plant Pathol. 120:177–188. 10.1007/s10658-007-9206-9 [DOI] [Google Scholar]
- 11.Vidal E, Yokomi RK, Moreno A, Bertolini E, Cambra M. 2012. Calculation of diagnostic parameters of advanced serological and molecular tissue-print methods for detection of citrus tristeza virus: a model for other plant pathogens. Phytopathology 102:114–121. 10.1094/PHYTO-05-11-0139 [DOI] [PubMed] [Google Scholar]
- 12.Baulcombe D. 2004. RNA silencing in plants. Nature 431:356–363. 10.1038/nature02874 [DOI] [PubMed] [Google Scholar]
- 13.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. 2001. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409:363–366. 10.1038/35053110 [DOI] [PubMed] [Google Scholar]
- 14.Lecellier CH, Voinnet O. 2004. RNA silencing: no mercy for viruses? Immunol. Rev. 198:285–303. 10.1111/j.0105-2896.2004.00128.x [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-Ferrer V, Voinnet O. 2009. Roles of plant small RNAs in biotic stress responses. Annu. Rev. Plant Biol. 60:485–510. 10.1146/annurev.arplant.043008.092111 [DOI] [PubMed] [Google Scholar]
- 16.Itaya A, Folimonov A, Matsuda Y, Nelson RS, Ding B. 2001. Potato spindle tuber viroid as inducer of RNA silencing in infected tomato. Mol. Plant Microbe Interact. 14:1332–1334. 10.1094/MPMI.2001.14.11.1332 [DOI] [PubMed] [Google Scholar]
- 17.Itaya A, Zhong X, Bundschuh R, Qi Y, Wang Y, Takeda R, Harris AR, Molina C, Nelson RS, Ding B. 2007. A structured viroid RNA is substrate for dicer-like cleavage to produce biologically active small RNAs but is resistant to RISC-mediated degradation. J. Virol. 81:2980–2994. 10.1128/JVI.02339-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papaefthimiou I, Hamilton AJ, Denti MA, Baulcombe DC, Tsagris M, Tabler M. 2001. Replicating potato spindle tuber viroid RNA is accompanied by short RNA fragments that are characteristic of post-transcriptional gene silencing. Nucleic Acids Res. 29:2395–2400. 10.1093/nar/29.11.2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez de Alba AE, Flores R, Hernández C. 2002. Two chloroplastic viroids induce the accumulation of small RNAs associated with posttranscriptional gene silencing. J. Virol. 76:13094–13096. 10.1128/JVI.76.24.13094-13096.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carbonell A, Martínez de Alba AE, Flores R, Gago S. 2008. Double-stranded RNA interferes in a sequence-specific manner with infection of representative members of the two viroid families. Virology 371:44–53. 10.1016/j.virol.2007.09.031 [DOI] [PubMed] [Google Scholar]
- 21.Schwind N, Zwiebel M, Itaya A, Ding B, Wang MB, Krczal G, Wassenegger M. 2009. RNAi-mediated resistance to Potato spindle tuber viroid in transgenic tomato expressing a viroid hairpin RNA construct. Mol. Plant Pathol. 10:459–469. 10.1111/j.1364-3703.2009.00546.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moissiard G, Voinnet O. 2004. Viral suppression of RNA silencing in plants. Mol. Plant Pathol. 5:71–82. 10.1111/j.1364-3703.2004.00207.x [DOI] [PubMed] [Google Scholar]
- 23.Lu R, Folimonov A, Shintaku M, Li WX, Falk BW, Dawson WO, Ding SW. 2004. Three distinct suppressors of RNA silencing encoded by a 20-kb viral RNA genome. Proc. Natl. Acad. Sci. U. S. A. 101:15742–15747. 10.1073/pnas.0404940101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Serio F, Martínez de Alba AE, Navarro B, Gisel A, Flores R. 2010. RNA-dependent RNA polymerase 6 delays accumulation and precludes meristem invasion of a viroid that replicates in the nucleus. J. Virol. 84:2477–2489. 10.1128/JVI.02336-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fagoaga C, López C, Moreno P, Navarro L, Flores R, Peña L. 2005. Viral-like symptoms induced by the ectopic expression of the p23 gene of Citrus tristeza virus are citrus specific and do not correlate with the pathogenicity of the virus strain. Mol. Plant Microbe Interact. 18:435–445. 10.1094/MPMI-18-0435 [DOI] [PubMed] [Google Scholar]
- 26.Domínguez A, Fagoaga C, Navarro L, Moreno P, Peña L. 2002. Regeneration of transgenic citrus plants under non selective conditions results in high-frequency recovery of plants with silenced transgenes. Mol. Genet. Genomics 267:544–556. 10.1007/s00438-002-0688-z [DOI] [PubMed] [Google Scholar]
- 27.Fagoaga C, Pensabene-Bellavia G, Moreno P, Navarro L, Flores R, Peña L. 2011. Ectopic expression of the p23 silencing suppressor of Citrus tristeza virus differentially modifies viral accumulation and tropism in two transgenic woody hosts. Mol. Plant Pathol. 12:898–910. 10.1111/j.1364-3703.2011.00722.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siddiqui SA, Sarmiento C, Truve E, Lehto H, Lehto K. 2008. Phenotypes and functional effects caused by various viral RNA silencing suppressors in transgenic Nicotiana benthamiana and N. tabacum. Mol. Plant Microbe Interact. 21:178–187. 10.1094/MPMI-21-2-0178 [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Turina M, Medina V, Falk BW. 2009. Synergistic interaction between the potyvirus, Turnip mosaic virus and the crinivirus, Lettuce infectious yellows virus in plants and protoplasts. Virus Res. 144:163–170. 10.1016/j.virusres.2009.04.017 [DOI] [PubMed] [Google Scholar]
- 30.Siddiqui SA, Sarmiento C, Kiisma M, Koivumaki S, Lemmetty A, Truve E, Lehto K. 2008. Effects of viral silencing suppressors on tobacco ringspot virus infection in two Nicotiana species. J. Gen. Virol. 89:1502–1508. 10.1099/vir.0.83621-0 [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Ruiz S, Navarro B, Gisel A, Peña L, Navarro L, Moreno P, Di Serio F, Flores R. 2011. Citrus tristeza virus infection induces the accumulation of viral small RNAs (21-24-nt) mapping preferentially at the 3′-terminal region of the genomic RNA and affects the host small RNA profile. Plant Mol. Biol. 75:607–619. 10.1007/s11103-011-9754-4 [DOI] [PubMed] [Google Scholar]
- 32.López C, Navas-Castillo J, Gowda S, Moreno P, Flores R. 2000. The 23-kDa protein coded by the 3′-terminal gene of citrus tristeza virus is an RNA-binding protein. Virology 269:462–470. 10.1006/viro.2000.0235 [DOI] [PubMed] [Google Scholar]
- 33.Ruiz-Ruiz S, Soler N, Sánchez-Navarro J, Fagoaga C, López C, Navarro L, Moreno P, Peña L, Flores R. 2013. Citrus tristeza virus p23: determinants for nucleolar localization and their influence on suppression of RNA silencing and pathogenesis. Mol. Plant Microbe Interact. 26:306–318. 10.1094/MPMI-08-12-0201-R [DOI] [PubMed] [Google Scholar]
- 34.Satyanarayana T, Gowda S, Ayllón MA, Albiach-Martí MR, Rabindram R, Dawson WO. 2002. The p23 protein of citrus tristeza virus controls asymmetrical RNA accumulation. J. Virol. 76:473–483. 10.1128/JVI.76.2.473-483.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gambino G, Navarro B, Vallania R, Gribaudo I, Di Serio F. 2011. Somatic embryogenesis efficiently eliminates viroid infections from grapevines. Eur. J. Plant Pathol. 130:511–519. 10.1007/s10658-011-9770-x [DOI] [Google Scholar]


