Abstract
Human tetherin is a host restriction factor that inhibits replication of enveloped viruses by blocking viral release. Tetherin has an unusual topology that includes an N-terminal cytoplasmic tail, a single transmembrane domain, an extracellular domain, and a C-terminal glycosylphosphatidylinositol anchor. Tetherin is not well conserved across species, so it inhibits viral replication in a species-specific manner. Thus, studies of tetherin activities from different species provide an important tool for understanding its antiviral mechanism. Here, we report cloning of equine tetherin and characterization of its antiviral activity. Equine tetherin shares 53%, 40%, 36%, and 34% amino acid sequence identity with feline, human, simian, and murine tetherins, respectively. Like the feline tetherin, equine tetherin has a shorter N-terminal domain than human tetherin. Equine tetherin is localized on the cell surface and strongly blocks human immunodeficiency virus type 1 (HIV-1), simian immunodeficiency virus (SIV), and equine infectious anemia virus (EIAV) release from virus-producing cells. The antiviral activity of equine tetherin is neutralized by EIAV envelope protein, but not by the HIV-1 accessory protein Vpu, which is a human tetherin antagonist, and EIAV envelope protein does not counteract human tetherin. These results shed new light on our understanding of the species-specific tetherin antiviral mechanism.
INTRODUCTION
Tetherin (also referred to as HM1.24, BST-2, or CD317) is a type II single-pass transmembrane protein. Human tetherin (huTHN) was first identified as a cellular restriction factor that blocks human immunodeficiency virus type 1 (HIV-1) particle release from infected cells in the absence of the HIV-1 accessory protein Vpu (1). Later, it was found that human tetherin has very broad antiviral activities, which also target many other enveloped viruses, including retroviruses, filoviruses, arenaviruses, paramyxoviruses, herpesviruses, and rhabdoviruses (2–9). Human tetherin orthologs have been isolated from several other species, including monkey, cat, pig, mouse, cattle, and sheep, which all show similar antiviral activities (10–17).
Tetherin has an unusual topology, which consists of an N-terminal cytoplasmic tail (CT), a single transmembrane domain, an extracellular domain, and a C-terminal glycosylphosphatidylinositol (GPI) membrane anchor (18). The extracellular coiled-coil domain promotes dimerization of adjacent tetherin molecules with disulfide links. This topology is rare and is shared only with an isoform of the prion protein (19). Accordingly, these structural features together determine tetherin's antiviral function. During viral infection, tetherins on the cell surface and viral envelope can prevent virion release either by direct cross-linking or by the formation of dimers between adjacent coiled-coil domains (20). Tetherin is constitutively expressed in mature B cells, some cancer cell lines, bone marrow stromal cells, monocyte-derived macrophages, and plasmacytoid dendritic cells, and its expression can be induced by type I and II interferon (IFN) treatment (21–28). Thus, tetherin may play a fundamental role in the initiation and perpetuation of a virus-specific immune response. Some viruses encode proteins to counteract tetherin. The known tetherin antagonists include the Vpu protein of HIV-1; the envelope proteins of HIV-2, simian immunodeficiency virus from tantalus monkeys (SIVtan), feline immunodeficiency virus (FIV), and Ebola virus; the Nef protein of SIV; and the K5 protein of the human herpesvirus Kaposi's sarcoma-associated herpesvirus (KSHV). These viral proteins antagonize the antiviral activity of tetherin by different mechanisms (1, 2, 16, 29–32). Equine infectious anemia virus (EIAV) is a macrophage-tropic lentivirus that causes a persistent infection characterized by recurring viremia, fever, thrombocytopenia, and wasting symptoms (33, 34). EIAV shares genetic and structural similarity with HIV, SIV, and FIV. In addition to the structural proteins encoded by gag, pol, and env, EIAV encodes three accessory proteins: Tat, Rev, and S2, making EIAV the least complex lentivirus. EIAV infection induces a reproducible clinical disease course, which provides a useful model for comparative study of restriction factors that are involved in resistance to lentiviral infection and a very useful system for lentivirus vaccine development.
Although tetherins from many different species have been well characterized as potent antiretroviral factors, whether equine cells encode an ortholog of tetherin and the interaction between equine tetherin (eqTHN) and EIAV or other retroviruses remain unknown. In this study, we isolated the equine tetherin gene and investigated its antiviral activity and how this activity is counteracted by EIAV. We found that equine tetherin has very broad antiviral activity and that its activity is only neutralized by the EIAV envelope protein.
MATERIALS AND METHODS
Identification and cloning of the equine tetherin gene.
Based on the genome data for Equus caballus, an equine tetherin gene was identified on the chromosome 21 genomic scaffold of Equus caballus breed thoroughbred, EquCab2.0 scaffold_73 (GenBank accession no. NW_001867390.1). Oligonucleotide primers were synthesized corresponding to the predicted start (forward primer 5′-ATGGGGGACCACAGGCTGCTGAGAT-3′) and stop (reverse primer 5′-TCAGGCCTGCAGATCCCAGAGGCCC-3′) codons of equine tetherin. Total RNA was extracted from equine macrophages using TRIzol reagent (Invitrogen) and subjected to reverse transcription (RT)-PCR. The amplified fragments were cloned into pEF-Flag-HA, which was constructed from pEF4/myc-His B (Invitrogen) to express a protein fused to a tandem FLAG-HA (hemagglutinin) tag at the N terminus. The expression plasmid for equine tetherin was named pEF-eqTHN. The truncated forms of equine tetherin lacking the putative CT or putative GPI anchor were also generated by RT-PCR using total RNA from equine macrophages. To determine the initiation site of equine tetherin and to identify the regulatory elements in the tetherin promoter region, 5′ rapid amplification of cDNA ends (RACE) was performed using a 5′-Full RACE Core Set (TaKaRa). Total RNA extracted from equine macrophages was used as a PCR template. The PCR product was cloned into the pMD18-T vector and subsequently sequenced.
Plasmids.
Human (huTHN) and macaque (macTHN) tetherin genes were cloned into the pEF-Flag-HA vector. The pcDNA-Vphu vector, which encodes a human codon-optimized form of NL4-3 Vpu (Vphu), was kindly provided by Klaus Strebel (NIH). This vpu gene was subsequently cloned into a pcDNA3.1 (+) vector (Invitrogen) that has two HA tags at the C terminus. The pNL-r-HSAS HIV-1 proviral construct was obtained from Beth Jamieson and Jerome Zack through the NIH AIDS Reagent Program. The SIVmac239 proviral vector pBR239E was kindly provided by Toshiaki Kodama. To create an Env and Nef deletion pBR239E (pBR239EΔEΔN), the env gene was first inactivated by blunting a HindIII site; then, the nef gene was inactivated by swapping with the NheI/EcoRI fragment from p239SpE3′, which contains a premature stop codon in the nef gene region and was also obtained from the NIH AIDS Reagent Program. The pLG3-8 vector expresses the full-length EIAV proviral genome of the EIAVFDDV12 strain. Using this construct as a template, the EIAV gag-pol gene was amplified and inserted into the VR-1012 vector (a gift kindly provided by Xiaofang Yu) by EcoRI and NotI digestion. The EIAV S2 gene was expressed with an N-terminal green fluorescent protein (GFP) tag in the pEGFP-C1 vector (BD Biosciences Clontech). The EIAV rev and env genes were amplified from pLG3-8 and cloned into the pcDNA3.1 mammalian expression vector (Invitrogen).
Cells and transfection.
Human embryonic kidney (HEK) 293T cells, equine dermal cells, equine vascular endothelial cells, and HeLa cells were cultured at 37°C in a 5% CO2 incubator in Dulbecco's modified Eagle's medium (Gibco, Invitrogen) supplemented with 10% fetal bovine serum and penicillin/streptomycin. These cells were transfected either by the calcium-phosphate method or with Lipofectamine 2000 reagent (Invitrogen), following the manufacturer's instructions. Equine primary macrophages were maintained in RPMI 1640, supplemented with 10% horse serum and 30% fetal bovine serum.
Measurement of tetherin expression by real-time PCR.
Cells were treated for 24 h with or without 1,000 units/ml alpha interferon (IFN-α) (Kingfisher Biotech), and the total RNA was extracted as described above. One hundred nanograms of total RNA was subjected to reverse transcription using SuperScript II reverse transcriptase and oligo(dT)12-18 as a primer (Invitrogen). Synthesized cDNAs were then subjected to real-time PCR using a SYBR green Master Mix kit (Invitrogen) according to the manufacturer's protocols. Real-time RT-PCR was performed using the tetherin primers 5′-GTGGTGGTGTTTCTGCTTGTG-3′ (forward) and 5′-GTTGCGACACTCCTGCTCTG-3′ (reverse) and the β-actin primers 5′-ACGGCATCGTCACCAACTG-3′ (forward) and 5′-CAAACATGATCTGGGTCATCTTCTC-3′ (reverse).
Virion release and Western blotting.
To produce virus-like particles (VLPs), a total of 1 × 106 293T cells cultured in six-well plates were transfected with 5 μg of EIAV Gag Pol expression vector, pNL-r-HSAS, or pBR239E and 0.1 to 2 μg of equine, human, or macaque tetherin expression vectors. The proper amount of pEF-Flag-HA empty vector (Invitrogen) was used in the transfection as a control DNA. At 48 h posttransfection, the culture supernatants were harvested and the cells were lysed in buffer containing 100 mM Tris-HCl (pH 7.5), 50 mM NaCl, 5 mM EDTA, and 1% Triton X-100. The supernatants were clarified by low-speed centrifugation, and the released VLPs were collected by centrifugation at 20,000 × g for 2 h. Both viral lysates and cell lysates were separated on 12% gels by SDS-polyacrylamide gel electrophoresis (PAGE). The separated proteins were then transferred onto nitrocellulose membranes and blocked with 5% dried milk in phosphate-buffered saline (PBS) containing 0.1% (vol/vol) Tween 20 for 2 h at room temperature. Membranes were incubated for 2 h with the appropriate primary antibodies, which included a monoclonal anti-Flag antibody (Sigma), a mouse anti-HA antibody (Sigma), anti-EIAV equine serum, anti-HIV-1 p24 monoclonal antibody, anti-SIV p27 monoclonal antibody, and anti-actin antibody (Sigma). The membranes were washed three times with Tris-buffered saline with Tween 20 (TBST) for 10 min each time and then incubated with Alexa Fluor 800-labeled goat anti-mouse IgG or goat anti-horse secondary antibody (Odyssey) for 1 h at room temperature. Specific proteins were detected and quantified using the Odyssey system (Li-Cor).
Coimmunoprecipitation assay.
293T cells were transfected with 1 μg of EIAV Env expression vector and 1 μg eqTHN–glutathione S-transferase (GST) or huTHN-GST. After 48 h, the cells were lysed in a buffer comprising 100 mM Tris-HCl (pH 7.5), 50 mM NaCl, 5 mM EDTA, and 1% Triton X-100 and then incubated with glutathione-Sepharose beads. The bead-associated proteins were analyzed by Western blotting with an anti-V5 antibody and an anti-EIAV antibody, as described above.
Confocal microscopy.
HEK 293T cells grown on glass coverslips were transiently transfected with appropriate tetherin and EIAV Gag Pol expression constructs, employing the Lipofectamine 2000 reagent (Invitrogen). The cells were then fixed with 4% paraformaldehyde-PBS and permeabilized for 10 min in 0.1% Triton X-100–PBS. The cells were incubated with anti-HA antibody for detection of tetherin or an anti-EIAV antiserum, followed by staining using secondary antibodies conjugated with fluorescein isothiocyanate (FITC) or tetramethyl rhodamine isocyanate (TRITC) (Sigma). After being mounted on slides using AntiFade-4 6-diamidino-2-phenylindole (DAPI) mounting solution (Sigma), the cells were visualized with a Leica DM-IRE2 confocal microscope.
RESULTS
Characterization of the equine tetherin gene.
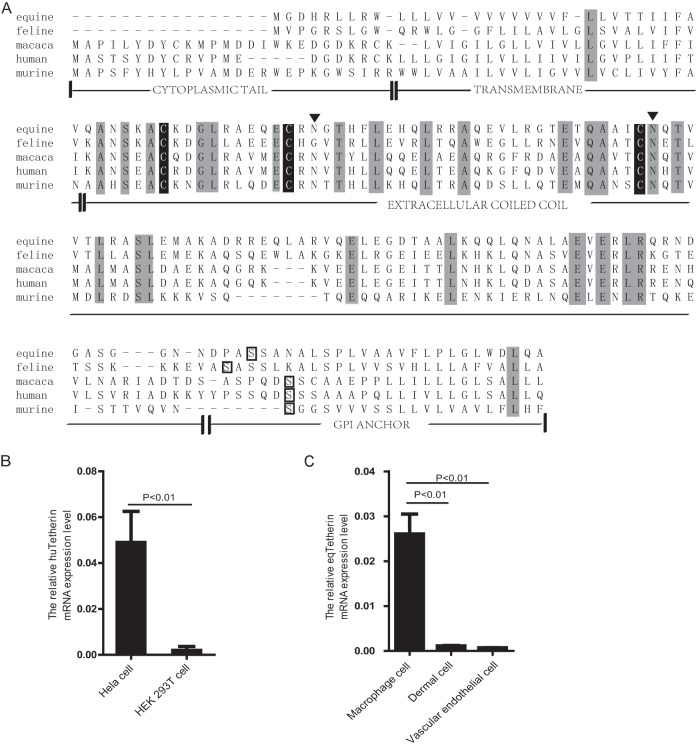
After screening the equine genome, a genomic sequence with significant homology to tetherin was identified on chromosome 21. To clone this gene, primers were designed, and a 498-bp fragment was amplified from equine macrophages by RT-PCR, which was confirmed as equine tetherin cDNA after cloning and sequencing. Both the nucleic acid and the amino acid sequences of the gene were compared to those from cat (GenBank accession number AB564550), human (NM_004335), rhesus macaque (HQ596987), and mouse (NM_198095) (Fig. 1A). The degrees of sequence identity of equine tetherin with those of cat, human, rhesus macaque, and mouse were 74%, 58%, 57%, and 48% in nucleic acid and 53%, 40%, 36%, and 34% in amino acid, respectively.
FIG 1.
Characterization of equine tetherin. (A) Amino acid sequence alignment of mammalian tetherins. Identical amino acids are shaded in light gray. The predicted topology of equine tetherin includes an amino-terminal CT, a transmembrane region (TM), an extracellular coiled-coil domain, and a predicted site for GPI anchor attachment, which are boxed in black. Three Cys residues in the extracellular domain are shaded in black. Two putative N-glycosylation sites in the extracellular domain are marked with black triangles. (B) Human tetherin and β-actin mRNA expression in HeLa cells and HEK 293T cells was quantified by real-time PCR. The numbers of tetherin mRNA copies were normalized to those of β-actin. The histograms represent the averages from three independent experiments (n = 3). (C) Equine tetherin and β-actin mRNA expression in equine macrophages, equine dermal cells, and equine vascular endothelial cells. The data represent the means and standard deviations (SD) for three independent experiments. A P value of <0.01 was considered very significant.
Based on bioinformatic prediction (CLUSTALW2, HMMTOP, big-PI Predictor GPI Modification Site Prediction, and NetNGlyc 1.0 Server), a C-terminal GPI anchor, an extracellular coiled-coil domain, and an N-terminal transmembrane domain were identified in equine tetherin. Three cysteine residues in the extracellular domain, which are important for dimer formation, are also conserved in the equine tetherin sequence. In addition, like feline tetherin, equine tetherin has a shorter N-terminal region than human and monkey tetherins, but this structural feature did not affect the protein's tethering function and subcellular localization (Fig. 2A; see Fig. 4). Thus, equine tetherin has a topology similar to those of other mammalian orthologs.
FIG 2.
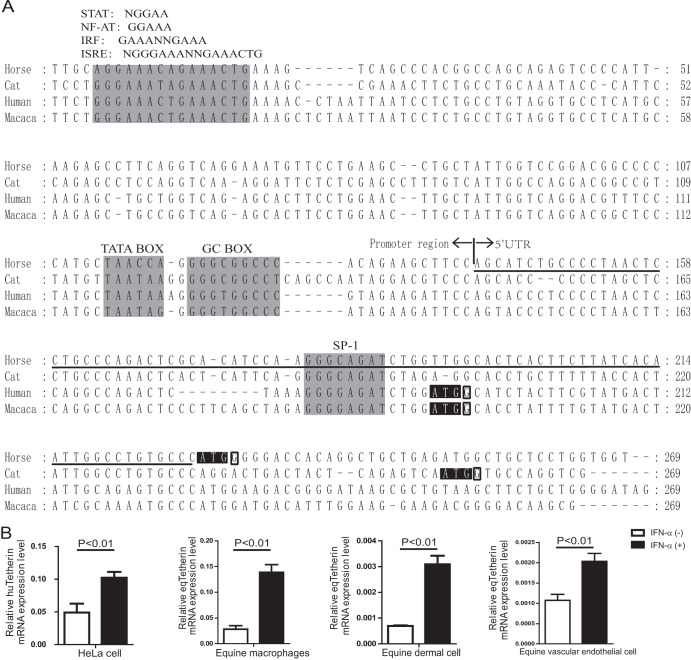
Analyses of the 5′ flanking region of the tetherin genes and their IFN-α-inducible expression. (A) Nucleotide sequence alignment of 5′ flanking regions between equine, feline, macaque, and human tetherins. The sequence from 5′ RACE of the equine tetherin gene (underlined) and the putative promoter region was used to compare with those of tetherin genes from the human, macaque, and feline genomes. Putative regulatory elements conserved among different species are shaded in light gray. The translational start codons are shaded in black. Conserved and important residues at the +4 position are outlined in black. (B) Induction of tetherin expression by IFN-α. Equine macrophages, equine dermal cells, equine vascular endothelial cells, and HeLa cells were cultured for 24 h in the presence (+) or absence (−) of equine IFN-α. Then, tetherin and β-actin mRNA expression levels were quantified by real-time PCR. The numbers of tetherin mRNA copies were normalized to those of β-actin. The data represent the means and SD for three independent experiments. A P value of <0.01 was considered very significant.
FIG 4.
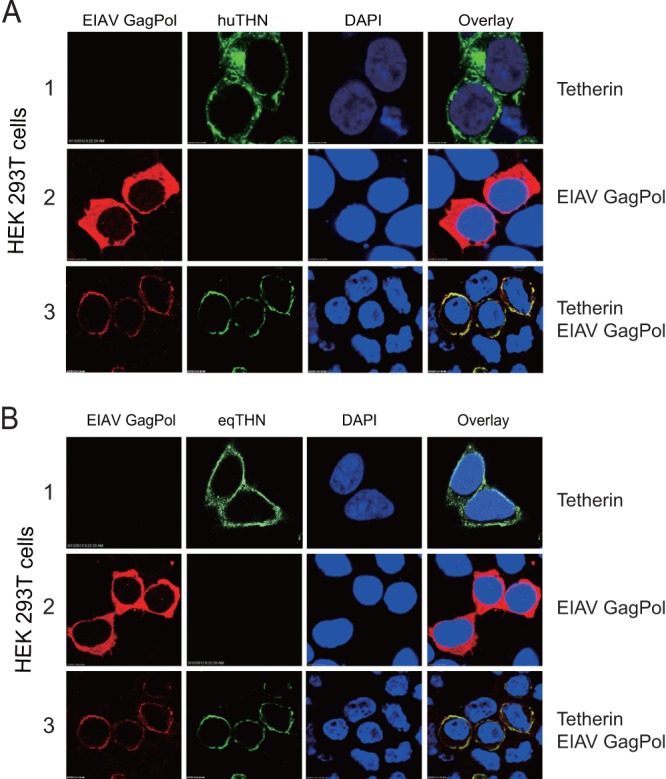
Colocalization of nascent EIAV VLPs and tetherin on the cell surface. 293T cells were transfected with an HA-tagged human tetherin expression construct (A) or an HA-tagged equine tetherin construct (B) and an EIAV Gag Pol expression construct. Twenty-four hours later, the cells were stained with an anti-HA antibody (green) and an anti-EIAV antibody (red), and nuclei were stained with DAPI (blue). Then, the cells were analyzed by confocal microscopy. Single deconvolved optical sections acquired at the cell-coverslip interface are shown.
Tetherin is constitutively expressed in monocyte-derived macrophages and plasmacytoid dendritic cells. To determine how tetherin transcripts are expressed in equine cells permissive for EIAV replication, the levels of tetherin expression in equine macrophages, equine dermal cells, and equine vascular endothelial cells were analyzed by real-time RT-PCR. As a control, we first compared tetherin mRNA expression in human HeLa and HEK 293T cells and found that HeLa cells expressed significantly higher levels of tetherin mRNA than 293T cells (P < 0.01) (Fig. 1B). This result is consistent with previous reports by other groups (1, 35). Next, we compared tetherin expression in equine cells. It was found that high levels of tetherin transcripts were detected in equine macrophages; the levels of tetherin mRNA in equine dermal cells and equine vascular endothelial cells were low, but they were still detectable (P < 0.01) (Fig. 1C). Because EIAV is macrophage-tropic, the high level of tetherin expression in equine macrophages and how EIAV overcomes this barrier are of particular interest.
It has been reported that the expression of tetherin is inducible by type I IFNs. To study this mechanism, we analyzed the nucleotide sequence of equine tetherin's promoter region and the 5′ untranslated region (5′ UTR) obtained from 5′-RACE analysis. The equine tetherin gene has an 88-bp 5′ UTR. Like the other mammalian orthologs, its promoter region contains a putative nontraditional TATA box, a GC box, and several potential transcription factor-binding sites that may bind to NF-AT and STAT (Fig. 2A). In addition, at least two IFN-responsive elements, IRF and ISRE, which contain multiple overlapping consensus sequence motifs, were also identified (Fig. 2A), To confirm that the expression of equine tetherin is induced by type I IFN, equine macrophages, equine dermal cells, and equine vascular endothelial cells were treated with IFN-α for 24 h at 1,000 units/ml, and the levels of tetherin expression were quantified by quantitative real-time RT-PCR. It was found that, as in human HeLa cells, IFN-α could significantly increase tetherin expression in all these equine cells (P < 0.01). However, the levels of increase (about 6-fold) were slightly higher in equine macrophages and equine dermal cells than in equine vascular endothelial cells (about 2-fold) (Fig. 2B). Altogether, these results confirmed that the expression of equine tetherin is also inducible by IFN-α.
Equine tetherin inhibits lentivirus release.
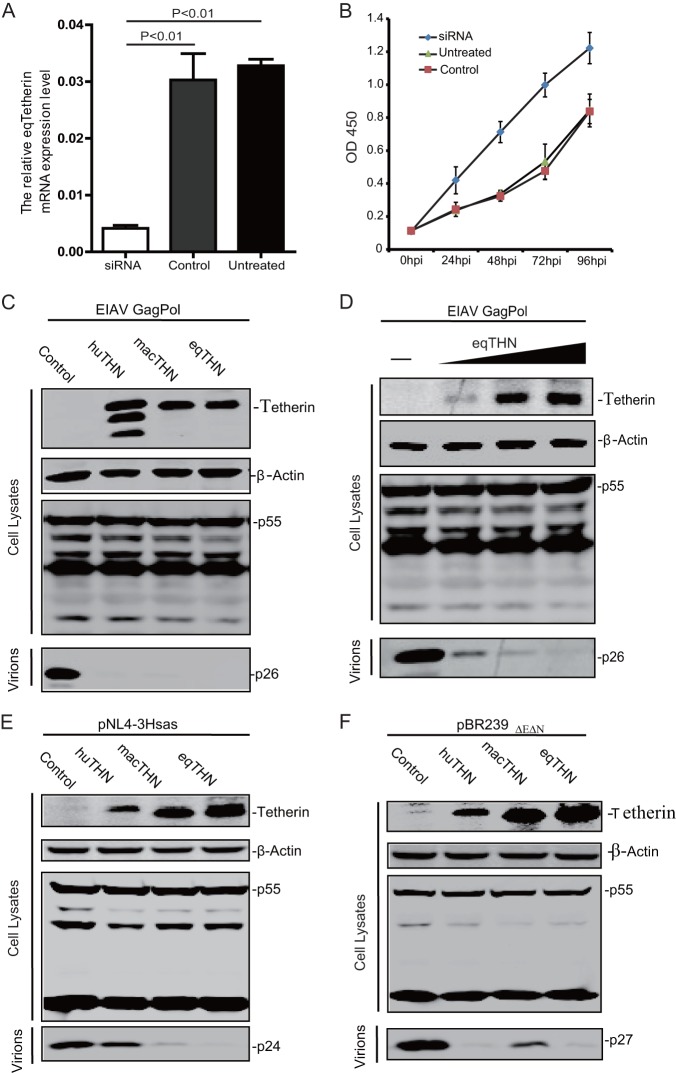
Since a high level of equine tetherin expression was observed in macrophages (Fig. 1C), we next asked if tetherin inhibits EIAV replication in macrophages. When we transfected equine macrophages with an equine-tetherin-specific small interfering RNA (siRNA), equine tetherin mRNA expression was significantly decreased compared to the relatively high level of equine tetherin expression in untreated cells or cells transfected with control siRNA (P < 0.01) (Fig. 3A). EIAV (1 × 103 50% tissue culture infective doses [TCID50]) was used to infect these macrophages, and viral replication was monitored by detecting viral reverse transcriptase in the supernatant. Increased EIAV replication was observed in the tetherin knockdown cells compared with the untreated and treated with control siRNA groups (Fig. 3B). This result indicated that equine tetherin may have the ability to block EIAV replication.
FIG 3.
Effect of equine tetherin on retrovirus replication. (A) Knockdown of equine tetherin expression. Equine monocyte-derived macrophages were transfected with 100 nM siRNA or 100 nM scrambled siRNA (Control) or not transfected (Untreated). The siRNA target sequence was GTGGAACGACTACGACAAA. Twenty-four hours posttransfection, tetherin and β-actin mRNA expression levels were quantified by real-time PCR. The number of tetherin mRNA copies was normalized to that of β-actin. The data represent the means and SD for three independent experiments. A P value of <0.01 was considered very significant. (B) Knockdown of tetherin promotes EIAV growth in equine macrophages. Equine monocyte-derived macrophages transfected with 100 nM siRNA against eqTHN or control siRNA (Control) for 24 h were infected with EIAV at 1 × 103 TCID50. The virus titers in the supernatants collected at 0 h postinfection (p.i.), 24 h p.i., 48 h p.i., 72 h p.i., and 96 h p.i. were determined by measuring the reverse transcriptase activity using an RT activity kit (Reverse Transcriptase Assay, Colorimetric kit; Roche, Switzerland) according to the manufacturer's protocol. (C) An EIAV Gag Pol-expressing construct (5 μg) was transfected into 293T cells alone (Control) or with a huTHN, an HA-tagged macTHN, or an eqTHN expression vector (1 μg). (D) An EIAV Gag Pol-expressing construct (3 μg) was transfected into 293T cells, along with increasing amounts of an equine tetherin expression vector (0, 0.2, 0.4, and 0.8 μg). (E) A HIV-1 provirus clone (pNL-r-HSAS) (5 μg) was transfected into 293T cells alone (Control) or with either huTHN, macTHN, or eqTHN expression vector (1 μg). (F) A SIV provirus clone (pBR239EΔEΔN) (5 μg) was transfected into 293T cells alone (Control) or with either huTHN, macTHN, or eqTHN expression vector (1 μg). Forty-eight hours posttransfection, the cells were lysed, and the viral particles in the culture supernatant were pelleted down by ultracentrifugation. Viral proteins in the transfected cells and released virions were analyzed by Western blotting using anti-EIAV serum, an HIV p24Gag monoclonal antibody (no. 3537), or a SIV p27 Gag monoclonal antibody (no. 1610) from the NIH AIDS Research and Reference Reagent Program. Intracellular expression of tetherin was confirmed by Western blotting using anti-HA antibody.
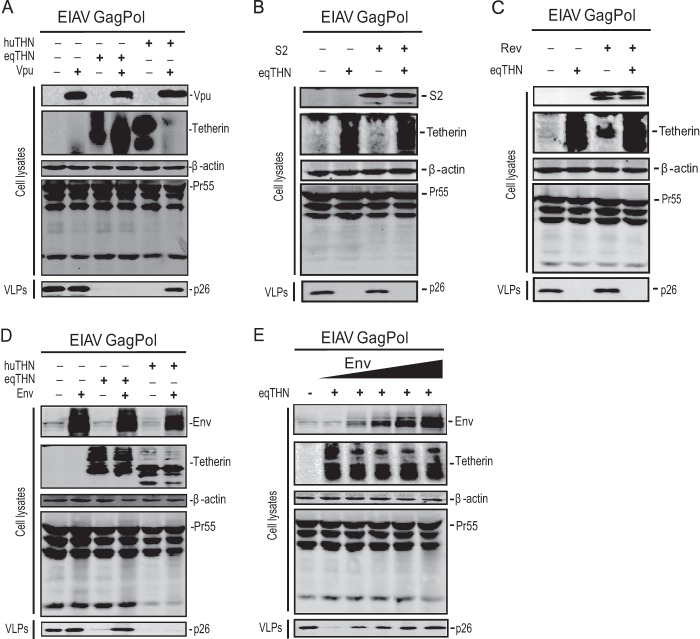
To determine whether equine tetherin blocks EIAV VLP release, an EIAV Gag Pol expression vector was transfected into HEK 293T cells in the presence of an equine, human, or macaque tetherin expression vector. After 48 h, VLPs were isolated from the supernatants, and Gag expression in VLPs and cells was analyzed by Western blotting. It was found that in the presence of a huTHN, a macTHN, or an eqTHN, no VLPs were detected in the supernatants (Fig. 3C), while tetherin did not affect viral Gag protein expression in the cells. It was confirmed that this effect of eqTHN was dose dependent (Fig. 3D). Thus, like huTHN and macTHN, eqTHN could strongly block EIAV VLP release.
Next, we determined whether equine tetherin could inhibit SIV or HIV particle release. To measure HIV-1 and SIV release, 293T cells were transfected with an HIV-1 or SIVmac239 (ΔEnv ΔNef) proviral construct in the presence of huTHN, macTHN, or eqTHN. After 48 h, VLPs were purified from cell supernatants and the viral particles released were analyzed. It was found that huTHN blocked SIV but not HIV-1 release, which is consistent with the fact that HIV-1 Vpu can neutralize huTHN; macTHN blocked HIV-1 VLP release, which is consistent with the fact that HIV-1 Vpu cannot neutralize macTHN (Fig. 3E and F); macTHN also inhibited SIV VLP release in the absence of Env and Nef (Fig. 3F). Notably, eqTHN could block both HIV-1 and SIV release, indicating that eqTHN has very broad antiviral activity and that HIV-1 cannot counteract this equine restriction factor.
Subcellular localization of equine tetherin.
To understand how equine tetherin blocks viral release, we determined whether it could retain EIAV VLPs on the cell surface. HEK 293T cells were transfected with a human (Fig. 4A) or an equine (Fig. 4B) tetherin expression vector in the presence or absence of an EIAV Gag Pol expression vector, and Gag and tetherin subcellular distribution was determined by fluorescence microscopy. Most of the human and equine tetherins were found on the cell surface (Fig. 4A and B, rows 1), and the EIAV Gag proteins were found in the cytoplasm when they were transfected individually (Fig. 4A and B, rows 2). However, when tetherin and Gag proteins were expressed together, the EIAV Gag proteins were colocalized on the plasma membrane with human or equine tetherin (Fig. 4A and B, rows 3). Taken together, these results further confirmed that equine tetherin could inhibit the release of EIAV particles from 293T cells, likely by tethering nascent virus particles on the cell surface.
Mapping of the active antiviral domain of equine tetherin.
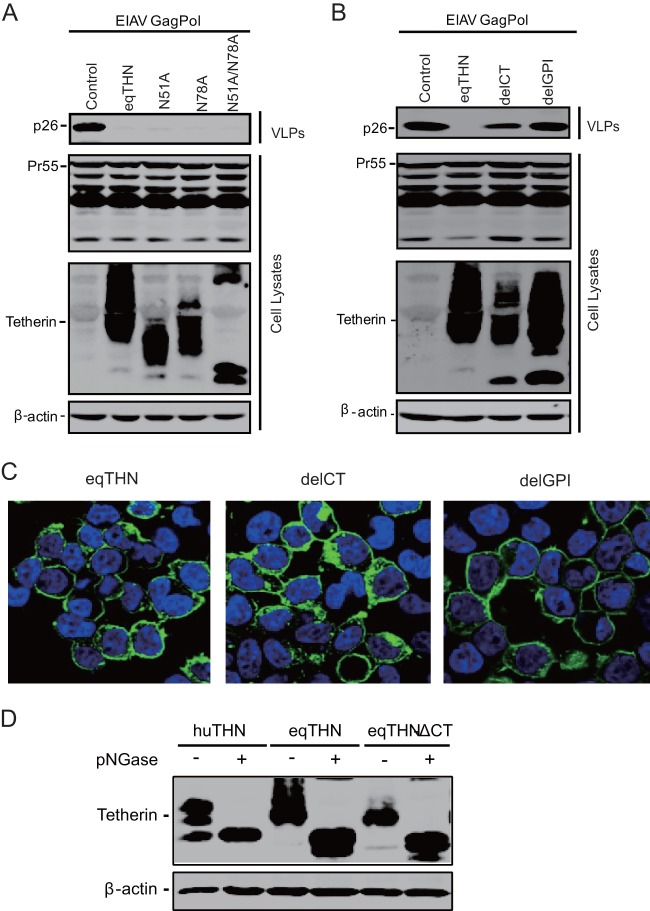
N-linked glycosylation sites of feline tetherin were shown to be important for its antiviral activity (14). We next asked if equine tetherin has a similar phenotype. Single or double mutations in the N-linked glycosylation sites (N51A, N79A, and N51A/N78A) were created and used to transfect 293T cells, together with the EIAV Gag Pol construct. We found that in fact it was not required for tetherin function (Fig. 5A). Both CT and GPI play important roles in maintaining tetherin's function. To dissect the roles of these two domains of equine tetherin in its antiviral capability, two equine tetherin mutants were created, which contained a deletion of the cytoplasmic tail (delCT) and GPI (delGPI), respectively. They were transfected with the EIAV Gag Pol expression vector into 293T cells, and after 48 h, viral release was measured. It was found that both delCT and delGPI showed slight molecular weight changes in Western blotting. The wild-type tetherin protein markedly decreased the viral particle production, and in contrast, the delGPI mutant almost completely lost its antiviral activity. The inhibitory activity of delCT was significantly reduced (Fig. 5B). Notably, the subcellular distribution of delCT, but not delGPI, was partially changed to the cytoplasmic compartment (Fig. 5C). Unlike human tetherin reported previously (1), delCT was well glycosylated (Fig. 5D). Thus, the cytoplasmic tail and GPI anchor, but not the glycosylation domain, are critical for equine tetherin antiviral activity.
FIG 5.
Identification of the crucial domains of equine tetherin. (A) An EIAV Gag Pol-expressing vector (5 μg) was transfected into 293T cells alone (Control) or with 1 μg of HA-tagged eqTHN (wild type [WT]), HA-tagged eqTHN (N51A), HA-tagged eqTHN (N78A), or HA-tagged eqTHN (N51A/N78A). (B) An EIAV Gag Pol-expressing vector (5 μg) was transfected into 293T cells alone (Control) or with 1 μg of eqTHN (WT), eqTHN (delCT), or eqTHN (delGPI). Forty-eight hours posttransfection, the cells were lysed and viral particles were spun down from the culture supernatants by ultracentrifugation. Viral proteins in the transfected cells and released virions were analyzed by Western blotting. (C) 293T cells were transfected with eqTHN (WT), eqTHN (delCT), or eqTHN (delGPI). Twenty-four hours later, the cells were stained with an anti-HA antibody (green), and nuclei were stained with DAPI stain (blue). After that, the cells were analyzed by confocal microscopy. Single deconvolved optical sections acquired at the cell-coverslip interface are shown. (D) 293T cells were transfected with huTHN (WT), eqTHN (WT), or eqTHN (delCT). The cells were lysed and then treated (+) or not (−) with PNGase for the de-N-glycosylated reaction, Tetherin was detected with anti-HA antibody by Western blotting.
Equine tetherin can be neutralized by EIAV Env protein, but not HIV-1 Vpu.
Lentiviruses have evolved various mechanisms to counteract tetherin restriction. For example, the antiviral activity of tetherin could be neutralized by several viral proteins, including Vpu of HIV-1, Nef of SIV, and the envelope proteins of HIV-2, SIVtan, and FIV. To understand how EIAV evades this host restriction, we tested whether any viral proteins could neutralize equine tetherin activity. First, we directly measured HIV-1 Vpu activity against equine tetherin. A Vpu expression construct was transfected into 293T cells along with the EIAV Gag Pol expression vector and the expression vector for human or equine tetherin, and viral release was determined. It was found that HIV-1 Vpu could overcome the human tetherin but not the equine tetherin inhibitory activity (Fig. 6A), which further confirmed our previous observation (Fig. 3C, E, and F). Thus, the activity of Vpu against tetherin is species specific. Second, we directly tested the activities of the EIAV S2, Rev, and Env proteins. An S2, Rev, or Env expression vector was transfected into 293T cells, along with the EIAV Gag Pol expression vector plus the expression vector for human or equine tetherin, and viral release was determined. It was found that neither S2 nor Rev could counteract equine tetherin antiviral activity (Fig. 6B and C). However, expression of the EIAV Env protein almost completely overcomes the antiviral activity of equine tetherin, but not human tetherin (Fig. 6D). Increasing the doses of Env expression proportionally rescued the release of VLPs (Fig. 6E). Similar results were found when we transfected equine dermal cells (data not shown).
FIG 6.
EIAV Env protein, but not HIV-1 Vpu protein, counteracts equine tetherin. (A) 293T cells were transfected with an EIAV Gag Pol expression plasmid (2.5 μg) and the tetherin expression vectors, as indicated, in the presence or absence of 1.5 μg HIV-1 Vpu expression vector. (B and C) 293T cells were transfected with an EIAV Gag Pol expression construct (2.5 μg) and the eqTHN expression vector, as indicated, in the presence or absence of 1.5 μg of EIAV S2 (B) or EIAV Rev (C) plasmid. (D) 293T cells were transfected with an EIAV Gag Pol expression construct (2.5 μg) and the tetherin expression vectors, as indicated, in the presence or absence of 1 μg of EIAV Env expression vector. (E) 293T cells were transfected with an EIAV Gag Pol expression construct (2.5 μg) only or along with an eqTHN expression vector in the presence of increasing amounts of the Env plasmid (0, 50, 100, 500, and 1,000 ng). Forty-eight hours posttransfection, the cells were lysed and viral particles were spun down from culture supernatants by ultracentrifugation. Viral proteins in the transfected cells and released virions were analyzed by Western blotting using anti-EIAV serum, Intracellular expression of tetherin was confirmed by Western blotting using anti-HA antibody.
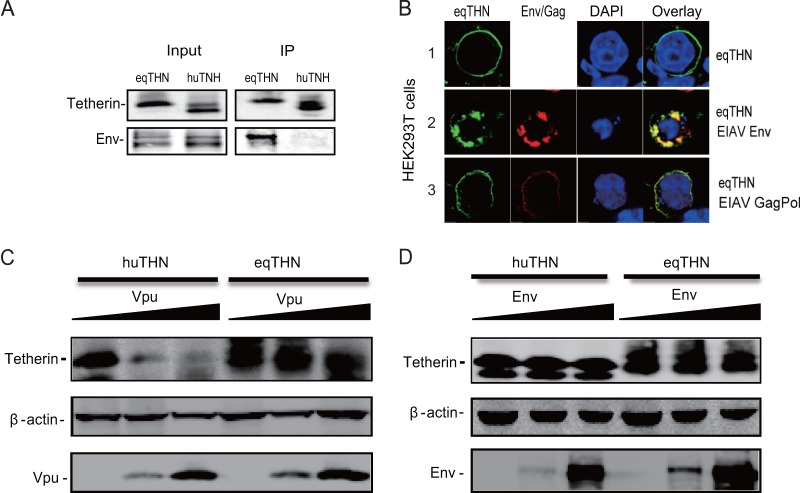
To confirm this finding, we determined how EIAV Env interacts with equine and human tetherins by a GST pulldown assay. Equine or human tetherin was fused with a GST tag, and these fusion proteins were coexpressed with EIAV Env proteins. Cellular proteins were pulled down by glutathione-agarose beads, and the bead-associated proteins were analyzed by Western blotting. It was found that the Env protein was copurified with equine, but not human, tetherin, indicating that EIAV Env specifically interacts with equine tetherin (Fig. 7A). To further confirm this specific interaction, we determined how EIAV Env affects equine tetherin subcellular localization. 293T cells were transfected with Env and tetherin expression vectors, and their subcellular distribution was determined by confocal microscopy. It was found that upon expression of EIAV Env, equine tetherin was redistributed from the cell surface to intracellular compartments, where the proteins were colocalized (Fig. 7B). Next, we asked whether EIAV Env could degrade the equine tetherin. Using increasing amounts of Vpu or Env expression plasmids, we found that Vpu could specifically degrade huTHN, but not eqTHN, in a dose-dependent manner (Fig. 7C). EIAV Env protein could not degrade huTHN and eqTHN (Fig. 7D), although it was able to bind to eqTHN specifically (Fig. 7A), indicating that a different mechanism may be used by EIAV Env to counteract eqTHN. Taken together, these results revealed that EIAV Env could specifically counteract equine tetherin antiviral activity.
FIG 7.
EIAV Env proteins antagonize the eqTHN antiviral activity by intracellular sequestration of equine tetherin from the plasma membrane. (A) 293T cells were transfected with an EIAV Env-expressing construct (1 μg) and an equine tetherin or human tetherin-GST fusion expression construct (1 μg). Forty-eight hours posttransfection, the cells were lysed and the cell lysates were incubated with glutathione-Sepharose beads. After washing, bead-associated proteins were analyzed by Western blotting using an anti-V5 antibody and anti-EIAV serum. IP, immunoprecipitation. (B) 293T cells were transfected with an eqTHN expression plasmid only or along with an EIAV Env or EIAV Gag Pol expression vector. Forty-eight hours later, the cells were stained with an anti-EIAV antibody (red) and an anti-HA antibody (green), while nuclei were stained with DAPI (blue), and the cells were analyzed by confocal microscopy. Single deconvolved optical sections acquired at the cell-coverslip interface are shown. (C and D) 293T cells were transfected with huTHN (100 ng) or eqTHN (100 ng) in the presence of increasing amounts of HIV-1 Vpu expression vector (0, 0.5, and 1 μg) (C) or EIAV Env expression vector (0, 0.5, and 1 μg) (D). Forty-eight hours posttransfection, the cells were lysed, and the cell lysates were analyzed by Western blotting using an anti-HA antibody and anti-EIAV serum.
DISCUSSION
Retroviruses are subject to a variety of host restrictions, which are created by a number of different cellular restriction factors. These factors inhibit viral replication at different steps of the viral life cycle, which represents a new layer of innate immunity to protect hosts from retroviral infection (36, 37). In the case of HIV-1, viral replication can be inhibited by many cellular proteins, such as APOBEC3, TRIM5α, tetherin, SAMHD1, and MOV10 (36). EIAV is a member of the lentiviruses, with the simplest genome structure within the family. EIAV infection is used as a model for the study of the immunology and pathology of lentiviruses and as an efficient delivery system for gene therapy (38, 39). So far, equine APOBEC is the only equine restriction factor that has been characterized (40, 41). Here, we demonstrate that equine tetherin is expressed in equine macrophages, fibroblasts, and other equine cell types and is able to inhibit the release of EIAV from transfected 293T cells in vitro as potently as human tetherin.
The discovery of tetherin antiviral activity has provided a new strategy for inhibition of enveloped virus replication. This novel antiviral mechanism needs to be further studied at the molecular level. Human tetherin has broad activity against a wide variety of viruses. Our data also showed that equine tetherin inhibits the release of several lentivirus particles from cells, including HIV, SIV, and EIAV. These findings indicated that tetherins derived from different species share similar components to interact with retroviruses. The evidence that tetherin colocalized with viral particles at the plasma membrane also supports this hypothesis. However, it is still unclear how tetherin retains virions on cell membranes.
A number of studies have shown that the unusual molecular topology of tetherin plays an important role in its biological function (23, 42). A dual-tyrosine motif (Y6-Y8) in the cytoplasmic domain of human tetherin is crucial for trafficking between lipid rafts on the plasma membrane and intracellular compartments, including the trans-Golgi Network (TGN), via adaptor complexes involved in clathrin-mediated endocytosis (43–45). We have found that equine tetherin bears a cytoplasmic N-terminal region, a transmembrane region, an ectodomain, and a C-terminal GPI anchor. Interestingly, its cytoplasmic tail lacks 16 amino acids compared with human tetherin, sharing similarity with feline tetherin (14, 16). Equine tetherin carries a shorter N-terminal region but displays strong inhibition of viral particle release. Interestingly, murine tetherin has a single-nucleotide polymorphism that mutated the first ATG, which was expressed at a higher level and showed more potent inhibition of Friend retrovirus release (17). There is also evidence that a shorter isoform of ovine tetherin displays stronger antiviral activity than that found in the longer one (11). Thus, the short forms of tetherins do not have a dual-tyrosine motif in their cytoplasmic domains, so we hypothesize that they may be internalized via a different pathway. It is not clear how these features affect the antiviral activity. It is apparent that the selective pressure from viruses has led to species specificity and diversity in restriction factors. The loss of the N-terminal region of tetherin in some species reflects a coevolution relationship between host and virus, and its significance needs to be further evaluated.
It has been reported that the GPI anchor-mediated localization to lipid rafts promoted clathrin-mediated endocytosis. The GPI anchor is crucial for the antiviral activity of tetherin (1, 46, 47). We also found that removal of the GPI anchor of equine tetherin abolished its antiviral function, while unlike human tetherin, deletion of CT in equine tetherin showed a partial impact on its antiviral activity, indicating a different function of the CT domain. In addition, we checked whether the two sites for posttranslational N-linked glycosylation were important for antiviral activity. In agreement with previous studies (5, 48), we found that introduction of mutations to the N-linked glycosylation sites had no effect on tetherin antiviral activity, although it had been reported that glycosylation of feline tetherin is crucial for tetherin to block virus release (14). Since the localization of glycosylation sites differs between species, whether the localization of glycosylation affects the antiviral function remains unknown.
Many viruses have evolved their own strategies to overcome restriction by tetherin. Among the primate lentiviruses, the Vpu protein of HIV-1 and the envelope glycoprotein of HIV-2 antagonize human tetherin, while most SIVs use Nef to antagonize the tetherin in the nonhuman primate hosts (27, 29, 30, 49, 50). Additional viral antagonists of tetherin include the KSHV K5 protein and the glycoproteins of SIV, FIV, and Ebola virus (2, 4, 16, 31, 32). The activities of these viral proteins as tetherin antagonists are species specific (51, 52). The mechanism by which HIV-1 Vpu counteracts human tetherin has been extensively studied. The major Vpu-responsive site in tetherin was mapped to an STS motif (positions 3 to 5) within the cytoplasmic tail, which is required for Vpu/β-TrCP-dependent ubiquitination (53). The EIAV genome does not carry a Vpu- or Nef-encoding sequence. However, like the HIV-1 Vpu protein, the EIAV S2 protein is also encoded by an open reading frame that overlaps with env, and its translation is also regulated by leaky ribosome scanning (54, 55). Thus, the HIV-1 vpu and EIAV S2 genes have very similar genomic localizations and share very similar expression strategies, indicating that they may have similar functions. Nevertheless, we found that EIAV S2 could not counteract the equine tetherin activity, indicating that EIAV S2 and HIV-1 Vpu may not necessarily have similar functions. The previous study showed that the EIAV S2 mutants replicated similarly to those of the parental virus in equine cell cultures, including macrophages, indicating that the S2 gene is dispensable for viral replication in vitro (56). Interestingly, we found that EIAV envelope protein can rescue EIAV VLP budding from the cell by interacting with equine tetherin, sharing similar phenotypes of interaction between FIV envelope and feline tetherin. Unlike HIV-1 Vpu, the EIAV Env protein could not degrade tetherin. Although EIAV Env, like Ebola virus glycoprotein (57), could bind to tetherin and sequestrate in the intracellular compartment, the details of how envelope proteins antagonize tetherin remain unknown.
In summary, studies on retrovirus restriction by tetherin in horses provide another useful opportunity to further understand the interaction between intrinsic immunity and retroviral infection. We will continue to study the role of tetherin in defending against EIAV infection in vivo and how EIAV Env counteracts this restriction to establish persistent infection.
ACKNOWLEDGMENTS
We thank Toshiaki Kodama for providing the SIVmac239 proviral vector pBR239E, Klaus Strebel (NIH) for providing pcDNA-Vphu, Beth Jamieson and Jerome Zack for providing pNL-r-HSAS, Xiaofang Yu for providing the VR-1012 vector, Bruce Chesebro and Kathy Wehrly for providing HIV-1 p24 monoclonal antibody (183-H12-5C), and Niels Pedersen for providing the SIVmac p27 monoclonal antibody (55-2F12) through the NIH AIDS Reagent Program.
This study was supported by grants from the National Natural Science Foundation of China to Xiaojun Wang (31072113 and 31222054), a grant from the Central Public Interest Scientific Institution Basal Research Fund (2012ZL080), and grants from the State Key Laboratory of Veterinary Biotechnology (SKLVBP201205 and SKLVBP201304).
Footnotes
Published ahead of print 13 November 2013
REFERENCES
- 1.Neil SJ, Zang T, Bieniasz PD. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425–430. 10.1038/nature06553 [DOI] [PubMed] [Google Scholar]
- 2.Pardieu C, Vigan R, Wilson SJ, Calvi A, Zang T, Bieniasz P, Kellam P, Towers GJ, Neil SJ. 2010. The RING-CH ligase K5 antagonizes restriction of KSHV and HIV-1 particle release by mediating ubiquitin-dependent endosomal degradation of tetherin. PLoS Pathog. 6:e1000843. 10.1371/journal.ppat.1000843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jouvenet N, Neil SJ, Zhadina M, Zang T, Kratovac Z, Lee Y, McNatt M, Hatziioannou T, Bieniasz PD. 2009. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 83:1837–1844. 10.1128/JVI.02211-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaletsky RL, Francica JR, Agrawal-Gamse C, Bates P. 2009. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 106:2886–2891. 10.1073/pnas.0811014106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakuma T, Noda T, Urata S, Kawaoka Y, Yasuda J. 2009. Inhibition of Lassa and Marburg virus production by tetherin. J. Virol. 83:2382–2385. 10.1128/JVI.01607-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansouri M, Viswanathan K, Douglas JL, Hines J, Gustin J, Moses AV, Fruh K. 2009. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi's sarcoma-associated herpesvirus. J. Virol. 83:9672–9681. 10.1128/JVI.00597-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radoshitzky SR, Dong L, Chi X, Clester JC, Retterer C, Spurgers K, Kuhn JH, Sandwick S, Ruthel G, Kota K, Boltz D, Warren T, Kranzusch PJ, Whelan SP, Bavari S. 2010. Infectious Lassa virus, but not filoviruses, is restricted by BST-2/tetherin. J. Virol. 84:10569–10580. 10.1128/JVI.00103-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones PH, Mehta HV, Maric M, Roller RJ, Okeoma CM. 2012. Bone marrow stromal cell antigen 2 (BST-2) restricts mouse mammary tumor virus (MMTV) replication in vivo. Retrovirology 9:10. 10.1186/1742-4690-9-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weidner JM, Jiang D, Pan XB, Chang J, Block TM, Guo JT. 2010. Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J. Virol. 84:12646–12657. 10.1128/JVI.01328-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattiuzzo G, Ivol S, Takeuchi Y. 2010. Regulation of porcine endogenous retrovirus release by porcine and human tetherins. J. Virol. 84:2618–2622. 10.1128/JVI.01928-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnaud F, Black SG, Murphy L, Griffiths DJ, Neil SJ, Spencer TE, Palmarini M. 2010. Interplay between ovine bone marrow stromal cell antigen 2/tetherin and endogenous retroviruses. J. Virol. 84:4415–4425. 10.1128/JVI.00029-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liberatore RA, Bieniasz PD. 2011. Tetherin is a key effector of the antiretroviral activity of type I interferon in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 108:18097–18101. 10.1073/pnas.1113694108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goffinet C, Schmidt S, Kern C, Oberbremer L, Keppler OT. 2010. Endogenous CD317/Tetherin limits replication of HIV-1 and murine leukemia virus in rodent cells and is resistant to antagonists from primate viruses. J. Virol. 84:11374–11384. 10.1128/JVI.01067-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuma A, Abe M, Morikawa Y, Miyazawa T, Yasuda J. 2011. Cloning and characterization of the antiviral activity of feline Tetherin/BST-2. PLoS One 6:e18247. 10.1371/journal.pone.0018247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietrich I, McMonagle EL, Petit SJ, Vijayakrishnan S, Logan N, Chan CN, Towers GJ, Hosie MJ, Willett BJ. 2011. Feline tetherin efficiently restricts release of feline immunodeficiency virus but not spreading of infection. J. Virol. 85:5840–5852. 10.1128/JVI.00071-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celestino M, Calistri A, Del Vecchio C, Salata C, Chiuppesi F, Pistello M, Borsetti A, Palu G, Parolin C. 2012. Feline tetherin is characterized by a short N-terminal region and is counteracted by the feline immunodeficiency virus envelope glycoprotein. J. Virol. 86:6688–6700. 10.1128/JVI.07037-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett BS, Smith DS, Li SX, Guo K, Hasenkrug KJ, Santiago ML. 2012. A single nucleotide polymorphism in tetherin promotes retrovirus restriction in vivo. PLoS Pathog. 8:e1002596. 10.1371/journal.ppat.1002596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kupzig S, Korolchuk V, Rollason R, Sugden A, Wilde A, Banting G. 2003. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 4:694–709. 10.1034/j.1600-0854.2003.00129.x [DOI] [PubMed] [Google Scholar]
- 19.Moore RC, Lee IY, Silverman GL, Harrison PM, Strome R, Heinrich C, Karunaratne A, Pasternak SH, Chishti MA, Liang Y, Mastrangelo P, Wang K, Smit AF, Katamine S, Carlson GA, Cohen FE, Prusiner SB, Melton DW, Tremblay P, Hood LE, Westaway D. 1999. Ataxia in prion protein (PrP)-deficient mice is associated with upregulation of the novel PrP-like protein doppel. J. Mol. Biol. 292:797–817. 10.1006/jmbi.1999.3108 [DOI] [PubMed] [Google Scholar]
- 20.Perez-Caballero D, Zang T, Ebrahimi A, McNatt MW, Gregory DA, Johnson MC, Bieniasz PD. 2009. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 139:499–511. 10.1016/j.cell.2009.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishikawa J, Kaisho T, Tomizawa H, Lee BO, Kobune Y, Inazawa J, Oritani K, Itoh M, Ochi T, Ishihara K, Hirano T. 1995. Molecular cloning and chromosomal mapping of a bone marrow stromal cell surface gene, BST2, that may be involved in pre-B-cell growth. Genomics 26:527–534. 10.1016/0888-7543(95)80171-H [DOI] [PubMed] [Google Scholar]
- 22.Vidal-Laliena M, Romero X, March S, Requena V, Petriz J, Engel P. 2005. Characterization of antibodies submitted to the B cell section of the 8th Human Leukocyte Differentiation Antigens Workshop by flow cytometry and immunohistochemistry. Cell. Immunol. 236:6–16. 10.1016/j.cellimm.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 23.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. 2006. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 177:3260–3265 http://www.jimmunol.org/content/177/5/3260 [DOI] [PubMed] [Google Scholar]
- 24.Mangeat B, Gers-Huber G, Lehmann M, Zufferey M, Luban J, Piguet V. 2009. HIV-1 Vpu neutralizes the antiviral factor Tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS Pathog. 5:e1000574. 10.1371/journal.ppat.1000574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyagi E, Andrew AJ, Kao S, Strebel K. 2009. Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc. Natl. Acad. Sci. U. S. A. 106:2868–2873. 10.1073/pnas.0813223106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245–252. 10.1016/j.chom.2008.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rong L, Zhang J, Lu J, Pan Q, Lorgeoux RP, Aloysius C, Guo F, Liu SL, Wainberg MA, Liang C. 2009. The transmembrane domain of BST-2 determines its sensitivity to down-modulation by human immunodeficiency virus type 1 Vpu. J. Virol. 83:7536–7546. 10.1128/JVI.00620-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erikson E, Adam T, Schmidt S, Lehmann-Koch J, Over B, Goffinet C, Harter C, Bekeredjian-Ding I, Sertel S, Lasitschka F, Keppler OT. 2011. In vivo expression profile of the antiviral restriction factor and tumor-targeting antigen CD317/BST-2/HM1.24/tetherin in humans. Proc. Natl. Acad. Sci. U. S. A. 108:13688–13693. 10.1073/pnas.1101684108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Tortorec A, Neil SJ. 2009. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 83:11966–11978. 10.1128/JVI.01515-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hauser H, Lopez LA, Yang SJ, Oldenburg JE, Exline CM, Guatelli JC, Cannon PM. 2010. HIV-1 Vpu and HIV-2 Env counteract BST-2/tetherin by sequestration in a perinuclear compartment. Retrovirology 7:51. 10.1186/1742-4690-7-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta RK, Mlcochova P, Pelchen-Matthews A, Petit SJ, Mattiuzzo G, Pillay D, Takeuchi Y, Marsh M, Towers GJ. 2009. Simian immunodeficiency virus envelope glycoprotein counteracts tetherin/BST-2/CD317 by intracellular sequestration. Proc. Natl. Acad. Sci. U. S. A. 106:20889–20894. 10.1073/pnas.0907075106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez LA, Yang SJ, Hauser H, Exline CM, Haworth KG, Oldenburg J, Cannon PM. 2010. Ebola virus glycoprotein counteracts BST-2/Tetherin restriction in a sequence-independent manner that does not require tetherin surface removal. J. Virol. 84:7243–7255. 10.1128/JVI.02636-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leroux C, Cadore JL, Montelaro RC. 2004. Equine Infectious Anemia Virus (EIAV): what has HIV's country cousin got to tell us? Vet. Res. 35:485–512. 10.1051/vetres:2004020 [DOI] [PubMed] [Google Scholar]
- 34.Leroux C, Craigo JK, Issel CJ, Montelaro RC. 2001. Equine infectious anemia virus genomic evolution in progressor and nonprogressor ponies. J. Virol. 75:4570–4583. 10.1128/JVI.75.10.4570-4583.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammonds J, Wang JJ, Yi H, Spearman P. 2010. Immunoelectron microscopic evidence for Tetherin/BST2 as the physical bridge between HIV-1 virions and the plasma membrane. PLoS Pathog. 6:e1000749. 10.1371/journal.ppat.1000749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng YH, Jeang KT, Tokunaga K. 2012. Host restriction factors in retroviral infection: promises in virus-host interaction. Retrovirology 9:112. 10.1186/1742-4690-9-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malim MH, Bieniasz PD. 2012. HIV restriction factors and mechanisms of evasion. Cold Spring Harb. Perspect. Med. 2:a006940. 10.1101/cshperspect.a006940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitrophanous K, Yoon S, Rohll J, Patil D, Wilkes F, Kim V, Kingsman S, Kingsman A, Mazarakis N. 1999. Stable gene transfer to the nervous system using a non-primate lentiviral vector. Gene Ther. 6:1808–1818. 10.1038/sj.gt.3301023 [DOI] [PubMed] [Google Scholar]
- 39.Balaggan KS, Binley K, Esapa M, Iqball S, Askham Z, Kan O, Tschernutter M, Bainbridge JW, Naylor S, Ali RR. 2006. Stable and efficient intraocular gene transfer using pseudotyped EIAV lentiviral vectors. J. Gene Med. 8:275–285. 10.1002/jgm.845 [DOI] [PubMed] [Google Scholar]
- 40.Zielonka J, Bravo IG, Marino D, Conrad E, Perkovic M, Battenberg M, Cichutek K, Munk C. 2009. Restriction of equine infectious anemia virus by equine APOBEC3 cytidine deaminases. J. Virol. 83:7547–7559. 10.1128/JVI.00015-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bogerd HP, Tallmadge RL, Oaks JL, Carpenter S, Cullen BR. 2008. Equine infectious anemia virus resists the antiretroviral activity of equine APOBEC3 proteins through a packaging-independent mechanism. J. Virol. 82:11889–11901. 10.1128/JVI.01537-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans DT, Serra-Moreno R, Singh RK, Guatelli JC. 2010. BST-2/tetherin: a new component of the innate immune response to enveloped viruses. Trends Microbiol. 18:388–396. 10.1016/j.tim.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masuyama N, Kuronita T, Tanaka R, Muto T, Hirota Y, Takigawa A, Fujita H, Aso Y, Amano J, Tanaka Y. 2009. HM1.24 is internalized from lipid rafts by clathrin-mediated endocytosis through interaction with alpha-adaptin. J. Biol. Chem. 284:15927–15941. 10.1074/jbc.M109.005124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rollason R, Korolchuk V, Hamilton C, Schu P, Banting G. 2007. Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif. J. Cell Sci. 120:3850–3858. 10.1242/jcs.003343 [DOI] [PubMed] [Google Scholar]
- 45.Boucrot E, Saffarian S, Zhang R, Kirchhausen T. 2010. Roles of AP-2 in clathrin-mediated endocytosis. PLoS One 5:e10597. 10.1371/journal.pone.0010597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dube M, Roy BB, Guiot-Guillain P, Binette J, Mercier J, Chiasson A, Cohen EA. 2010. Antagonism of tetherin restriction of HIV-1 release by Vpu involves binding and sequestration of the restriction factor in a perinuclear compartment. PLoS Pathog. 6:e1000856. 10.1371/journal.ppat.1000856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Habermann A, Krijnse-Locker J, Oberwinkler H, Eckhardt M, Homann S, Andrew A, Strebel K, Krausslich HG. 2010. CD317/tetherin is enriched in the HIV-1 envelope and downregulated from the plasma membrane upon virus infection. J. Virol. 84:4646–4658. 10.1128/JVI.02421-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrew AJ, Miyagi E, Kao S, Strebel K. 2009. The formation of cysteine-linked dimers of BST-2/tetherin is important for inhibition of HIV-1 virus release but not for sensitivity to Vpu. Retrovirology 6:80. 10.1186/1742-4690-6-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neil SJ, Sandrin V, Sundquist WI, Bieniasz PD. 2007. An interferon-alpha-induced tethering mechanism inhibits HIV-1 and Ebola virus particle release but is counteracted by the HIV-1 Vpu protein. Cell Host Microbe 2:193–203. 10.1016/j.chom.2007.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jia B, Serra-Moreno R, Neidermyer W, Rahmberg A, Mackey J, Fofana IB, Johnson WE, Westmoreland S, Evans DT. 2009. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 5:e1000429. 10.1371/journal.ppat.1000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McNatt MW, Zang T, Hatziioannou T, Bartlett M, Fofana IB, Johnson WE, Neil SJ, Bieniasz PD. 2009. Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog. 5:e1000300. 10.1371/journal.ppat.1000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goffinet C, Allespach I, Homann S, Tervo HM, Habermann A, Rupp D, Oberbremer L, Kern C, Tibroni N, Welsch S, Krijnse-Locker J, Banting G, Krausslich HG, Fackler OT, Keppler OT. 2009. HIV-1 antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host Microbe 5:285–297. 10.1016/j.chom.2009.01.009 [DOI] [PubMed] [Google Scholar]
- 53.Tokarev AA, Munguia J, Guatelli JC. 2011. Serine-threonine ubiquitination mediates downregulation of BST-2/tetherin and relief of restricted virion release by HIV-1 Vpu. J. Virol. 85:51–63. 10.1128/JVI.01795-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fagerness AJ, Flaherty MT, Perry ST, Jia B, Payne SL, Fuller FJ. 2006. The S2 accessory gene of equine infectious anemia virus is essential for expression of disease in ponies. Virology 349:22–30. 10.1016/j.virol.2005.12.041 [DOI] [PubMed] [Google Scholar]
- 55.Li F, Leroux C, Craigo JK, Cook SJ, Issel CJ, Montelaro RC. 2000. The S2 gene of equine infectious anemia virus is a highly conserved determinant of viral replication and virulence properties in experimentally infected ponies. J. Virol. 74:573–579. 10.1128/JVI.74.1.573-579.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li F, Puffer BA, Montelaro RC. 1998. The S2 gene of equine infectious anemia virus is dispensable for viral replication in vitro. J. Virol. 72:8344–8348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuhl A, Banning C, Marzi A, Votteler J, Steffen I, Bertram S, Glowacka I, Konrad A, Sturzl M, Guo JT, Schubert U, Feldmann H, Behrens G, Schindler M, Pohlmann S. 2011. The Ebola virus glycoprotein and HIV-1 Vpu employ different strategies to counteract the antiviral factor tetherin. J. Infect. Dis. 204(Suppl 3):S850–S860. 10.1093/infdis/jir378 [DOI] [PMC free article] [PubMed] [Google Scholar]