Abstract
Atypical and classical scrapie-infected sheep brain tissue was monolaterally injected into the tonsils of lambs to investigate their role as a prion entry point. We first detected classical PrPSc within the inoculated tonsil and in the ipsilateral retropharyngeal lymph node at 3 months postinoculation (p.i.). At 7 months p.i., PrPSc colonized other lymphoid tissues bilaterally, including ileal Peyer's patches. The earliest PrPSc deposition within the brain was ipsilaterally observed at 9 months p.i. in the substantia reticularis of the medulla oblongata. At 12 months p.i., PrPSc deposition was present bilaterally in the nucleus parasympathicus nervi vagi, as well as in the intermediolateral cell column of the thoracolumbar spinal cord. No PrPSc was detected in the lambs inoculated with atypical scrapie. These findings suggest that neuroinvasion may naturally occur from the tonsil after a widespread prion replication within the lymphoid tissues during classical scrapie only, thus mimicking the pathogenesis after oral ingestion.
INTRODUCTION
Classical and atypical sheep and goat scrapie are two distinct transmissible spongiform encephalopathies (TSEs) caused by an unconventional agent (prion) corresponding largely, if not entirely, to the disease-associated isoform (PrPSc) of the normal host-encoded cellular prion protein (PrPC), which accumulates in the brain of sick animals (1).
An increasing body of experimental evidence in mouse models and field observations in sheep suggests that the classical scrapie agent gains entry into the host's body through the gut's ileal mucosa, with the earliest PrPSc deposition occurring in Peyer's patches (PPs) and then in other lymphoreticular system (LRS) districts, including palatine tonsils (PTs) (2–5). From this initial site of replication, prions gain access to the central nervous system (CNS) through the sympathetic and parasympathetic fibers of the autonomic nervous system, with the nucleus parasympathicus nervi vagi (NPNV) and the intermediolateral (IML) cell column of the thoracic spinal cord representing the first PrPSc deposition sites within the CNS (2, 6–8).
Notwithstanding this, PrPSc from atypical scrapie cases is not detectable within LRS tissues and is also immunobiochemically different from that found in classical scrapie (9). Furthermore, much less information is available on the pathogenesis of atypical scrapie, and whether it is a spontaneous rather than acquired or contagious disease remains unclear.
Nevertheless, oral transmission of atypical scrapie has been recently achieved in AHQ/AHQ sheep (10). In that study, although no detectable PrPSc was found outside the brain during the preclinical and the clinical stages of the disease, prion infectivity was found in the distal ileum. Another interesting work, albeit not showing immunohistochemical evidence of PrPSc outside the CNS, demonstrated that LRS tissues from atypical scrapie-affected sheep can harbor prion infectivity (11). These findings indicate in some way that LRS involvement may also occur in atypical scrapie, thereby suggesting that it could be a contagious pathological entity.
Studies in rodents have also shown that neuroinvasion may occur from the oral and nasal cavities through the loco-regional nervous fibers, with or without regional LRS involvement (12, 13), with susceptibility being enhanced by small lesions on the epithelial surface of the tongue (14). Whether a similar pathogenic behavior may also characterize classical and atypical scrapie under experimental or natural conditions is a matter of speculation, although the documented presence of PrPSc and/or infectivity in the retina, tongue, and nasal mucosa during scrapie and BSE infection (15, 16) would suggest that these highly innervated head districts could represent potential prion entry sites from which neuroinvasion may occur directly through the loco-regional nerve fibers, or after prion replication in the LRS. Furthermore, ruminant PTs, which are not present in rodents (17), exhibit a specialized epithelium, along with B and T lymphocytes, as well as follicular dendritic cells, which are organized into well-defined secondary follicles, thus likely creating a suitable microenvironment for the replication and the subsequent spread of prions, including the sheep scrapie agent (18). With this in mind, the present study had the main objective of defining the role of PTs in prion neuroinvasion from the oral cavity in classical and atypical scrapie.
MATERIALS AND METHODS
Experimental scrapie infection design.
Sarda breed lambs were acquired from historically scrapie-free flocks in Sardinia and sorted by the prion genotype into two groups, one including 24 ARQ/ARQ animals and the other including 6 ARQ/AHQ animals. PrP genotyping was carried out by sequencing of PCR-amplified products (19). No mutation at codon 141 or in other codons were found in any of the sheep under investigation. In the Sarda breed, scrapie is consistently observed only in sheep carrying the ARQ/ARQ genotype (19), whereas sheep with the ARQ/AHQ genotype are more at risk for atypical scrapie (10).
To exclude potential environmental scrapie sources, lambs were housed inside a newly constructed sheep barn. The lambs were kept with their dams and separated from each other until they were weaned at 25 days of age; after weaning, they were housed in groups according to genotype.
At 25 to 30 days of age, both ARQ/ARQ and ARQ/AHQ animals were infected by the intratonsillar route with classical or atypical scrapie-infected brain tissue, respectively. The genotype of the scrapie brain homogenate was identical to that of the inoculated lambs. Three ARQ/ARQ lambs were euthanized at 7 days postinoculation (p.i.), followed by three additional lambs, at 1, 3, 5, 7, 9, and 12 months p.i., respectively. The remaining three lambs were euthanized at the clinical onset of scrapie. The six ARQ/AHQ lambs inoculated with atypical scrapie were euthanized at 17 and 27 months p.i., three at a time.
Inoculum preparation.
One homogenate for inoculum was prepared from a pool of brains of scrapie-affected ARQ/ARQ sheep collected in the framework of the passive surveillance plan and identified as classical scrapie by the Italian National Reference Laboratory for TSE strain typing. A second homogenate was prepared from a pool of brains of AR(H)Q/AHQ sheep collected within the framework of the European active surveillance plan (fallen stock) and identified as atypical scrapie-affected by the aforementioned laboratory.
Experimental scrapie infection procedure.
Before the inoculation procedure, lambs were starved during the 24 h preceding the surgery. They were sedated using atropine sulfate (0.04 mg/kg, administered intramuscularly [i.m.]), xylazine hydrochloride (Rompun; 0.22 mg/kg i.m.), and acepromazine maleate (Prequillan; 0.07 mg/kg i.m.). After recumbence and venous catheterization, induction and deepening of anesthesia were achieved via a rapid injection of thiopental sodium (Pentothal sodium; 10 mg/kg, administered intravenously). Finally, 50 μl of the 25% classical or atypical scrapie-infected sheep brain homogenate was slowly injected only into the right PT crypts with a microsyringe after opening the mouth and illuminating the PTs by means of a straight-blade laryngoscope.
As controls, five genetically resistant (ARR/ARR) lambs were inoculated using the same surgical procedure, while an additional two ARQ/ARQ lambs were mock inoculated with a healthy sheep brain homogenate.
Sampling.
During postmortem examination, the brain and spinal cord, together with the ganglia innervating the ovine PTs (20), namely, the cervical cranial ganglia (CCG), proximal glossopharyngeal ganglia (PGG), proximal vagal ganglia (PVG), distal vagal ganglia (DVG), trigeminal ganglia (TG), and cervicothoracic ganglia (CTG), were adequately sampled. The gut-associated lymphoid tissue, including PTs, along with several lymph nodes, were also promptly collected (Tables 1, 2, and 3). All of these samples were fixed in buffered formaldehyde solution (4% [wt/vol]) or in part stored at −20°C when the size of the organ was adequate.
TABLE 1.
Time-dependent progression of experimental scrapie infection after intratonsillar inoculation of a scrapie-brain homogenate, by immunohistochemical PrPSc detection in LRS tissues from the heads of lambs under study at different days and months p.i.a
| Organ (body side)b | PrPSc-positive sheep/PrPSc-negative sheep at various times p.i. |
||||||
|---|---|---|---|---|---|---|---|
| 7 days | 1 mo | 3 mo | 5 mo | 7 mo | 9 mo | 12 mo | |
| Palatine tonsil (IPL) | 0/3 | 0/3 | 2 (++)/1 | 2 (+)/1 | 2 (++)/1 | 3 (+++)/0 | 3 (+++)/0 |
| Palatine tonsil (COL) | 0/3 | 0/3 | 0/3 | 1 (+)/2 | 2 (++)/1 | 3 (+++)/0 | 3 (+++)/0 |
| RPLN (IPL) | 0/3 | 0/3 | 3 (+++)/0 | 3 (+++)/0 | 2 (+++)/1 | 3 (+++)/0 | 3 (+++)/0 |
| RPLN (COL) | 0/3 | 0/3 | 0/3 | 2 (+)/1 | 2 (+++)/1 | 3 (+++)/0 | 3 (+++)/0 |
| Third eyelid (IPL) | NA | NA | NA | NA | 2 (+)/1 | 2 (++)/1 | 3 (++)/0 |
| Third eyelid (COL) | NA | NA | NA | NA | 0/3 | 3 (++)/0 | NA |
| SML (IPL) | NA | NA | NA | NA | 0/3 | 3 (++)/0 | 3 (++)/0 |
| SML (COL) | NA | NA | NA | NA | 0/3 | 3 (++)/0 | 3 (++)/0 |
All animals were clinically healthy. The intensity of PrPSc immunolabeling is indicated as 1 (+), 2 (++), and 3 (+++). NA, not available.
IPL, ipsilateral; COL, contralateral; RPLN, retropharyngeal lymph node; SML, submandibular lymph node.
TABLE 2.
Time-dependent progression of experimental scrapie infection after intratonsillar inoculation of a scrapie-brain homogenate, by immunohistochemical PrPSc detection in LRS tissues from the lambs under study at different days and months p.i.a
| Organ (body side)b | PrPSc-positive sheep/PrPSc-negative sheep at various times p.i. |
||||||
|---|---|---|---|---|---|---|---|
| 7 days | 1 mo | 3 mo | 5 mo | 7 mo | 9 mo | 12 mo | |
| Cranial cervical LN (IPL) | 0/3 | 0/3 | 0/3 | 0/3 | 2 (+++)/1 | 3 (+++)/0 | 3 (+++)/0 |
| Cranial cervical LN (COL) | 0/3 | 0/3 | 0/3 | 0/3 | 2 (+)/1 | 3 (+++)/0 | 3 (++)/0 |
| Mediastinal LN | 0/3 | 0/3 | 0/3 | 0/3 | 2 (++)/1 | 3 (+++)/0 | 3 (++)/0 |
| Prescapular LN (IPL) | 0/3 | 0/3 | 0/3 | 0/3 | 2 (+)/1 | 3 (+++)/0 | 3 (++)/0 |
| Prescapular LN (COL) | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 3 (+++)/0 | 3 (++)/0 |
| Spleen | 0/3 | 0/3 | 0/3 | 0/3 | 2 (+)/1 | 3 (++)/0 | 3 (++)/0 |
| Peyer's patches | 0/3 | 0/3 | 0/3 | 0/3 | 2 (+)/1 | 3 (++)/0 | 3 (++)/0 |
| Mesenteric LN | 0/3 | 0/3 | NA | NA | 2 (+)/1 | 3 (+++)/0 | 3 (+++)/0 |
| Popliteal LN (IPL) | NA | NA | NA | NA | 0/3 | 3 (+)/0 | 3 (++)/0 |
| Popliteal LN (COL) | NA | NA | NA | NA | 0/3 | 3 (++)/0 | 3 (++)/0 |
All animals were clinically healthy. The intensity of PrPSc immunolabeling is indicated as 1 (+), 2 (++), and 3 (+++). NA, not available.
LN, lymph node; IPL, ipsilateral; COL, contralateral.
TABLE 3.
Time-dependent progression of experimental scrapie infection after intratonsillar inoculation of a scrapie-brain homogenate, by immunohistochemical PrPSc detection in different nervous tissues from the lambs under study at different days and months p.i.a
| Organ | PrPSc-positive sheep/PrPSc-negative sheep at various times p.i.b |
||||||
|---|---|---|---|---|---|---|---|
| 7 days | 1 mo | 3 mo | 5 mo | 7 mo | 9 mo | 12 mo | |
| Obex region | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 2*/1 | 3†/0 |
| Rostral medulla | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 3‡/0 |
| Cerebellum | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| Midbrain | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 3‡/0 |
| Thalamus | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| Spinal cord | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 2/1 |
| Peripheral nervous system | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
All animals were clinically healthy.
*, All were PrPSc positive monolaterally only in the substantia reticularis; †, PrPSc was observed bilaterally in the nucleus parasympathicus nervi vagi and substantia reticularis; ‡, PrPSc deposition was observed bilaterally in the substantia reticularis.
Histopathology and PrPSc immunohistochemistry (IHC).
A histologic brain examination was performed for the detection of the characteristic spongiform lesions. Paraffin-embedded lymphoid and nervous tissue sections (5 μm thick) were stained with hematoxylin and eosin and subsequently examined under a light microscope.
For PrPSc IHC, 4 μm-thick, paraffin-embedded lymphoid and nervous tissue sections were autoclaved at 121°C for 20 min in a solution of 0.01 M citric acid (pH 6.1) and then immersed in a solution of 0.3% H2O2 for 10 min at room temperature. Further steps included utilization of a biotin-streptavidin detection method (Vector Laboratories, Inc., USA), using F99 (VMRD, Inc., USA) as a primary anti-PrP monoclonal antibody.
Immune reactions were visualized by 3-3′-diaminobenzidine (DAB) chromogen solution (Dako, Denmark). Appropriate negative controls were set up by omitting the primary monoclonal antibody.
Phenotype of PrPSc deposition in the brain.
In order to define the phenotype of PrPSc deposition in the brain of the inoculated animals, the whole brains from the three ARQ/ARQ sheep with clinical signs (see results) were collected at postmortem examination. The phenotypes of these sheep were compared to those of five naturally scrapie-affected sheep at the terminal disease stage. These sheep carried the ARQ/ARQ genotype at the PrP gene and were collected from different flocks. Each sheep's brain was sagitally divided into two halves; one half was fixed in formaldehyde for IHC investigations, while the other was frozen at −20°C for immunobiochemical analysis. From the formalin-fixed portion, seven distinct coronal sections were cut, representative of the frontal cerebral cortex, basal nuclei, thalamus, midbrain, cerebellum, pons, and medulla oblongata (obex). PrPSc deposition was scored to create a profile by using a method described elsewhere (21).
In short, the magnitude of PrPSc accumulation was scored from 0 to 3 among eight different patterns of PrPSc accumulation, which were defined as follows: granular (coarse and punctate), intraneuronal, glial, linear, perivascular, coalescing, subependymal, and subpial. The profiles of PrPSc values represented the respective mean scores of the sheep group inoculated by the intratonsillar route, as well as of the naturally scrapie-affected group.
PrPSc WB analysis.
Western blot (WB) analysis for PrPSc detection in nervous and LRS tissues was carried out with a modified Prionics check test (Prionics AG, Switzerland), in accordance with the protocol described elsewhere (22).
Ethics statement.
The experimental procedures involving the sheep investigated here were officially approved by the Ethics Committee of the Istituto Zooprofilattico Sperimentale of Sardinia (permission 207/2012), in strict agreement with the guidelines of Italian National Law 116/1992.
RESULTS
No evidence of PrPSc immunolabeling was found in lymphoid and nervous tissues from the lambs euthanized at 7 days and 1 month p.i. This was also true for the injected PTs, which appeared to be histologically normal.
In two of the three lambs euthanized at 3 months p.i., we detected PrPSc within the inoculated PT and in the ipsilateral retropharyngeal lymph node (RPLN), while in the remaining one, PrPSc was observed in the ipsilateral RPLN but not in the inoculated PT (Fig. 1 and Table 1). At 5 months p.i., PrPSc was detected in the PT and RPLN, including the contralateral ones.
FIG 1.
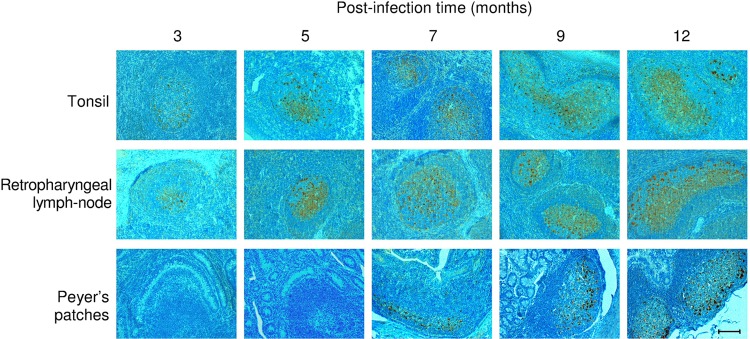
Representative immunohistochemical patterns of PrPSc deposition in the tonsil, retropharyngeal lymph node and ileal Peyer's patches (PPs) of lambs serially sacrificed at different times postinfection. The earliest PrPSc deposition was observed at 3 months p.i., while PPs displayed PrPSc at 7 months p.i. Magnitude of PrPSc deposition increased, along with the incubation time. Scale bar, 100 μm.
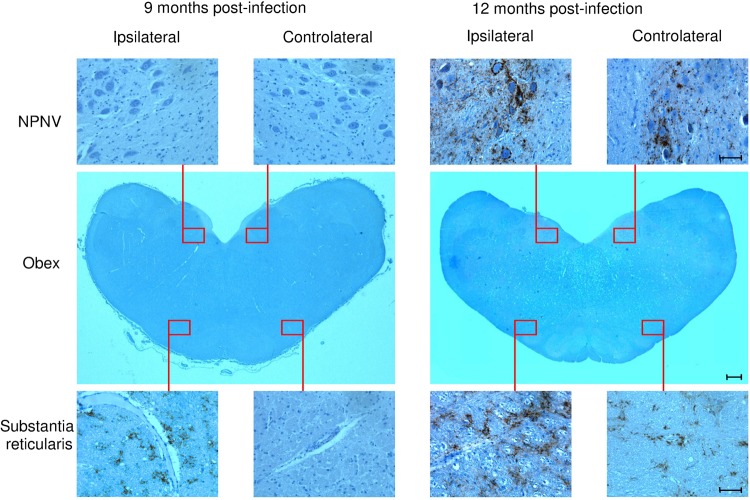
Subsequently, at 7 months p.i., PrPSc deposition bilaterally involved other LRS districts, including ileal PPs (Fig. 1, Table 2). The earliest PrPSc deposition within the brain was observed at 9 months p.i. in two of three lambs. In these cases, PrPSc immunostaining was apparent only ipsilaterally to the inoculated PT, with a focal glial pattern in proximity to vessels, and exclusively in the substantia reticularis of the obex region (Fig. 2, Table 3). At this time p.i., no PrPSc immunolabeling could be detected throughout the spinal cord; on the contrary, a widespread PrPSc deposition occurred within LRS tissues (Fig. 1, Table 2). At 12 months p.i., granular punctuate and perineuronal PrPSc aggregates were bilaterally and symmetrically observed by IHC along the ventral border of the NPNV (Fig. 2). In addition, PrPSc was bilaterally evident in the substantia reticularis, at the level of the medulla oblongata (Fig. 2) and of the middle brain, as well as in the IML cell column of the thoracolumbar segments (T10-L2) of the spinal cord (Table 3). Interestingly, the amount of PrPSc in the substantia reticularis was noticeably greater in the side ipsilateral-to-inoculated PT than in the other one (Fig. 2).
FIG 2.
Representative immunohistochemical patterns of PrPSc deposition within the brain at the obex level of preclinically scrapie affected sheep following intratonsillar inoculation. At 9 months p.i., the earliest PrPSc deposition was observed in the obex as glial-perivascular patterns in the substantia reticularis ipsilaterally to the inoculum side. The corresponding symmetrically contralateral area, as well as both the ipsilateral and contralateral nucleus parasympathicus nervi vagi (NPNV), were negative for PrPSc. At 12 months postinfection, a PrPSc deposition was found bilaterally in the obex at the level of both the NPNV and the substantia reticularis. Micrographs are higher magnifications of the red line-enclosed area of the transversal section of the obex (macrophotograph). Scale bars, 1,000 μm (macrophotograph) and 100 μm (micrographs).
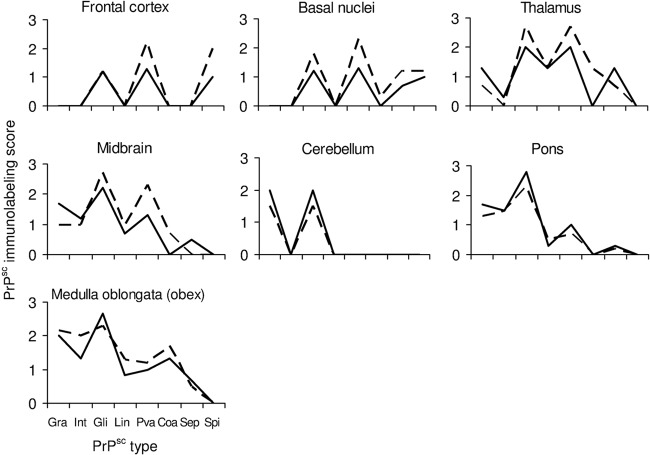
Abundant PrPSc aggregates were diffusely observed in a range of LRS districts (Fig. 1, Tables 1 and 2). The remaining three lambs developed clear neurological signs of scrapie at 20, 22, and 24 months p.i., respectively. In these animals, the histological brain lesions and PrPSc IHC deposition profiles were comparable to those seen in natural scrapie cases (Fig. 3). WB examinations confirmed the results obtained by IHC (data not shown).
FIG 3.
Distribution and magnitude of the different PrPSc immunolabeling patterns in clinically scrapie-affected sheep after intratonsillar inoculation (solid line) or natural infection (dashed line). Note the similarity of the profiles, despite slight differences in magnitude. Gra, granular (coarse and punctate); Int, intraneuronal; Gli, glial; Lin, linear; Pva, perivascular; Coa, coalescing; Sep, subependymal; Spi, subpial.
No evidence of PrPSc deposition was found in the sympathetic and parasympathetic ganglia connected to PTs in all of the sheep examined before the onset of clinical scrapie. On the contrary, scanty PrPSc deposits were detected in the cervical cranial ganglia (CCG) of the three sheep showing clinical signs of scrapie. As far as concerns the lambs carrying the ARQ/AHQ genotype and inoculated with atypical scrapie-infected brain tissue, we observed no detectable PrPSc in the animals euthanized at 17 and 27 months p.i. Finally, none of the sheep either carrying the resistant ARR/ARR genotype or inoculated with healthy ovine brain homogenates showed any evidence of PrPSc deposition in either LRS or nervous tissues.
DISCUSSION
Previous studies using murine models have shown that different prion strains can directly invade the brain from the buccal cavity through the trigeminal nerve after inoculation into the tooth pulp (23) or by trafficking along lingual and facial nerves subsequent to topical application onto the lingual mucosa (14). In such studies, neuroinvasion occurred independently from LRS involvement, even when the lymphotropic chronic wasting disease strain was used (14). Neuroinvasion from the tongue can also be independent from LRS involvement and is partially dependent on the prion strain and/or the host species (13). Conversely, after inoculation of the transmissible mink encephalopathy agent into the tongue, it has been shown (12) that PrPSc deposition first occurs in the locoregional lymph nodes before prion neuroinvasion via the hypoglossal nerve and that this entry route is more efficient than oral ingestion. Taken together, these data from experimentally infected mice suggest that the oral cavity may serve as an efficient route for prion entry from which neuroinvasion may directly occur through the regional neural network, with LRS being inconstantly involved at a locoregional level.
In contrast to the above findings, our study of experimentally challenged ovines shows that a consistent prion (PrPSc) replication occurs widely in the LRS tissues of the body before the neuroinvasion process develops. This suggests that scrapie prion neuroinvasion after intratonsillar infection is preceded by the agent's replication in the LRS, as reported in the course of oral scrapie infection (2, 5). Our results, markedly diverging from those obtained in mice (12–14), could be explained by the fact that sheep, unlike mice, possess well-developed PTs which likely support early prion access to the remaining LRS body sites, including ileal PPs.
Indeed, due to their location, PTs play a key role in immunity as the site where vast amounts of antigens enter the body during feeding (17). In this regard, the high efficiency and effectiveness of the low infectivity titers of the scrapie brain homogenate utilized in our study underscore the role likely played by PTs as the entry and first replication site of the scrapie agent; this is also expected to hold true under natural conditions. In addition, foreign body ingestion may cause integrity loss of the mucosal barrier in ruminants, thus exposing PTs exposure to minute prion infectivity amounts from the environment and facilitating prion infection acquirement (14).
In our experiment, one of the lamb showed, at the early stage postinfection, PrPSc accumulation in the RPLN but not in the PT. This suggests that PTs may represent mostly an entry site from which the scrapie prion traffics to the RPLN, which is the putative lymphoid district draining the PTs (24), where the earliest immunohistochemically detectable PrPSc replication occurs. As a proof of the concept, we did not find any evidence of PrPSc deposition in the PT after the first week p.i. Interestingly, RPLNs were found to be the earliest site of PrPSc accumulation after oral experimental chronic wasting disease infection in deer (25).
In our study, the neuroinvasion process during classical scrapie was characterized, at 9 months p.i., by an early glial, ipsilateral-to-inoculated-PT, PrPSc deposition within the substantia reticularis of the obex, thereby suggesting that the sheep scrapie prion may gain its first CNS access exclusively by the neural network innervating the inoculated PT and RPLN in which evidence of PrPSc deposition had been previously found at 3 months. In this respect, since no detectable PrPSc occurred in the CCG, PGG, PVG, DVG, TG, and CTG ganglia, we could not identify the precise nervous pathway followed by the scrapie agent to reach the substantia reticularis. Nevertheless, we speculate that the vagal afferent fibers could serve as the prion entry route. In fact, sensory vagal terminations scattered in the substantia reticularis between the NPNV and the nucleus ambiguus in the ventrolateral medulla were observed after injection of CTB-HRP tracer into the DVG (also termed nodose ganglia) of lambs (26). Furthermore, vagal efferent neurons are located not only in the NPNV but also in the reticular formation of the ovine obex (27). Our classical scrapie-infected sheep exhibited a PrPSc deposition phenotype associated with brain astrocytes, a finding which is not surprising since it has been previously observed as an early IHC deposition pattern in scrapie-infected mouse (28) and also frequently seen in the substantia reticularis in natural cases of scrapie (29). Similarly to what has been reported in previous experimental and natural classical scrapie studies, after PrPSc colonization of ileal PPs we found evidence of bilateral PrPSc deposition in the NPNV and IML cell column of the thoracolumbar spinal cord (2, 6). This strongly supports the involvement of the parasympathetic vagal and splanchnic nerves in prion neuroinvasion even in our experimental model. Consequently, ileal PPs, along with vagal and splanchnic nerves, seem to represent the favorite and early pathway for classical scrapie agent's neuroinvasion, independently from its entry site into the host's body.
The successful transmission of atypical scrapie by oral ingestion (10) and the unexpected finding of prion infectivity in peripheral tissues, including LRS districts (11), have suggested in some way that classical and atypical scrapie share common biopathogenic disease patterns. However, our study highlights that neuroinvasion may occur from the PT only during classical scrapie, followed by a widespread LRS involvement, thus mimicking the pathogenesis of natural oral infection.
Interestingly, the similar PrPSc deposition pattern observed in the brains of sheep inoculated by the intratonsillar route and of naturally scrapie-affected sheep, along with the similar incubation time observed by us after oral inoculation of ARQ/ARQ Sarda sheep (30), suggest that the natural and the experimental intratonsillar infections are de facto indistinguishable at the terminal stage.
Notably, by applying conventional methods, we found no evidence of PrPSc deposition in either the LRS or nervous tissues from all of the atypical scrapie-inoculated sheep which were euthanized after 17 and 27 months p.i. In this respect, it should also be emphasized that we used lambs younger than 3 months, similarly to what was done in a successful oral transmission study (10), although the lack of F141 mutation of the PrP gene makes these animals less susceptible to atypical scrapie. To confirm these findings, we also inoculated transgenic mice with lymphoid and nervous tissues from the atypical scrapie-inoculated sheep. The experiment is still under way, and the data will not be available for another 12 months. Nevertheless, the present investigation's results do not argue in favor of an efficient transmissibility and self-propagation of the atypical scrapie agent in nature.
ACKNOWLEDGMENTS
We thank Cinzia Santucciu, Davide Pintus, and Claudia Contu for technical assistance. We also thank Romolo Nonno for carrying out the mouse bioassay.
This study was supported by the EMIDA project and grant IZS SA 01/11 from the Italian Ministero della Salute.
Footnotes
Published ahead of print 6 November 2013
REFERENCES
- 1.Prusiner SB. 1998. Prions. Proc. Natl. Acad. Sci. U. S. A. 95:13363–13383. 10.1073/pnas.95.23.13363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andréoletti O, Berthon P, Marc D, Sarradin P, Grosclaude J, van Keulen L, Schelcher F, Elsen JM, Lantier F. 2000. Early accumulation of PrPSc in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J. Gen. Virol. 81:3115–3126 [DOI] [PubMed] [Google Scholar]
- 3.McCulloch L, Brown KL, Bradford BM, Hopkins J, Bailey M, Rajewsky K, Manson JC, Mabbott NA. 2011. Follicular dendritic cell-specific prion protein (PrPSc) expression alone is sufficient to sustain prion infection in the spleen. PLoS Pathog. 7:e1002402. 10.1371/journal.ppat.1002402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kujala P, Raymond CR, Romeijn M, Godsave SF, van Kasteren SI, Wille H, Prusiner SB, Mabbott NA, Peters PJ. 2011. Prion uptake in the gut: identification of the first uptake and replication sites. PLoS Pathog. 7:e1002449. 10.1371/journal.ppat.1002449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Keulen LJ, Schreuder BE, Meloen RH, Mooij-Harkes G, Vromans ME, Langeveld JP. 1996. Immunohistochemical detection of prion protein in lymphoid tissues of sheep with natural scrapie. J. Clin. Microbiol. 34:1228–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Keulen LJ, Schreuder BE, Vromans ME, Langeveld JP, Smits MA. 2000. Pathogenesis of natural scrapie in sheep. Arch. Virol. Suppl. 16:57–71 [DOI] [PubMed] [Google Scholar]
- 7.Beekes M, McBride PA, Baldauf E. 1998. Cerebral targeting indicates vagal spread of infection in hamster fed with scrapie. J. Gen. Virol. 79:601–607 [DOI] [PubMed] [Google Scholar]
- 8.McBride PA, Schulz-Schaeffer WJ, Donaldson M, Bruce M, Diringer H, Kretzschmar HA, Beekes M. 2001. Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J. Virol. 75:9320–9327. 10.1128/JVI.75.19.9320-9327.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Dur A, Béringue V, Andréoletti O, Reine F, Laï TL, Baron T, Bratberg B, Vilotte JL, Sarradin P, Benestad SL, Laude HA. 2005. Newly identified type of scrapie agent can naturally infect sheep with resistant PrP genotype. Proc. Natl. Acad. Sci. U. S. A. 102:16031–16036. 10.1073/pnas.0502296102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmons MM, Moore SJ, Konold T, Thurston L, Terry LA, Thorne L, Lockey R, Vickery C, Hawkins SA, Chaplin MJ, Spiropoulos J. 2011. Experimental oral transmission of atypical scrapie to sheep. Emerg. Infect. Dis. 17:848–854. 10.3201/eid1705.101654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andréoletti O, Orge L, Benestad SL, Beringue V, Litaise C, Simon S, Le Dur A, Laude H, Simmons H, Lugan S, Corbière F, Costes P, Morel N, Schelcher F, Lacroux C. 2011. Atypical/Nor98 scrapie infectivity in sheep peripheral tissues. PLoS Pathog. 7:e1001285. 10.1371/journal.ppat.1001285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartz JC, Kincaid AE, Bessen RA. 2003. Rapid prion neuroinvasion following tongue infection. J. Virol. 77:583–591. 10.1128/JVI.77.1.583-591.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bessen RA, Martinka S, Kelly J, Gonzalez D. 2009. Role of the Lymphoreticular System in Prion Neuroinvasion from the oral and nasal mucosa. J. Virol. 83:6435–6445. 10.1128/JVI.00018-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denkers ND, Telling GC, Hoover EA. 2011. Minor oral lesions facilitate transmission of Chronic Wasting Disease. J. Virol. 85:1396–1399. 10.1128/JVI.01655-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casalone C, Corona C, Crescio MI, Martucci F, Mazza M, Ru G, Bozzetta E, Acutis PL, Caramelli M. 2005. Pathological prion protein in the tongues of sheep infected with naturally occurring scrapie. J. Virol. 79:5847–5849. 10.1128/JVI.79.9.5847-5849.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corona C, Porcario C, Martucci F, Iulini B, Manea B, Gallo M, Palmitessa C, Maurella C, Mazza M, Pezzolato M, Acutis P, Casalone C. 2009. Olfactory system involvement in natural scrapie disease. J. Virol. 83:3657–3667. 10.1128/JVI.01966-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casteleyn C, Breugelma S, Simoens P, van Broeck W. 2011. The tonsils revisited: review of the anatomical localization and histological characteristics of the tonsils of domestic and laboratory animals. Clin. Dev. Immunol. 2011:ID 472460. 10.1155/2011/472460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breugelmans S, De Spiegelaere W, Casteleyn C, Simoens P, Van den Broeck W. 2011. Differences between the ovine tonsils based on an immunohistochemical quantification of the lymphocyte subpopulations. CIMID 34:217–225. 10.1016/j.cimid.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 19.Maestrale C, Carta A, Attene S, Galistu A, Santucciu C, Cancedda MG, Saba M, Sechi S, Patta C, Bandino E, Ligios C. 2009. p.Asn176Lys and p.Met137Thr dimorphisms of the PRNP gene significantly decrease the susceptibility to classical scrapie in ARQ/ARQ sheep. Anim. Genet. 40:982–985. 10.1111/j.1365-2052.2009.01943.x [DOI] [PubMed] [Google Scholar]
- 20.Russo D, Fantaguzzi CM, Di Guardo G, Clavenzani P, Costerbosa GL, Ligios C, Chiocchetti R. 2009. Characterization of sheep (Ovis aries) palatine tonsil innervations. Neuroscience 161:813–826. 10.1016/j.neuroscience.2009.04.015 [DOI] [PubMed] [Google Scholar]
- 21.González L, Martin S, Houston FE, Hunter N, Reid HW, Bellworthy SJ, Jeffrey M. 2005. Phenotype of disease-associated PrP accumulation in the brain of bovine spongiform encephalopathy experimentally infected sheep. J. Gen. Virol. 86:827–838. 10.1099/vir.0.80299-0 [DOI] [PubMed] [Google Scholar]
- 22.Maestrale C, Di Guardo G, Cancedda MG, Marruchella G, Masia M, Sechi S, Macciocu S, Santucciu C, Petruzzi M, Ligios C. 2013. A lympho-follicular microenvironment is required for pathological prion protein deposition in chronically inflamed tissues from scrapie-affected sheep. PLoS One 8:e62830. 10.1371/journal.pone.0062830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingrosso L, Flavio Pisani, Maurizio Pocchiari. 1999. Transmission of the 263K scrapie strain by the dental route. J. Gen. Virol. 80:3043–3047 [DOI] [PubMed] [Google Scholar]
- 24.Barone R. 2003. Angiologia parte seconda: vene e sistema linfatico, p 328–346 In Anatomia comparata dei mammiferi domestici, vol 5 Edagricole, Bologna, Italy [Google Scholar]
- 25.Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O'Rourke KI, Hoover EA. 1999. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). J. Gen. Virol. 80:2757–2764 [DOI] [PubMed] [Google Scholar]
- 26.Wild JM, Johnston BM, Gluckman PD. 1991. Central projections of the nodose ganglion and the origin of vagal efferents in the lamb. J. Anat. 175:105–129 [PMC free article] [PubMed] [Google Scholar]
- 27.Chiocchetti R, Clavenzani P, Barazzoni AM, Grandis A, Bombardi C, Costerbosa GL, Petrosino G, Avoni GB, Bortolami R. 2003. Viscerotopic representation of the subdiaphragmatic tracts of the digestive apparatus within the vagus complex in the sheep. Brain Res. 961:32–44. 10.1016/S0006-8993(02)03836-2 [DOI] [PubMed] [Google Scholar]
- 28.Diedrich JF, Bendheimt PE, Kimt YS, Carps RI, Haase AT. 1991. Scrapie-associated prion protein accumulates in astrocytes during scrapie infection. Proc. Natl. Acad. Sci. U. S. A. 88:375–379. 10.1073/pnas.88.2.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryder SJ, Spencer YI, Bellerby PJ, March SA. 2001. Immunohistochemical detection of PrP in the medulla oblongata of sheep: the spectrum of staining in normal and scrapie-affected sheep. Vet. Rec. 148:7–13. 10.1136/vr.148.1.7 [DOI] [PubMed] [Google Scholar]
- 30.Ligios C, Cancedda MG, Carta A, Santucciu C, Maestrale C, Demontis F, Saba M, Patta C, DeMartini JC, Aguzzi A, Sigurdson CJ. 2011. Sheep with scrapie and mastitis transmit infectious prions through the milk. J. Virol. 85:1136–1139. 10.1128/JVI.02022-10 [DOI] [PMC free article] [PubMed] [Google Scholar]