Abstract
The purpose of this study was to characterize the mechanical behavior of tendon slices with different thicknesses. Tendon slices of 100, 200, 300, 400, and 500 μm thickness were mechanically tested. The 300 μm slices were further tested for strength and modulus after 21,000-cycle fatigue testing under different applied strain levels (0, 1, 3, 5, 8, 10, and 12%). The tendon slice structure, morphology, and viability of bone marrow stromal cells (BMSCs) seeded onto the slices were also examined with histology, scanning electron microscopy, and vital cell labeling, respectively. Tendon slices 300 μm or more in thickness had similar ultimate tensile strength and Young's modulus to the intact tendon bundle. A strain of 5% or less did not cause any structural damage, nor did it change the mechanical properties of a 300 μm-thick tendon slice after 21,000-cycle fatigue testing. BMSCs were viable between and on the tendon slices after 2 weeks in tissue culture. This study demonstrated that, if tendon slices are used as a scaffold for tendon tissue engineering, slices 300 μm or more in thickness would be preferable from a mechanical strength point of view. If mechanical stimulation is performed for seeded-cell preparations, 5% strain or less would be appropriate.
Keywords: tendon, slice, mechanical test, cell viability, fatigue
INTRODUCTION
Tendon and ligament injuries, such as rotator cuff tear,1,2 flexor tendon laceration,3,4 and anterior cruciate ligament rupture5,6 are common and disabling. Restoration of tendon and ligament function remains a clinical challenge. Given the frequency and increasing cost of tendon and ligament injuries, as well as the uneven quality of surgical interventions, new and innovative strategies like tissue engineering have become more attractive. Current tissue engineering strategies have relied predominantly upon scaffolds derived from both synthetic and naturally derived materials. However, synthetic scaffolds produce breakdown products that have been shown to be antimitotic and cytotoxic in vivo.7 Most naturally derived scaffolds also lack initial mechanical strength to permit immediate motion and rehabilitation after implantation, which has been shown to lead to subsequent adhesion formation, decreased range of motion, and poor functional outcomes.8,9 Engineered tendons produced in vitro using similar scaffolds or via self-assembly also have been shown to be weaker than native adult tendons and would not be expected to withstand rehabilitation after implantation.10 An ideal scaffold for tendon and ligament regeneration must be able to meet the physiologic demand of the native tendon or ligament by matching their mechanical properties and biocompatibility, promoting host cell mediated healing and biodegradation in order to be gradually replaced by new tissue while maintaining physiologically relevant mechanical properties.11
Recently, decellularized native tendon has been developed as a potential tendon scaffold after treatment with different detergents, such as Triton-X, sodium dodecyl sulfate, or tri-nitro-butyl-phosphate. However, the cell seeding was limited to the tendon surface.12 More recently, a novel scaffold with multilayer tendon slices (MTS) was reported.13 Although the seeded cells survived and functioned well after 2 weeks of culture, the mechanical properties of the MTS scaffold have not been characterized.
The purpose of this study was to characterize the mechanical behavior of native tendon slices with different thicknesses, to select the minimal thickness of a single tendon slice for MTS that maintains the mechanical properties of the native tendon. The cyclic fatigue testing of tendon slices 300 μm in thickness was also investigated at different strain levels to identify a strain level for mechanical stimulation of a cell-seeded tendon slice without significant damage to the tendon tissue. Additionally, the viability of bone marrow stromal cells (BMSCs) seeded onto the MTS was assessed.
MATERIALS AND METHODS
Mechanical evaluation of single tendon slice
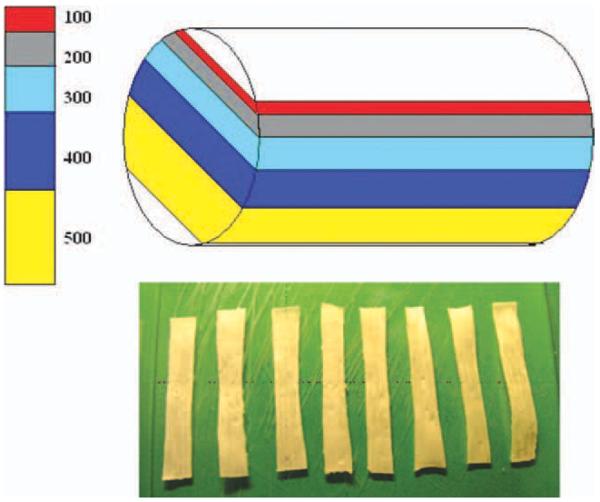
Four right hind limbs were obtained from four adult (1–2 yr old) mongrel dogs weighing 20–25 kg. The dogs were euthanized for other Institutional Animal Care and Use Committee (IACUC) approved studies, which did not affect the musculoskeletal tissues. Three bundles (lateral, central, and medial bundles) of each Achilles tendon (AT) were dissected from one hind limb (Figure 1). The tendon bundles were placed in liquid nitrogen for 10 min and then were thawed in room temperature. This procedure was repeated three times to disrupt all the cells in the tendon.14 This processing technique diminishes the antigenicity of the tendon without a significant decrease in its mechanical strength.15 Each bundle (40 mm in length) was fixed on a cryostat (Leica CM1850, Germany) with Tissue-Tek® optimal cutting temperature compound (Sakura Finetek USA, Torrance, CA) and longitudinally sliced into thicknesses of 100, 200, 300, 400, and 500 μm (Figure 2). A total of 12 AT bundles and 60 AT slices (n = 12 for each thickness) were used. The ends of each bundle and slice (about 10 mm) were wrapped with saline-soaked sandpaper and mounted into custom made grips on a uniaxial load frame (MTS 312, MTS, MA) for failure testing. Cross-sectional area and the distance between the grips were measured prior to testing with digital calipers (Mitutoyo Corporation, Japan). Each specimen of the bundles and slices was preloaded to 0.1N and then loaded to failure at a rate of 12 mm/min (equivalent to strain rate of 1%/s) following three preconditioning cycles in which the specimen was stretched to 2% strain at 0.5 Hz. Specimens were kept moist during testing with PBS. Ultimate tensile stress (UTS) and Young's modulus were calculated from the load and displacement obtained from the testing. Specimens that failed at the grips were excluded to eliminate artificial damage of the slice by gripping during testing.
FIGURE 1.
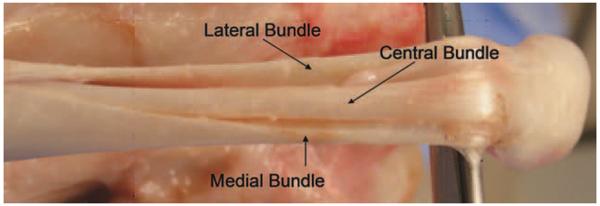
Canine AT is composed of three isolated tendon bundles of similar size.
FIGURE 2.
Each AT bundle was longitudinally cut into varied thicknesses from 100 to 500 μm in 100 μm increments.
Fatigue testing of single tendon slice
Slices 300 μm in thickness were selected for fatigue testing based on the above study. We used 56 tendon slices from 15 AT bundles, harvested from 5 hind limbs. After freeze-thaw treatment, the tendon bundles were sliced at 300 μm thickness. The 56 slices were divided into 7 groups (n = 8 for each group), each tested at a different applied strain (0, 1, 3, 5, 8, 10, and 12%) during tensile fatigue testing. The ends of the slices were wrapped with saline-soaked sandpaper, immersed into a bath filled with minimal essential medium (MEM) with Earle's salts (GIBCO, Grand Island, NY) and clamped onto Bose ELF 3200 system, BOSE Co, (Figure 3) with a 0.1N preload. After 100 cycles of preconditioning, the slices were reciprocated at 5 Hz to the prescribed applied strain for 21,000 cycles. During the fatigue testing, the load and displacement of each slice were sampled at the 100th cycle and the 21,000th cycle for each applied strain. The stress amplitude induced by the applied strain was calculated from the real-time sampling data. After the fatigue testing, the tendon slices were then loaded to failure at a rate of 12 mm/min. The load and displacement of each slice were recorded to calculate the ultimate tensile strength and Young's modulus for analysis.
FIGURE 3.

Fatigue testing of the tendon slice with 300 μm thickness at different strains.
BMSCs harvesting, seeding, and labeling
A total of 18 mL of bone marrow was aspirated from both proximal tibias from dogs being euthanized for other IACUC approved studies. Immediately prior to sacrifice, the dogs were anesthetized with intravenous ketamine (13 mg/kg) and diazepam (6 mg/kg) and maintained under anesthesia with 1.5% isoflurane. The heparinized bone marrow extract was added to 5.0 mL PBS and centrifuged at 1500 rpm (380 g) for 5 min at room temperature. The bone marrow pellet was then resolubilized in 10 mL of MEM with Earle's salts (GIBCO, Grand Island, NY), 10% fetal bovine serum (GIBCO), and 5% antibiotics (Antibiotic-Antimycotic, GIBCO). The pellet from one dog was divided into four equal aliquots, placed in four 100-mm culture dishes and incubated at 37°C with 5% CO2 and 95% air at 100% humidity. After 5 days, the medium and any floating cells were removed and new medium was added to the remaining adherent cells. These adherent cells were defined as BMSCs.16 The medium was then changed every other day until the adherent cells reached confluence. The cells in the second passage were then released with trypsin-EDTA solution (0.25% trypsin, 0.1% EDTA in HBSS, Mediatech, Manassas, VA) to produce a cell suspension and centrifuged at 1500 rpm for 5 min to remove the trypsin-EDTA solution. The concentrated cell suspension from each dog was then gathered in one tube. The cells were counted with a hemacytometer and the concentration of the cell suspension was adjusted to 5.0 × 105 cells/mL by adding additional medium. The collected BMSCs were labeled with the fluorescent marker PKH26-GL (PKH26 Red Fluorescent Cell Linker Kit for General Cell Membrane Labeling, Sigma, St. Louis, MO) following the manufacturer's instructions. To assess cell seeding and viability, 100 μL of labeled BMSCs suspension was seeded onto the surface of each slice 300 μm in thickness. Then the slices seeded with BMSCs were put in pair to create two-layered composite with BMSCs in between and cultured for 14 days. Total four samples of two-layered composite (n = 4) with the labeled BMSCs were transversely cut into fast frozen sections using a cryostat (Leica CM1850, Germany). The sections and slices seeded with BMSCs were examined by a laser scanning confocal microscope (LSM310, Zeiss).
Histology and scanning electron microscope
Two tendon bundles were fixed in 10% neutral buffered formalin. Longitudinal and cross-sectional images were stained with Masson trichrome to examine the structure of the tendon fascicles.
Two samples from each strain group were prepared for scanning electron microscopy after fatigue testing, in order to evaluate tendon integrity. These samples were fixed in a solution of buffered glutaraldehyde and osmium tetroxide. After dehydration in graded acetone, the specimens were coated with gold:palladium alloy and examined with a Hitachi 4700 scanning electron microscope at 10KV (Hitachi Scientific Instruments, Pleasanton, CA).
Statistics
The results were calculated as mean ± standard deviation. The mechanical testing results were tested for normality using the Kolmogorov-Smirnov test (with a Lillierfors' correction using SPSS software version 16.0), and the data displayed a normal distribution, so significance of differences among groups was assessed by one-way ANOVA followed by Tukey's post hoc multiple-comparison test. All results with p < 0.05 were considered significant.
RESULTS
Mechanical characteristics of single MTS
The ultimate tensile strength of the entire bundle and the slices with thicknesses of 500, 400, and 300 μm was significantly higher than the tendon slices with thicknesses of 200 and 100 μm (p < 0.05). There was neither significant difference in UTS among the intact tendon bundle and the 500, 400, and 300 μm tendon slices, nor was there a significant difference in UTS between the 200 and 100 μm thicknesses (Figure 4). The Young's modulus of the entire bundle was significantly higher than the 200 and 100 μm thickness tendon slices (p < 0.05). There was no significant difference in Young's modulus among the intact tendon bundle and the slices with 500, 400, and 300 μm thickness. The Young's modulus of the 500, 400, and 300 μm tendon slices was significantly higher than that of the 100 μm slices (p < 0.05), but not the 200 μm slices. There was no significant difference in modulus between the 200 and 100 μm slices (Figure 5).
FIGURE 4.
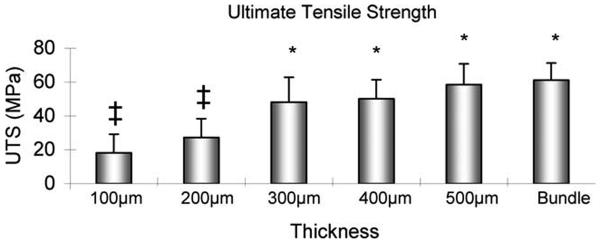
The ultimate tensile strength of the tendon slices with different thicknesses. ‡: Significantly different from 300 μm slice, 400 μm slice, 500 μm slice or Bundle. *: Significantly different from 100 μm slice or 200 μm slice.
FIGURE 5.
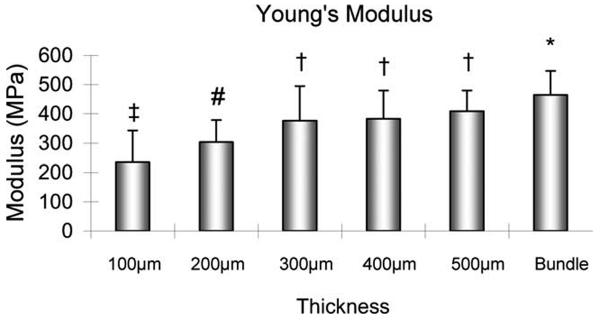
The Young's modulus of the tendon slices with different thicknesses. ‡: Significantly different from 300 μm slice, 400 μm slice, 500 μm slice or Bundle. #: Significantly different from Bundle. †: Significantly different from 100 μm slice. *: Significantly different from 100 μm slice or 200 μm slice.
Mechanical behavior of single MTS during and after fatigue
During the fatigue testing, there was no significant difference in mean stress amplitude induced by applied strain between the 100th cycle and 21,000th cycle among the 1, 3, and 5% strain levels. However, the mean stress amplitude at the 21,000th cycle was significantly lower than that at the 100th cycle among applied strain levels of 8, 10, and 12% (p < 0.05; Figure 6).
FIGURE 6.
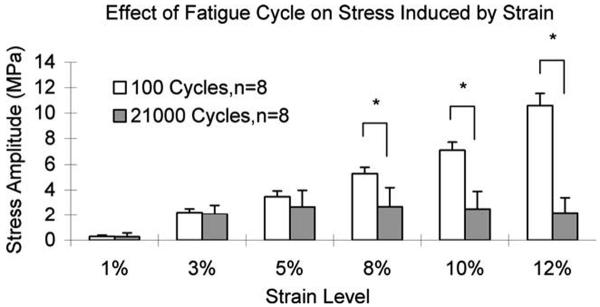
The mean stress amplitude induced by applied strain during fatigue testing. *: Indicates significant difference between the stress amplitudes of the 100th cycle and 21,000th cycle (p < 0.05).
After the 21,000-cycle fatigue testing, the UTS of the slices tested at 0% strain was significantly higher than that at each strain level of 8, 10, or 12% (p < 0.05; Figure 7). There was no significant difference in UTS among the 1, 3, and 5% strain levels, but all of these had a UTS, which was significantly higher than the UTS at each strain level of 10 or 12% (p < 0.05). The Young's modulus after cyclic testing at 0% strain was significantly higher than that at 8, 10, or 12% strain level (p < 0.05). There was no significant difference in Young's modulus among the 0, 1, 3, or 5% strain level. There was also no significant difference in Young's modulus among the 1, 3, 5, 8, 10, and 12% strain levels (Figure 8).
FIGURE 7.
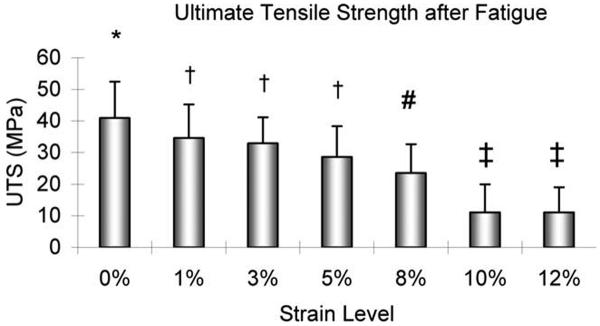
The ultimate tensile strength of the tendon slices with 300 μm thickness after 21,000-cycle fatigue testing. *: Significantly different from 8, 10, or 12%. †: Significantly different from 10 or 12%. #: Significantly different from 0%. ‡: Significantly different from 0, 1, 3, or 5%.
FIGURE 8.
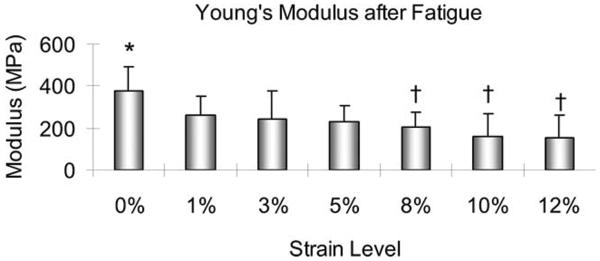
The Young's modulus of the tendon slices with 300 μm thickness after 21,000-cycle fatigue testing. *: Significantly different from 8, 10, or 12%. †: Significantly different from 0%.
Cell viability, histology, and scanning electron microscope
Under light microscopy, the tendon fascicles size was 200–300 μm depending upon the angle of the section (Figure 9). Therefore, a 100 μm thick slice is too thin to include any undamaged large tendon fascicles. Although a 200 μm thick slice may contain some larger fascicles, the largest fascicles will be damaged by the sectioning process, thereby weakening the slice. In contrast, slices that are more than 300 μm thick are more likely to contain undamaged, larger fascicles.
FIGURE 9.
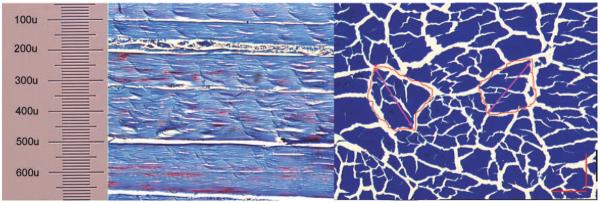
Tendon fascicles display irregular shape with variable size in longitudinal section (left) and cross section (right), Masson Trichrome staining under ×100 magnification.
Under SEM, the cyclically loaded slices at 8% strain or more displayed tendon fascicle damage. The cyclically loaded slices at 5% strain or less did not show any evidence of structural changes, compared with the images of the native tendon slice without fatigue testing (Figure 10).
FIGURE 10.
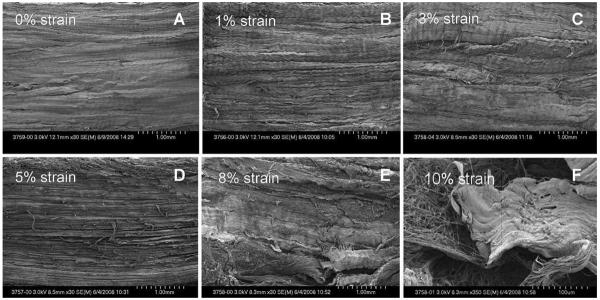
SEM (×30) of a tendon slice (300 μm) after 21,000-cycle fatigue testing at 1, 3, and 5% strains (B, C, and D) showed no changes in the structure compared with the tendon slice at 0% strain (A). The slices at 8 and 10% strains (E and F) demonstrated collagen fiber disruption.
Viable BMSCs labeled with PKH26 were observed between and on the tendon slices by confocal laser microscopy after 2 weeks in tissue culture (Figure 11).
FIGURE 11.
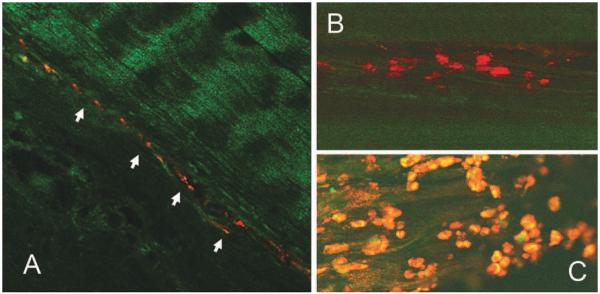
The BMSCs retained the fluorescent marker PKH26-GL after 2 weeks in tissue culture, seeded between two layers of 300 μm tendon slice (A: ×100; B: ×400). BMSCs were observed alive on the tendon slice surface under confocal microscopy (C: ×400).
DISCUSSION
One of the most critical elements to consider in tissue engineering is the structural scaffold. The ideal scaffold for tendon and ligament regeneration must be able to meet the physiologic conditions of the native tendon or ligament by matching their mechanical properties and biocompatibility, promoting host cell mediated healing and, ideally, biodegrading to be gradually replaced by host tissue while maintaining physiologically relevant mechanical properties.11 Biodegradable synthetic polymers such as polylactic acid, polyglycolic acid, and polycaprolactone have been well developed and used for tissue engineering as scaffolds for bone, cartilage, tendon, and ligament regeneration.17–20 However, regulation of the surface for cell attachment, porosity for cell survival, mechanical properties for functional application, and degradation for new tissue regeneration has been difficult to achieve simultaneously.21,22 Furthermore, the particles released during polymer degradation result in a highly acidic environment, which affects cellular function and tissue remodeling.23,24 Although naturally derived collagen scaffolds present a more native surface relative to synthetic polymer scaffolds, the mechanical properties of fabricated collagen scaffolds are weaker than those of a normal tendon.25,26
Decellularized native tendon tissue has been recently studied as a scaffold for tendon tissue engineering in a tissue culture model. However, cell seeding was limited to the tendon surface.12 Although cell penetration was improved by detergent treatment, the loss of mechanical properties and damage to extracellular matrix are disadvantages of this preparation method.27–29
More recently, a novel composite for tendon tissue engineering was proposed, consisting of MTS seeded with BMSCs.13 In that study, the BMSCs successfully seeded onto the tendon slices, survived for 2 weeks in tissue culture and expressed a marker of tendon phenotype, tenomodulin. The new tissue engineering approach provided a possibility to use native tendon slices as the scaffold to generate tendon or ligament tissues. The thickness of the tendon slice was 50 μm in that study. However, the mechanical properties of the tendon slices were not studied. Therefore, in this study, we selected five different thicknesses of tendon slices to investigate their mechanical characteristics and found out that the tendon slice with 300 μm or more in thickness had similar ultimate tensile strength and Young's modulus to the intact tendon bundle.
In this study, when the AT bundles were longitudinally cut into varied thicknesses from 100 to 500 μm in 100 μm increments, we found that slices 300 μm or thicker maintain similar mechanical properties to an intact tendon bundle. However, the slices thinner than 300 μm displayed decreased mechanical strength. Tendon mechanical properties are determined by the integrity of the tendon fascicles. According to our histological examination of transverse-sections of canine ATs, the diameter of the fascicles varies from 100 to 280 μm, which is similar to dimensions noted in a previous study (80–320 μm)30 and consistent with our finding of preservation of material properties in slices of 300 μm or thicker. When the tendon is longitudinally sliced thinner than 300 μm, the integrity of most fascicles within the same slice is damaged, which leads to tendon slice weakening. By contrast, when the slice thickness is 300 μm or more, the majority of the intact fascicles are preserved, thus the basic mechanical properties of the entire tendon bundle are maintained.
We believe that native tendon slices might serve as potential cell seeded tissue-engineering scaffolds. It is reasonable to consider that these scaffolds might be mechanically stimulated in tissue culture prior to implantation, to promote cell proliferation, differentiation, and extracellular matrix synthesis.31,32 In this study, to provide valuable data for a tissue culture under mechanical stimulation using the screened tendon slice (300 μm in thickness) as a tissue engineering scaffold, we designed the fatigue testing at different applied strains. We found that 5% strain or less did not alter the mechanical properties of the 300 μm slices, even over 20,000 loading cycles. These results are similar to previous reports that tendon behaves in an elastic fashion and returns to its original length when unloaded if the strain remains <4%33 and that beyond 8% strain, macroscopic failure occurs from intrafibril damage by molecular slippage.34,35
There are several limitations to this study. First, although the mechanical properties of a single tendon slice were studied, the material properties of the MTS composite were not measured. The construction of an MTS composite with individual slices may affect the mechanical properties of engineered tendon. This should be investigated in future studies. Second, the cells survived 2 weeks in tissue culture, but the cell biological behaviors were not studied, such as TGF- β response and collagen synthesis. Third, parameters during fatigue testing, such as frequency and duration, were not studied beyond 5.0 Hz with 21,000 cycles. This was determined based on a future cell-stimulation protocol, in which the slices would be subjected to a 7-day loading regime of cyclic uniaxial strain at 0.2 Hz with 3.6% elongation for 20 min/h, 12h/day. Clearly, other loading regimens should also be tested. Fourth, we did not quantitatively count the cell number under confocal examination. Finally, in this study, the fascicle diameter was observed based on both longitudinal and cross-sectional histology of tendon bundles. However, we did not quantitatively count the number of intact fascicles at cross-sectional area of the slice.
CONCLUSION
This study demonstrated that a tendon slice of 300 μm thickness was the thinnest to conserve the ultimate tensile strength and Young's modulus of normal tendons and that BMSCs seeded between the tendon slices were viable after 2 weeks in tissue culture. We also found that a 5% strain or less would be appropriate if mechanical stimulation of the scaffold is desired. This study provides useful information for future development of an MTS scaffold for tendon tissue engineering.
Acknowledgments
Contract grant sponsor: Mayo Foundation; contract grant number: 90608011
Contract grant sponsor: Scientific Research Foundation for the Returned Overseas Chinese Scholars of State Education Ministry; contract grant number: 2010609
REFERENCES
- 1.Cofield RH, Parvizi J, Hoffmeyer PJ, Lanzer WL, Ilstrup DM, Rowland CM. Surgical repair of chronic rotator cuff tears. A prospective long-term study. J Bone Joint Surg Am. 2001;83-A:71–77. doi: 10.2106/00004623-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A:219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Manske PR. History of flexor tendon repair. Hand Clin. 2005;21:123–127. doi: 10.1016/j.hcl.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Amadio PC. What's new in hand surgery. J Bone Joint Surg Am. 2009;91:496–502. doi: 10.2106/JBJS.H.01697. [DOI] [PubMed] [Google Scholar]
- 5.Fu FH, Schulte KR. Anterior cruciate ligament surgery 1996. State of the art? Clin Orthop. 1996;325:19–24. doi: 10.1097/00003086-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Hantes ME, Basdekis GK, Varitimidis SE, Giotikas D, Petinaki E, Malizos KN. Autograft contamination during preparation for anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2008;90:760–764. doi: 10.2106/JBJS.G.00806. [DOI] [PubMed] [Google Scholar]
- 7.Garvin J, Qi J, Maloney M, Banes AJ. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 2003;9:967–979. doi: 10.1089/107632703322495619. [DOI] [PubMed] [Google Scholar]
- 8.Buckwalter JA, Hunziker EB. Orthopaedics. Healing of bones, cartilages, tendons, and ligaments: A new era. Lancet. 1996;348(Suppl 2):sII18. doi: 10.1016/s0140-6736(96)98028-9. [DOI] [PubMed] [Google Scholar]
- 9.Qin TW, Zhang SJ, Yang ZM, Mo XT, Li XQ. Mechanical properties and related histological alterations of engineered tendons in vivo. Key Eng Mater. 2005;288:11–14. [Google Scholar]
- 10.Calve S, Dennis RG, Kosnik PEII, Baar K, Grosh K, Arruda EM. Engineering of functional tendon. Tissue Eng. 2004;10:755–761. doi: 10.1089/1076327041348464. [DOI] [PubMed] [Google Scholar]
- 11.Moffat KL, Wang INE, Rodeo SA, Lu HH. Orthopedic interface tissue engineering for the biological fixation of soft tissue grafts. Clin Sports Med. 2009;28:157–176. doi: 10.1016/j.csm.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angelidis IK, Thorfinn J, Connolly ID, Lindsey D, Pham HM, Chang J. Tissue engineering of flexor tendons: the effect of a tissue bioreactor on adipoderived stem cell-seeded and fibroblast-seeded tendon constructs. J Hand Surg Am. 2010;35:1466–1472. doi: 10.1016/j.jhsa.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Omae H, Zhao C, Sun YL, An KN, Amadio PC. Multilayer tendon slices seeded with bone marrow stromal cells: a novel composite for tendon engineering. J Orthop Res. 2009;27:937–942. doi: 10.1002/jor.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnoczky SP, DiCarlo EF, O'Brien SJ, Warren RF. Cellular repopulation of deep-frozen meniscal autografts: An experimental study in the dog. Arthroscopy. 1992;8:428–436. doi: 10.1016/0749-8063(92)90003-t. [DOI] [PubMed] [Google Scholar]
- 15.Minami A, Ishii S, Ogino T, Oikawa T, Kobayashi H. Effect of the immunological antigenicity of the allogeneic tendons on tendon grafting. Hand. 1982;14:111–119. doi: 10.1016/s0072-968x(82)80001-6. [DOI] [PubMed] [Google Scholar]
- 16.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 17.Lu L, Yaszemski MJ, Mikos AG. TGF-beta1 release from biodegradable polymer microparticles: Its effects on marrow stromal osteoblast function. J Bone Joint Surg Am. 2001;83-A(Suppl 1(Part 2)):S82–91. [PubMed] [Google Scholar]
- 18.Hoshi K. Present state and prospects of cartilage regenerative medicine. Clin Calcium. 2008;18:1701–1706. [PubMed] [Google Scholar]
- 19.Ishimoto Y, Hattori K, Ohgushi H. Articular cartilage regeneration using scaffold. Clin Calcium. 2008;18:1775–1780. [PubMed] [Google Scholar]
- 20.Sahoo S, Cho-Hong JG, Siew-Lok T. Development of hybrid polymer scaffolds for potential applications in ligament and tendon tissue engineering. Biomed Mater. 2007;2:169–173. doi: 10.1088/1748-6041/2/3/001. [DOI] [PubMed] [Google Scholar]
- 21.Butler DL, Hunter SA, Chokalingam K, Cordray MJ, Shearn J, Juncosa-Melvin N, Nirmalanandhan S, Jain A. Using functional tissue engineering and bioreactors to mechanically stimulate tissue-engineered constructs. Tissue Eng Part A. 2009;15:741–749. doi: 10.1089/ten.tea.2008.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinatier C, Mrugala D, Jorgensen C, Guicheux J, Noel D. Cartilage engineering: A crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009;27:307–314. doi: 10.1016/j.tibtech.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Wake MC, Gerecht PD, Lu L, Mikos AG. Effects of biodegradable polymer particles on rat marrow-derived stromal osteoblasts in vitro. Biomaterials. 1998;19:1255–1268. doi: 10.1016/s0142-9612(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 24.Kohn DH, Sarmadi M, Helman JI, Krebsbach PH. Effects of pH on human bone marrow stromal cells in vitro: Implications for tissue engineering of bone. J Biomed Mater Res. 2002;60:292–299. doi: 10.1002/jbm.10050. [DOI] [PubMed] [Google Scholar]
- 25.Faraj KA, van Kuppevelt TH, Daamen WF. Construction of collagen scaffolds that mimic the three-dimensional architecture of specific tissues. Tissue Eng. 2007;13:2387–2394. doi: 10.1089/ten.2006.0320. [DOI] [PubMed] [Google Scholar]
- 26.Glowacki J, Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. 2008;89:338–344. doi: 10.1002/bip.20871. [DOI] [PubMed] [Google Scholar]
- 27.Cartmell JS, Dunn MG. Development of cell-seeded patellar tendon allografts for anterior cruciate ligament reconstruction. Tissue Eng. 2004;10:1065–1075. doi: 10.1089/ten.2004.10.1065. [DOI] [PubMed] [Google Scholar]
- 28.Tischer T, Salzmann GM, Imhoff AB. Rotator cuff tears and internal impingement in athletes. Orthopade. 2007;36:950, 952–956. doi: 10.1007/s00132-007-1150-z. [DOI] [PubMed] [Google Scholar]
- 29.Ingram JH, Korossis S, Howling G, Fisher J, Ingham E. The use of ultrasonication to aid recellularization of acellular natural tissue scaffolds for use in anterior cruciate ligament reconstruction. Tissue Eng. 2007;13:1561–1572. doi: 10.1089/ten.2006.0362. [DOI] [PubMed] [Google Scholar]
- 30.Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6:11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- 31.Benhardt HA, Cosgriff-Hernandez EM. The role of mechanical loading in ligament tissue engineering. Tissue Eng Part B Rev. 2009;15:467–475. doi: 10.1089/ten.TEB.2008.0687. [DOI] [PubMed] [Google Scholar]
- 32.Yang G, Crawford RC, Wang JHC. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J Biomech. 2004;37:1543–1550. doi: 10.1016/j.jbiomech.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Sharma P, Maffulli N. Tendon injury and tendinopathy: Healing and repair. J Bone Joint Surg Am. 2005;87:187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien M. Structure and metabolism of tendons. Scand J Med Sci Sports. 1997;7:55–61. doi: 10.1111/j.1600-0838.1997.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 35.Butler DL, Grood ES, Noyes FR, Zernicke RF. Biomechanics of ligaments and tendons. Exerc Sport Sci Rev. 1978;6:125–181. [PubMed] [Google Scholar]