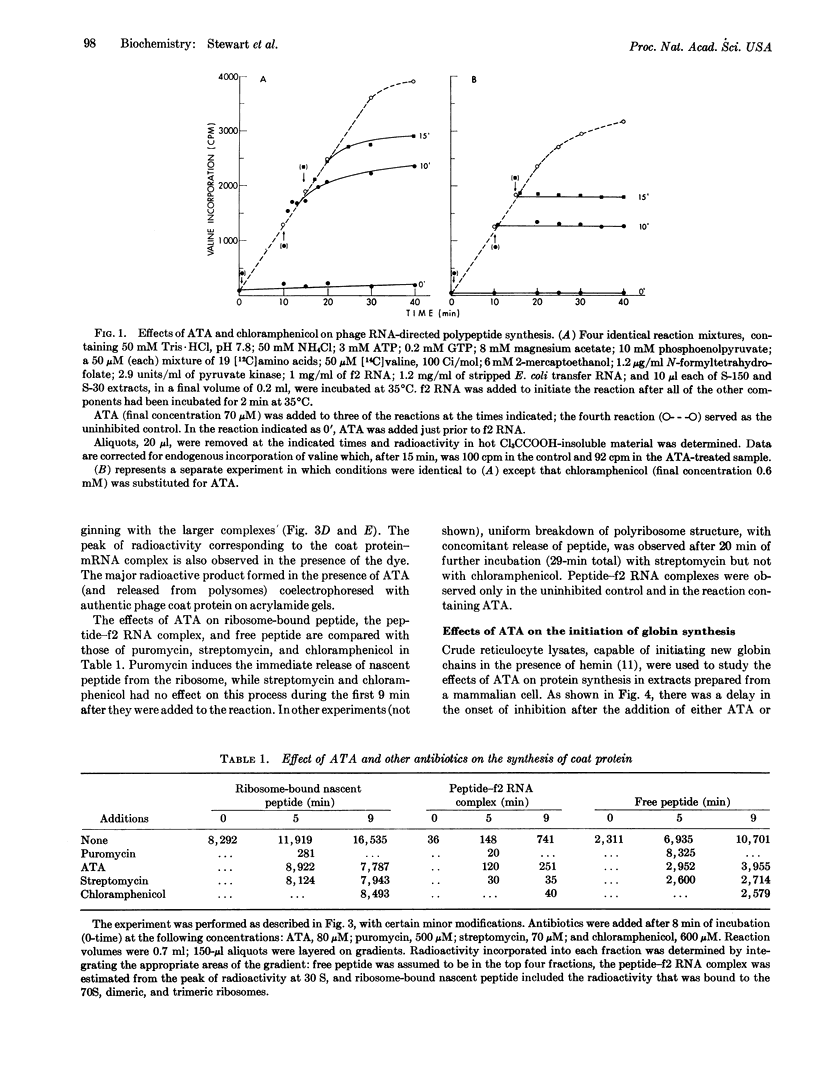
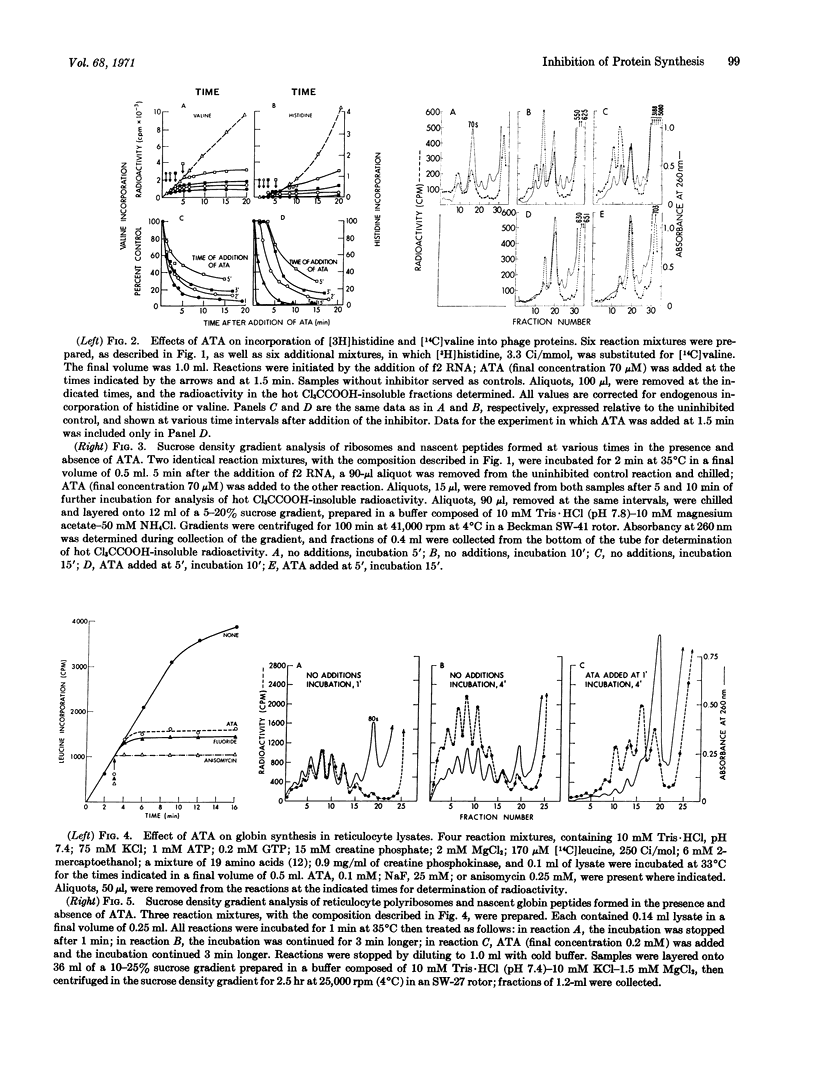
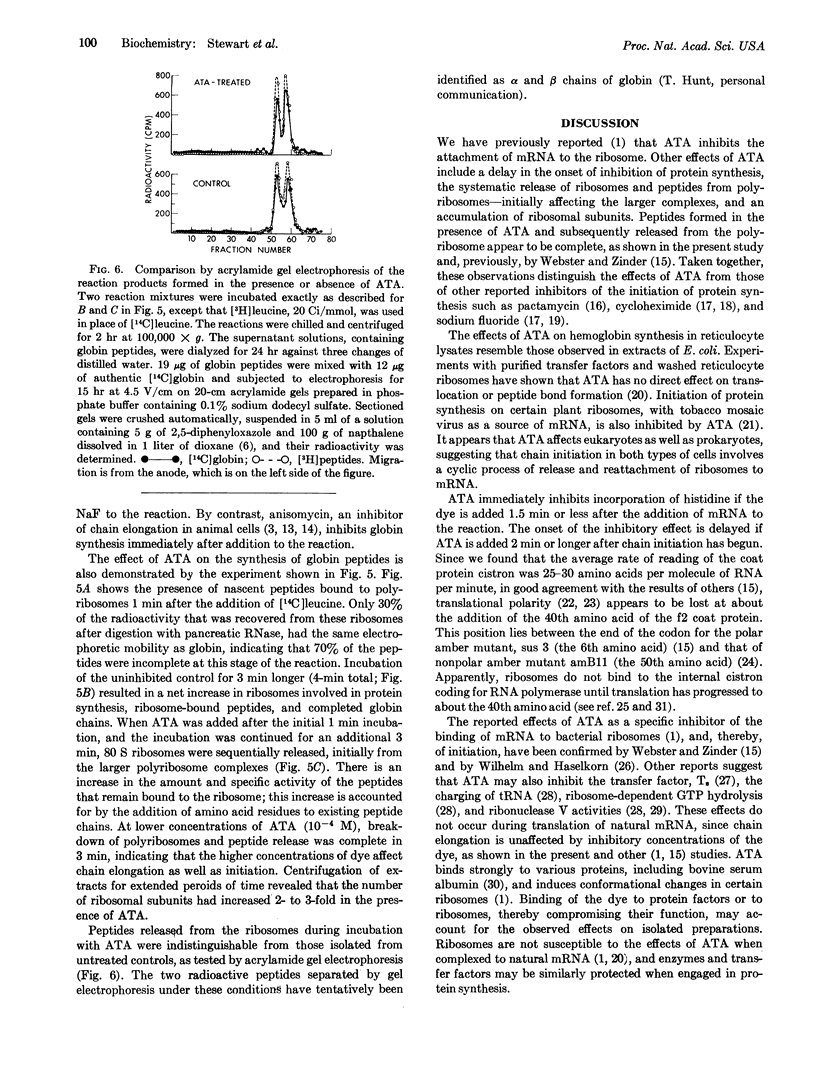
Abstract
Aurintricarboxylic acid prevents the attachment of bacteriophage messenger RNA to ribosomes. As a consequence, initiation of protein synthesis in cell-free extracts prepared from Escherichia coli or rabbit reticulocytes is inhibited at concentrations of dye that do not prevent chain extension. Its properties can be distinguished from other agents that inhibit protein synthesis, including sodium fluoride, cycloheximide, and pactamycin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britten R. J., Roberts R. B. High-Resolution Density Gradient Sedimentation Analysis. Science. 1960 Jan 1;131(3392):32–33. doi: 10.1126/science.131.3392.32. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Cell-free protein synthesis programmed with R17 RNA: identification of two phage proteins. J Mol Biol. 1966 Oct 28;21(1):173–193. doi: 10.1016/0022-2836(66)90086-6. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Polarity in vitro. J Mol Biol. 1967 Nov 28;30(1):213–217. doi: 10.1016/0022-2836(67)90254-9. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Herner A. E., Goldberg I. H. Inhibition by pactamycin of the initiation of protein synthesis. Binding of N-acetylphenylalanyl transfer ribonucleic acid and polyuridylic acid to ribosomes. Biochemistry. 1969 Apr;8(4):1312–1326. doi: 10.1021/bi00832a004. [DOI] [PubMed] [Google Scholar]
- Engelhardt D. L., Webster R. E., Zinder N. D. Amber mutants and polarity in vitro. J Mol Biol. 1967 Oct 14;29(1):45–58. doi: 10.1016/0022-2836(67)90180-5. [DOI] [PubMed] [Google Scholar]
- Grollman A. P. Inhibitors of protein biosynthesis. II. Mode of action of anisomycin. J Biol Chem. 1967 Jul 10;242(13):3226–3233. [PubMed] [Google Scholar]
- Grollman A. P., Stewart M. L. Inhibition of the attachment of messenger ribonucleic acid to ribosomes. Proc Natl Acad Sci U S A. 1968 Oct;61(2):719–725. doi: 10.1073/pnas.61.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerz W., McCarty K. S. Evidence for a proposed initiation complex for protein synthesis in reticulocyte polyribosome profiles. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1206–1213. doi: 10.1073/pnas.63.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano M., Schlessinger D., Apirion D. Ribonuclease V of Escherichia coli requires ribosomes and is inhibited by drugs. Nature. 1970 May 9;226(5245):514–516. doi: 10.1038/226514a0. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Mosteller R. D., Hardesty B. The mechanism of sodium fluoride and cycloheximide inhibition of hemoglobin biosynthesis in the cell-free reticulocyte system. J Mol Biol. 1966 Oct 28;21(1):51–69. doi: 10.1016/0022-2836(66)90079-9. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Bacteriophage f2 RNA: control of translation and gene order. Nature. 1968 Oct 26;220(5165):345–350. doi: 10.1038/220345a0. [DOI] [PubMed] [Google Scholar]
- Marcus A., Bewley J. D., Weeks D. P. Aurintricarboxylic acid and initiation factors of wheat embryo. Science. 1970 Mar 27;167(3926):1735–1736. doi: 10.1126/science.167.3926.1735. [DOI] [PubMed] [Google Scholar]
- Maxwell C. R., Rabinovitz M. Evidence for an inhibitor in the control of globin synthesis by hemin in a reticulocyte lysate. Biochem Biophys Res Commun. 1969 Apr 10;35(1):79–85. doi: 10.1016/0006-291x(69)90485-9. [DOI] [PubMed] [Google Scholar]
- McKeehan W., Hardesty B. The mechanism of cycloheximide inhibition of protein synthesis in rabbit reticulocytes. Biochem Biophys Res Commun. 1969 Aug 15;36(4):625–630. doi: 10.1016/0006-291x(69)90351-9. [DOI] [PubMed] [Google Scholar]
- NATHANS D., NOTANI G., SCHWARTZ J. H., ZINDER N. D. Biosynthesis of the coat protein of coliphage f2 by E. coli extracts. Proc Natl Acad Sci U S A. 1962 Aug;48:1424–1431. doi: 10.1073/pnas.48.8.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Weber K. Isolation and characterization of amber mutants of bacteriophage R17. J Mol Biol. 1967 Sep 14;28(2):311–330. doi: 10.1016/s0022-2836(67)80012-3. [DOI] [PubMed] [Google Scholar]
- Vazquez D., Battaner E., Neth R., Heller G., Monro R. E. The function of 80 S ribosomal subunits and effects of some antibiotics. Cold Spring Harb Symp Quant Biol. 1969;34:369–375. doi: 10.1101/sqb.1969.034.01.043. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Zinder N. D. Fate of the message-ribosome complex upon translation of termination signals. J Mol Biol. 1969 Jun 28;42(3):425–439. doi: 10.1016/0022-2836(69)90234-4. [DOI] [PubMed] [Google Scholar]
- Weissbach H., Brot N. Inhibition of transfer factor Ts by aurintricarboxylic acid. Biochem Biophys Res Commun. 1970;39(6):1194–1198. doi: 10.1016/0006-291x(70)90687-x. [DOI] [PubMed] [Google Scholar]
- Wilhelm J. M., Haselkorn R. The chain growth rate of T4 lysozyme in vitro. Proc Natl Acad Sci U S A. 1970 Feb;65(2):388–394. doi: 10.1073/pnas.65.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker W. V., Schulman H. M. Stimulation of globin-chain initiation by hemin in the reticulocyte cell-free system. Proc Natl Acad Sci U S A. 1968 Feb;59(2):582–589. doi: 10.1073/pnas.59.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]


