Abstract
The gH/gL heterodimer represents two of the four herpes simplex virus glycoproteins necessary and sufficient for membrane fusion. We generated deletions and point mutations covering gL residues 24 to 43 to investigate that region's role in gH/gL intracellular trafficking and in membrane fusion. Multiple mutants displayed a 40 to 60% reduction in cell fusion with no effect on gH/gL trafficking. The amino terminus of gL plays an important role in the gH/gL contribution to membrane fusion.
TEXT
Herpes simplex virus (HSV) is enveloped by a lipid bilayer containing approximately a dozen or more envelope glycoproteins. The virus relies upon its envelope glycoproteins to fuse the viral membrane with a cellular membrane to gain access to a host cell. The envelope glycoproteins that are necessary and sufficient to mediate membrane fusion are glycoproteins B, D, H, and L (gB, gD, gH, and gL) (1–3). The smallest of the HSV fusion glycoproteins is gL, which comprises 224 amino acids, with no obvious transmembrane domain. The integral membrane protein gH binds gL, probably in the endoplasmic reticulum (ER), and the corresponding gH/gL heterodimer then transits to sites of viral envelopment and the plasma membrane (4, 5). An intact HSV type 1 (HSV-1) gH will not exit the ER unless it is bound to gL (4–7). The gH/gL heterodimer has been postulated to have the hallmarks of a viral fusion protein and to play a direct role in membrane fusion (8–13). However, the HSV-2 gH/gL structure does not resemble any known viral fusion protein, and there is recent evidence that HSV gH/gL plays more of a regulatory and/or structural role in membrane fusion executed by the class III fusion protein gB (14–17).
The crystal structure has been determined for the HSV-2 gH (residues 48 to 803)/gL (residues 20 to 224) heterodimer (16). Three domains, H1 to H3, were assigned to the gH structure, with domain H1 further subdivided into H1A and H1B (16). The H1A and H1B subdomains (residues 49 to 115 and 13 to 327, respectively) form a vise to clamp onto gL. The majority of gL does not adopt an identifiable secondary structure, with three helices and two β-sheets comprising only 30% of gL residues. There are extensive areas of contact between gL and gH, such that most gL residues interact with or are in very close proximity to subdomains H1A and H1B. Regions of gL that extend outward from the gH/gL heterodimer and do not appear to interact with gH include residues at the amino terminus (residues 26 to 44) and a small β-strand near the carboxy terminus, residues 197 to 203 (16).
To investigate gH/gL trafficking and function in membrane fusion by using targeted mutagenesis, we were most interested in regions predicted by the crystal structure that project outwards. We adopted this strategy to minimize the chance we would generate gH or gL mutants that did not stably associate, because such mutants would be unlikely to provide information on gH/gL function in membrane fusion. HSV-1 gL residues 162 to 224 (including β-strand residues 197 to 203) have been deleted in previous studies without resulting in a reduction in gH/gL trafficking and function (7, 18, 19). Therefore, we focused on a mutational analysis of the HSV-1 gL amino terminus.
To facilitate an initial deletion mutagenesis of the gL amino terminus downstream of the signal sequence, we introduced an EcoRI restriction endonuclease site by a substitution mutation at nucleotides 77 and 78 (numbering starting with initiator ATG; GenBank accession number U53683) into the gL expression plasmid pMN116 (20). This mutation resulted in a conservative tyrosine-to-phenylalanine change at residue 26, Y26F (numbering starting with the initiator methionine; GenBank accession number AAA99790), that did not reduce gH/gL trafficking or HSV-1 glycoprotein-induced membrane fusion compared to the HSV-1(KOS) gL parent (Table 1). The resulting plasmid, pgLRI, was used to construct the deletion mutants shown in Fig. 1 by using PCR amplification and insertion into the EcoRI site. The deletion mutants were named to indicate the amino acids deleted, such that Δ27-34 has residues 27 to 34 deleted from gLRI (Fig. 1). We used the QuikChange site-directed mutagenesis system (Agilent Technologies) to produce the mutants in Fig. 1 that contained deletions starting at residue 24 or 26. While sequencing mutant genes used for this study, we discovered an S22P change in all of our mutants and our wild-type HSV-1(KOS) gL gene in pMN116. The proline at position 22 was present in 16 of 22 HSV-1 gL sequences from a variety of isolates for which data are available in GenBank.
TABLE 1.
Activities of gL mutants
| Mutant | Activity relative to wild typea |
|
|---|---|---|
| gH/gL traffickingb | Cell-cell fusion | |
| Y26F | +++++ | +++++ |
| Y26A | +++++ | ++++ |
| Y26E | +++++ | +++ |
| V27A | +++++ | ++++ |
| V27D | +++++ | +++ |
| R29A | +++++ | +++++ |
| R31A | +++++ | +++++ |
| V32A | ++++ | ++++ |
| R34A | +++++ | +++++ |
| E35A | +++++ | +++++ |
| V36A | ++++ | ++++ |
| D38A | +++ | +++ |
| L40A | ++++ | ++++ |
| L40D | ++++ | ++++ |
| K41A | ++++ | ++++ |
| K41E | +++ | +++ |
Result (percentage) relative to wild type gL: +++++, 80 to 100%; ++++, 60 to 79%; +++, 40 to 59%; ++, 20 to 39%; +, 0 to 19%.
gH/gL cell surface expression.
FIG 1.

Amino acid sequence of the HSV-1 gL amino terminus. SP, signal peptide; HA, influenza A virus hemagglutinin epitope tag. Residues are listed by their single-letter amino acid abbreviations. The sequence is from NCBI, accession number AAA99790, starting with the initiator methionine as residue 1. Our sequence differs from that of AAA99790 by a proline at residue 22 rather a serine. Arrows indicate predicted β-strands and the cylinder a predicted α-helix. Numbers above the sequence indicate the amino acid numbers. Underlined residues were singly mutated (listed in Table 1). Deletion mutants listed below the gL sequence with highlighted text represent residues deleted in a particular mutant. An asterisk indicates a Y-to-F mutation that occurred as a result of inserting an EcoRI site into the gL open reading frame in pgLRI.
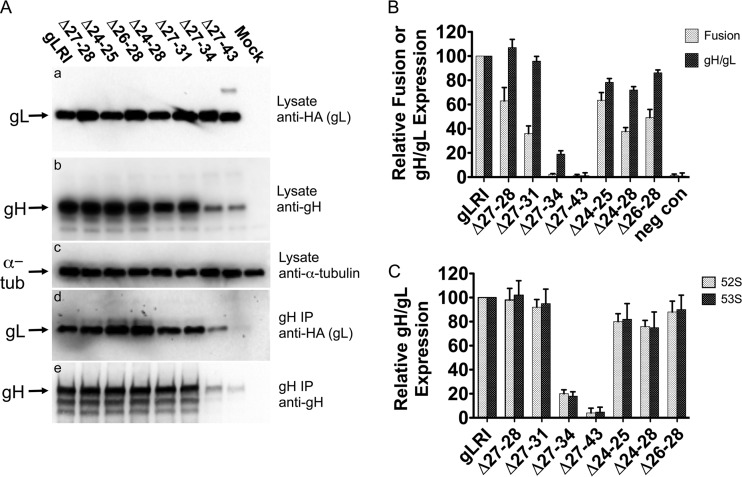
We tested the deletion mutants for their cellular expression and ability to interact with gH by Western blotting and coimmunoprecipitation as previously described (7, 18). The mutants were coexpressed with gH in Chinese hamster ovary (CHO) cells, and after 2 days lysates were obtained. The lysates were analyzed by Western blotting for gL expression by using a monoclonal antibody to detect the hemagglutinin (HA) epitope tag at the carboxy terminus of all forms of gL (Fig. 2A, panel a) and for gH expression by using rabbit R137 anti-gH/gL serum (provided by R. Eisenberg and G. Cohen, University of Pennsylvania) (19) (Fig. 2A, panel b). Another portion of the lysate was immunoprecipitated with the 52S anti-gH monoclonal antibody and analyzed for the presence of gL by Western blotting by using an HA monoclonal antibody (Fig. 2A, panel d) and for gH by using R137 antiserum (Fig. 2A, panel e). The results showed that all deletion mutants were expressed in cells and all interacted with gH. However, there was a decrease in the level of the Δ27-34 and the Δ27-43 mutants that coimmunoprecipitated with gH (Fig. 2A, panel d). The decrease corresponded to a decrease in gH precipitated by 52S (Fig. 2A, panel e) and a decrease in overall gH expression in Δ27-34- and Δ27-43-coexpressing cells (Fig. 2A, panel b). If the Δ27-34 and Δ27-43 mutants were to fail to associate appropriately with gH, there could be an increase in gH degradation to account for this reproducible reduction in gH coexpression. A similar reduction in overall gH levels in cells coexpressing gL mutants that do not interact with gH has been previously reported (21).
FIG 2.
HSV-1 gH/gL deletion mutant expression, coimmunoprecipitation, and function in cell-cell fusion. (A) Cells were transfected with plasmids expressing genes for gH and gLRI or one of the deletion mutants. After 48 h, the cells were processed for Western blotting with the following antibodies: (a) an anti-HA monoclonal antibody to detect wild-type or mutant gL expression; (b) R137 rabbit antiserum to detect gH expression; (c) anti-alpha-tubulin monoclonal antibody to monitor alpha-tubulin expression as a control. In parallel, the lysates from the transfected cells listed above were immunoprecipitated (IP) with anti-gH monoclonal antibody 52S, and the resulting immunoprecipitates were subjected to Western blotting with the following antibodies: (d) anti-HA monoclonal antibody to detect wild-type or mutant gL associated with gH; (e) R137 rabbit antiserum to detect levels of immunoprecipitated gH. Antibody binding to blots was detected using chemiluminescence. Mock cells were transfected with a plasmid expressing a negative control protein, angiotensin-converting enzyme 2. This experiment was performed three independent times, and the results of one representative replicate are shown. (B) Cell-cell fusion and gH/gL cell surface expression for gL mutants. CHO cells expressing HSV-1 gB, gD, or gH or T7 polymerase and either gLRI, a gL deletion mutant, or pcDNA3 (negative control [neg con]) were mixed with CHO cells expressing nectin-1 and the T7 polymerase-inducible β-Gal. The microgram ratio, gB:gD:gH:gL:T7, for transfected expression plasmids was 1:1:1:0.13:1. The β-Gal activity was measured as an indication of cell-cell fusion. Cell surface gH/gL expression was measured in envelope glycoprotein-expressing cells via CELISA with the anti-gH 52S monoclonal antibody. Mean relative values and mean standard deviations from at least three independent experiments are shown. Within each experiment, values are expressed as the percentage of the results of the transfection expressing gLRI. There was a significant difference between the mean cell fusion activity for gLRI and Δ27-28 (P = 0.015) and for gLRI and Δ27-31 (P = 0.0009), as determined by using a two-tailed unpaired t test. (C) Monoclonal antibody binding to gH in the presence of gL or one of the deletion mutants. CHO cells expressing gH and either gLRI or a deletion mutant were analyzed for cell surface gH/gL expression via CELISA. Two anti-gH monoclonal antibodies were used in parallel, 52S or 53S. Mean relative values and mean standard deviations from at least three independent experiments are shown. For each experiment, values are expressed as a percentage of the transfection expressing gLRI.
We next tested the deletion mutants for the ability to mediate gH/gL cell surface expression and for contributions to cell fusion induced by HSV-1 fusion glycoproteins. Each deletion mutant was separately expressed with HSV-1 gH, gB, gD, or T7 RNA polymerase in CHO cells, and these fusion effector cells were mixed with CHO cells expressing the gD receptor nectin-1 and T7-inducible β-galactosidase (β-Gal), largely as previously described (7, 18). CHO cells are resistant to membrane fusion induced by HSV-1 glycoproteins unless a gD receptor is expressed. The mixed cells were incubated overnight, and β-Gal activity was measured as an indication of the extent of cell-cell fusion. Because gH/gL cell surface expression is a key parameter for HSV cell-cell fusion, we also examined the ability of each deletion mutant to mediate heterodimer trafficking to the cell surface by performing a cell-based enzyme-linked immunosorbent assay (CELISA) on the fusion effector cells in parallel with the cell-cell fusion assay, as previously described (7, 18). The CELISA was performed by incubating the fusion effector cells with the anti-gH monoclonal antibody 52S, and the level of gH/gL cell surface expression was determined by measuring the level of bound 52S. The results are shown in Fig. 2B. For the deletion mutants starting at residue 27, as the deletions grew in size there was a progressive loss of cell fusion activity compared to gLRI. Cell fusion experiments with the Δ27-28 mutant showed a 40% reduction, Δ27-31 showed a >60% reduction, and there was no fusion activity observed for mutants Δ27-34 and Δ27-43. Interestingly, the Δ27-28 and Δ27-31 mutants mediated heterodimer cell surface expression similarly to that seen with gLRI, indicating that the defect in cell-cell fusion is not due to defects in heterodimer trafficking. The Δ27-34 and Δ27-43 mutants, however, displayed 80% and 100% reductions, respectively, in heterodimer cell surface expression, which may be expected because of a reduction in gH coexpression and possible defects in gH association compared to gLRI (Fig. 2A). The coexpression of the Δ27-34 mutant with gH consistently yielded heterodimer cell surface expression that was approximately 20% of that for gLRI and gH, yet Δ27-34 did not function in cell-cell fusion (Fig. 2B). We wondered if the Δ27-34 mutant was not producing enough cell surface heterodimer to allow cell-cell fusion, or if gH/gLΔ27-34 was defective in promoting cell-cell fusion. We reduced the amount of pgLRI used in the cell-cell fusion assay mixture until the gH/gL cell surface expression was approximately 20% of the normal level (equivalent to the level with gH/gLΔ27-34) and cell-cell fusion was clearly observed (data not shown). Therefore, we believe the gH/gLΔ27-34 heterodimer at the cell surface is defective in cell-cell fusion. The deletion mutants starting at residue 24 also displayed a disproportionate reduction in cell-cell fusion activity compared to gH/gL cell surface expression (Fig. 2B). The results were most clear for the Δ24-28 and Δ26-28 mutants, for which there were 10 to 20% reductions in gH/gL cell surface expression levels but correspondingly 50 to 60% reductions in cell-cell fusion. Taken together, the results for the deletion mutants indicate that gL residues 24 to 31 are important for the role gH/gL plays in HSV-1 glycoprotein-induced membrane fusion, and these residues are not required for heterodimer trafficking.
A study analyzing HSV-1 gL linker insertion mutants noted that some of the mutants altered the monoclonal antibody binding profile and, most likely the conformation, of the corresponding gH/gL heterodimers (21). The monoclonal antibody 53S binds the gH/gL heterodimer but not gH when expressed alone, while the 52S monoclonal antibody binds gH regardless of gL binding (22, 23). The mutant with the linker insertion nearest the gL amino terminus, residue 48, shows a marked defect in the ability to confer 53S binding (21). The 5-amino-acid insertion at residue 48 could affect gH/gL interactions and gH folding to reduce 53S binding and cell fusion, but not gH/gL trafficking. We analyzed our gL amino-terminal deletion mutants for their ability to promote 53S binding when coexpressed with gH to see if the altered 53S binding was a feature of all heterodimers containing amino-terminal gL mutants or all heterodimers that have defects in fusion independent of trafficking. CHO cells transiently expressing gH and gLRI or one of the gL deletion mutants were analyzed by CELISA for cell surface binding of 52S versus 53S monoclonal antibodies. The results in Fig. 2C show no relative difference in binding of 53S compared to 52S for any of our gL deletion mutants coexpressed with gH. Therefore, the gH/gL heterodimers described here featuring a gL amino-terminal mutant and defects in cell fusion did not show reduced 53S binding.
To identify specific residues in the gL amino terminus involved in the defect in cell-cell fusion, we generated point mutations in many of the residues between amino acids 26 and 41 (Fig. 1 and Table 1) by using the QuikChange site-directed mutagenesis system. The gH/gL trafficking as determined by CELISA and cell-cell fusion data is summarized in Table 1. Most of the mutants promoted heterodimer cell surface expression and cell-cell fusion with similar efficiencies (results for a few are shown in Fig. 3). However, mutants resulting from substitutions at two positions displayed a reduction in cell-cell fusion without an effect on gH/gL trafficking (Fig. 3 and Table 1). The Y26A mutant resulted in a slight reduction in cell-cell fusion, while the Y26E mutant showed a nearly 50% reduction in cell-cell fusion without affecting gH/gL cell surface levels. A similar phenotype was observed for substitutions at residue 27, with a slight reduction in cell-cell fusion for the V27A mutant while the V27D mutant yielded a greater reduction in cell fusion (>40%) without affecting gH/gL trafficking. Residues Y26 and V27 are important for the gH/gL contribution to membrane fusion. Both residues are 100% conserved in over 20 HSV-1 gL sequences and 4 HSV-2 sequences (data not shown).
FIG 3.

Cell-cell fusion and gH/gL cell surface expression activity for wild-type HSV-1 (KOS) gL and gL point mutants. CHO cells expressing HSV-1 gB, gD, gH, T7 polymerase and either HSV-1 (KOS) gL (wild type), a gL point mutant, or pcDNA3 (negative control [neg con]) were mixed with CHO cells expressing nectin-1 and the T7 polymerase-inducible β-Gal. The microgram ratio for gB:gD:gH:gL:T7 transfected expression plasmids was 1:1:1:0.13:1. The β-Gal activity was measured as an indication of cell-cell fusion. Cell surface gH/gL expression was measured in envelope glycoprotein-expressing cells via CELISA with the anti-gH 52S monoclonal antibody. Mean relative values and mean standard deviations from at least three independent experiments are shown. Within each experiment, values are expressed as the percentage of the transfection expressing wild-type KOS gL. There was a significant difference between the mean cell fusion activity for gLRI and that for Y26A (P = 0.041), Y26E (P = 0.0016), V27A (P = 0.024), and V27D (P = 0.0032) when the mean activity for each mutant was separately compared to that for gLRI by using a two-tailed unpaired t test.
As part of this report examining the gH/gL heterodimer for gL residues that are exposed or project outward, we identified five gH residues that appeared to have side chains projecting outward from the top or crown of H1A in a structural model of HSV-1 gH/gL (Fig. 4A and B) based upon the HSV-2 crystal structure (16). We generated R49A, N73A, L81A, and R83A single-gH mutants and constructed a double mutant with E87A and R88A. None of the substitution mutations had a deleterious effect on gH/gL cell surface expression or cell-cell fusion induced by HSV-1 glycoproteins (data not shown).
FIG 4.

Homology model of HSV-1 gH/gL. (A) gH/gL heterodimer. gH domains: H1A (red), H1B (yellow), H2 (green), H3 (orange), gL (white). The dashed white circle highlights domain H1 and gL. (B) Domain H1 and gL. Residues where single-substitution mutations were created are indicated. (C) Amino acid residues within 4 Å of tyrosine 26 and valine 27 are shown at the β1- and β2-strand regions. Each residue is colored to indicate its hydrophobic (green), polar (cyan), acidic (red), or basic (blue) property.
The HSV-2 crystal structure shows two β-strands (β1 and β2) near the gL amino terminus (16), both of which are located on the solvent-exposed surface. The β1-strand formed by gL residues 26 to 28 assembles into a small β-sheet with two other gL β-strands, β3 and β7. The β2 strand (residues 31 and 32) forms a β-sheet with three other gL β-strands (β4, β5, and β6) and two β-strands from gH (16). Point mutations in the HSV-1 version of the β1-strand (residues 26 and 27) reduce cell-cell fusion by HSV-1 glycoproteins without affecting gH/gL cell surface expression. Deletion of part or all of the β1-strand (Δ24-28, Δ26-28, Δ27-28, and Δ27-31) reduces cell-cell fusion with no or minor effects on gH/gL trafficking. The Δ27-31 mutant deletes portions of the putative β1- and β2-strands and displays the greatest reduction in cell-cell fusion without affecting gH/gL cell surface expression, suggesting that the β2-strand may also be important for cell-cell fusion.
To better rationalize the effects of the substitution mutations based on their specific locations on the protein structure, we constructed a homology model of the HSV-1 gH/gL (NCBI accession numbers NP_044623 for gH and NP_044602 for gL) based on the X-ray structure of HSV-2 gH/gL (16), by using previously described methods (24, 25). The modeling was done using the Prime module of the Schrodinger modeling package. Alignment of the HSV-1 and -2 nucleotide sequences revealed sequence identities of 75% and 80% for gL and gH, respectively (data not shown). All missing side chains and internal loops were first homology modeled to generate the most complete HSV-1 gH/gL model template. The HSV-1 gH/gL homology model was refined by restraint energy minimization and molecular dynamics simulation with an OPLS-AA 2005 force field (26) using the generalized Born implicit solvent model (27) to remove any possible remaining steric clashes. The final model was further subjected to a 45-nanosecond molecular dynamics simulation in explicit transferable intermolecular potential 3P solvent under constant pressure and temperature conditions within periodic boundary conditions for consistent long-range electrostatic energy and forces, solvent effects, and protein flexibility, by using the Desmond software package. The overall α-carbon (Cα) root mean square deviation was 3.1Å for the model, compared to the original X-ray structure of HSV-2 gH/gL at 3.0 Å resolution. The final model consisted of HSV-1 gH residues 49 to 797 and gL residues 23 to 203, with all secondary structure and disulfide bridge assignments conserved (Fig. 4). Examination of gL Y26 and V27 via the HSV-1 homology model showed both residues are located within a hydrophobic cluster consisting of I28, L52, W54, Y56, P196, P197, I198, and A200, with only the hydroxyl group of Y26 solvent exposed (Fig. 4C). It is likely that hydrophobicity is more important than the presence of the exposed hydroxyl group, because a phenylalanine substitution into that position, Y26F, did not affect function (Table 1). Since both Y26 and V27 are mostly buried, the mutation to alanine may not have a large effect on the surface topology of the hydrophobic cluster, while a mutation to acidic residues Y26E or Y27D is likely to have a more disruptive effect on the integrity and the surface packing of that region.
An unresolved question is how do mutations at the HSV-1 gL amino terminus affect the contribution of gH/gL to cell-cell fusion? One possibility is that the mutations disrupt protein-protein interactions important for membrane fusion. These interactions could be with a viral protein other than gH, or with a cellular protein. However, no direct evidence has yet been published regarding any interaction of HSV-1gL with any protein other than gH. Mutation of the gL amino terminus could disrupt protein-protein interactions within the gH/gL heterodimer itself. The resulting mutant heterodimer would be competent for trafficking but not for fusion. There is evidence for an interaction between the HSV-1 gL amino terminus and gH. Antibodies prepared against the HSV-1 gL amino terminus do not bind gL in the presence of gH (5, 28), suggesting a region of the gH/gL heterodimer blocks antibody access. Unfortunately, the HSV-2 gH/gL crystal structure is missing two major regions that could be positioned near the gL amino terminus, the gH amino terminus (residues 23 to 31) and the gL carboxy terminus (residues 166 to 196) (16). If the gH and gL amino termini interact, then the disruption of that interaction via mutagenesis could hinder the gH/gL contribution to fusion. The gH amino terminus can be deleted with very little effect on fusion, yet the region is clearly important because monoclonal antibodies binding it can block fusion (14, 29, 30). The region is currently thought to be flexible and must shift its conformation for gH/gL to contribute to fusion (14). This suggests that the gH amino terminus is not directly involved in the fusion process but covers a region of gH/gL that is. Deletion of the gH amino terminus exposes the gL carboxy terminus (14). The gL carboxy terminus can also be deleted without affecting fusion (7), but monoclonal antibodies to the region can block fusion, indicating some role in the HSV fusion process (14, 20, 30). Our work described here has identified the gL amino terminus as another region of gH/gL involved in HSV-1-mediated membrane fusion, and further study is required to understand how these various regions help mediate the gH/gL contribution to viral fusion events.
ACKNOWLEDGMENTS
We thank Sravanthi Kolla for technical assistance and the University of Minnesota Supercomputing Institute for providing the computational resources. We also thank R. Eisenberg and G. Cohen for the gift of the R137 antiserum.
These studies were supported in part by Public Health Service grants AI051476 (R.J.G.) and AI078079 (R.J.G.) from the National Institute of Allergy and Infectious Diseases and also by the Center for Drug Design.
Footnotes
Published ahead of print 23 October 2013
REFERENCES
- 1.Muggeridge MI. 2000. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J. Gen. Virol. 81:2017–2027 http://vir.sgmjournals.org/content/81/8/2017.long [DOI] [PubMed] [Google Scholar]
- 2.Pertel PE, Fridberg A, Parish ML, Spear PG. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313–324. 10.1006/viro.2000.0713 [DOI] [PubMed] [Google Scholar]
- 3.Turner A, Bruun B, Minson T, Browne H. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubin G, Jiang H. 1995. Expression of herpes simplex virus type 1 glycoprotein L (gL) in transfected mammalian cells: evidence that gL is not independently anchored to cell membranes. J. Virol. 69:4564–4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchinson L, Browne H, Wargent V, Davis-Poynter N, Primorac S, Goldsmith K, Minson AC, Johnson DC. 1992. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J. Virol. 66:2240–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browne H, Bell S, Minson T, Wilson DW. 1996. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J. Virol. 70:4311–4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klyachkin YM, Stoops KD, Geraghty RJ. 2006. Herpes simplex virus type 1 glycoprotein L mutants that fail to promote trafficking of glycoprotein H and fail to function in fusion can induce binding of glycoprotein L-dependent anti-glycoprotein H antibodies. J. Gen. Virol. 87:759–767 http://vir.sgmjournals.org/content/87/4/759.long [DOI] [PubMed] [Google Scholar]
- 8.Galdiero S, Falanga A, Vitiello M, Browne H, Pedone C, Galdiero M. 2005. Fusogenic domains in herpes simplex virus type 1 glycoprotein H. J. Biol. Chem. 280:28632–28643. 10.1074/jbc.M505196200 [DOI] [PubMed] [Google Scholar]
- 9.Galdiero S, Falanga A, Vitiello M, D'Isanto M, Collins C, Orrei V, Browne H, Pedone C, Galdiero M. 2007. Evidence for a role of the membrane-proximal region of herpes simplex virus type 1 glycoprotein H in membrane fusion and virus inhibition. Chembiochem 8:885–895. 10.1002/cbic.200700044 [DOI] [PubMed] [Google Scholar]
- 10.Gianni T, Martelli PL, Casadio R, Campadelli-Fiume G. 2005. The ectodomain of herpes simplex virus glycoprotein H contains a membrane α-helix with attributes of an internal fusion peptide, positionally conserved in the herpesviridae family. J. Virol. 79:2931–2940. 10.1128/JVI.79.5.2931-2940.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gianni T, Menotti L, Campadelli-Fiume G. 2005. A heptad repeat in herpes simplex virus 1 gH, located downstream of the alpha-helix with attributes of a fusion peptide, is critical for virus entry and fusion. J. Virol. 79:7042–7049. 10.1128/JVI.79.11.7042-7049.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gianni T, Piccoli A, Bertucci C, Campadelli-Fiume G. 2006. Heptad repeat 2 in herpes simplex virus 1 gH interacts with heptad repeat 1 and is critical for virus entry and fusion. J. Virol. 80:2216–2224. 10.1128/JVI.80.5.2216-2224.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanian RP, Geraghty RJ. 2007. Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc. Natl. Acad. Sci. U. S. A. 104:2903–2908. 10.1073/pnas.0608374104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atanasiu D, Cairns TM, Whitbeck JC, Saw WT, Rao S, Eisenberg RJ, Cohen GH. 2013. Regulation of herpes simplex virus gB-induced cell-cell fusion by mutant forms of gH/gL in the absence of gD and cellular receptors. mBio 4(2):e00046–13. 10.1128/mBio.00046-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atanasiu D, Saw WT, Cohen GH, Eisenberg RJ. 2010. Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J. Virol. 84:12292–12299. 10.1128/JVI.01700-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdary TK, Cairns TM, Atanasiu D, Cohen GH, Eisenberg RJ, Heldwein EE. 2010. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat. Struct. Mol. Biol. 17:882–888. 10.1038/nsmb.1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson JO, Longnecker R. 2010. Reevaluating herpes simplex virus hemifusion. J. Virol. 84:11814–11821. 10.1128/JVI.01615-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klyachkin YM, Geraghty RJ. 2008. Mutagenic analysis of herpes simplex virus type 1 glycoprotein L reveals the importance of an arginine-rich region for function. Virology 374:23–32. 10.1016/j.virol.2007.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng T, Ponce de Leon M, Novotny MJ, Jiang H, Lambris JD, Dubin G, Spear PG, Cohen GH, Eisenberg RJ. 1998. Structural and antigenic analysis of a truncated form of the herpes simplex virus glycoprotein gH-gL complex. J. Virol. 72:6092–6103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novotny MJ, Parish ML, Spear PG. 1996. Variability of herpes simplex virus 1 gL and anti-gL antibodies that inhibit cell fusion but not viral infectivity. Virology 221:1–13. 10.1006/viro.1996.0347 [DOI] [PubMed] [Google Scholar]
- 21.Fan Q, Lin E, Spear PG. 2009. Insertional mutations in herpes simplex virus type 1 gL identify functional domains for association with gH and for membrane fusion. J. Virol. 83:11607–11615. 10.1128/JVI.01369-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gompels UA, Minson AC. 1986. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology 153:230–247. 10.1016/0042-6822(86)90026-7 [DOI] [PubMed] [Google Scholar]
- 23.Gompels UA, Minson AC. 1989. Antigenic properties and cellular localization of herpes simplex virus glycoprotein H synthesized in a mammalian cell expression system. J. Virol. 63:4744–4755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang J, Maddali K, Pommier Y, Sham YY, Wang Z. 2010. Scaffold rearrangement of dihydroxypyrimidine inhibitors of HIV integrase: docking model revisited. Bioorg. Med. Chem. Lett. 20:3275–3279. 10.1016/j.bmcl.2010.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Sham YY, Rajamani R, Gao J, Portoghese PS. 2005. Homology modeling and molecular dynamics simulations of the mu opioid receptor in a membrane-aqueous system. Chembiochem 6:853–859. 10.1002/cbic.200400207 [DOI] [PubMed] [Google Scholar]
- 26.Jorgensen WL, Maxwell DS, Tirado-Rives J. 1996. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118:11225–11236 [Google Scholar]
- 27.Ghosh A, Rapp CS, Friesner RA. 1998. Generalized Born model based on a surface integral formulation. J. Phys. Chem. B 102:10983–10990 [Google Scholar]
- 28.Roop C, Hutchinson L, Johnson DC. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 67:2285–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cairns TM, Friedman LS, Lou H, Whitbeck JC, Shaner MS, Cohen GH, Eisenberg RJ. 2007. N-terminal mutants of herpes simplex virus type 2 gH are transported without gL but require gL for function. J. Virol. 81:5102–5111. 10.1128/JVI.00097-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cairns TM, Shaner MS, Zuo Y, Ponce-de-Leon M, Baribaud I, Eisenberg RJ, Cohen GH, Whitbeck JC. 2006. Epitope mapping of herpes simplex virus type 2 gH/gL defines distinct antigenic sites, including some associated with biological function. J. Virol. 80:2596–2608. 10.1128/JVI.80.6.2596-2608.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]