Abstract
Infections with hemorrhagic fever viruses are characterized by increased permeability leading to capillary leakage. Hantavirus infection is associated with endothelial dysfunction, and the clinical course is related to the degree of vascular injury. Circulating endothelial progenitor cells (cEPCs) play a pivotal role in the repair of the damaged endothelium. Therefore, we analyzed the number of cEPCs and their mobilizing growth factors in patients suffering from hantavirus disease induced by infection with Puumala virus. The numbers of EPCs of 36 hantavirus-infected patients and age- and gender-matched healthy controls were analyzed by flow cytometry. Concentrations of cEPC-mobilizing growth factors in plasma were determined by enzyme-linked immunosorbent assay. Laboratory parameters were correlated with the number of cEPCs. In patients infected with hantavirus, the number of cEPCs was significantly higher than that in healthy controls. Levels of mobilizing cytokines were upregulated in patients, and the mobilization of cEPCs is paralleled with the normalization of clinical parameters. Moreover, higher levels of cEPCs correlated with higher serum albumin levels and platelet concentrations. Our data indicate that cEPCs may play a role in the repair of hantavirus-induced endothelial damage, thereby influencing the clinical course and the severity of symptoms.
INTRODUCTION
Viral hemorrhagic fevers are characterized by severe endothelial dysfunction that often impairs the functions of multiple organs (1–3). Pathogens affect endothelial cells directly by infection and replication, or the function of cells lining the vessels is impaired indirectly via immune-mediated effects (4–6). Endothelial dysfunction results in disturbed permeability. The consequences of capillary leakage are hemorrhages, edema, hypotension, and organ failure. Pathogen-induced loss of endothelial function has a major impact on the clinical course and outcome of the infection. The control of the passage of ions, water, and molecules requires an intact endothelial monolayer. Maintenance and restoration of alterations of endothelial integrity are crucial processes for proper endothelial function. Detached endothelial cells may be replaced by the proliferation and migration of adjacent endothelial cells. Another mechanism of repair of the injured endothelium is the mobilization and differentiation of bone marrow-derived progenitor cells to endothelial cells. Endothelial progenitor cells (EPCs) contribute to vasculogenesis and reendothelialization by differentiation toward endothelial cells (7, 8). Upregulation of cytokines induces the release of EPCs from the bone marrow. Several growth factors, such as angiopoietin 1 (Ang-1) and Ang-2, vascular endothelial growth factor (VEGF), stroma-derived factor 1α (SDF-1α), granulocyte-macrophage colony-stimulating factor, granulocyte colony-stimulating factor, and erythropoietin (EPO), have been identified as crucial in the mobilization and proliferation of circulating EPCs (cEPCs) (9–16). The list of proteins, drugs, and behavioral factors that influence the complex process of mobilization of EPCs is increasing. Estrogen and physical exercise elevate the number of EPCs, whereas increasing age, diabetes, and smoking are associated with the depletion of cEPCs (17–21). Furthermore, drugs for the treatment of cardiovascular pathologies, such as statins and angiotensin-converting enzyme (ACE) inhibitors positively modulate EPC levels, thereby improving reendothelialization (22–24). The role of EPCs in different pathological conditions with vascular alteration and their impact on the outcome have been described in many studies, and their potential use as a predictive marker and as a therapeutic approach is under investigation (25–29). However, in contrast to the field of cardiovascular research, the significance of EPCs in the repair of endothelial damage in infectious diseases is not well characterized thus far. Many infectious diseases are associated with endothelial damage leading to organ failure. For example, multiple organ failure in sepsis is a consequence of altered endothelial function due to infection and the subsequent host response. Recently, several studies have identified the key role of EPCs in the outcome of severe sepsis (30–32). The number of cEPCs in septic patients correlates with survival and inversely with the severity of the clinical course.
Hantaviruses are members of the Bunyaviridae family. Pathogenic hantaviruses are transmitted to humans via inhalation of aerosols that contain virions because of contamination with rodent excreta. Hantavirus disease is characterized by capillary leakage and is manifested predominantly in the lung (hantavirus cardiopulmonary syndrome [HCPS]) or the kidney (hemorrhagic fever with renal syndrome [HFRS]). The severity of infection varies among the different hantaviruses. Furthermore, the clinical picture differs between individuals and ranges from asymptomatic infection to severe courses with a fatal outcome. Hallmarks of hantavirus disease are increased vascular permeability and thrombocytopenia. Common signs of hantavirus-induced vascular dysfunction are hypotension, hypoalbuminemia, hemorrhages, pleural effusion, and edema (33, 34). Although hantaviral disease shows high variability in the intensity and manifestation of symptoms, endothelial dysfunction seems to be the common underlying mechanism of hantavirus infection. Viral replication and immune-mediated effects both contribute to tissue damage in hantavirus infection, and the mechanisms of regenerative repair are not known (35–42). Therefore, we analyzed the role of EPCs in the clinical course of hantavirus infection.
MATERIALS AND METHODS
Study design.
Patients with serologically confirmed Puumala virus infection (n = 36) were included. All patients met the case definition of acute hantavirus infection of the German Robert Koch Institute (43). For a control, we recruited an age- and gender-matched group of 29 healthy volunteers. Clinical data were collected through a review of medical charts of the Department of Nephrology. Neither patients nor controls were treated with statins or ACE inhibitors. This study was approved by the Ethics Committee of the University of Heidelberg, Heidelberg, Germany, and it adhered to the Declaration of Helsinki. Written informed consent was obtained from all of the participants.
Isolation of peripheral blood mononuclear cells (PBMCs) and flow cytometry.
Fifteen-milliliter samples of blood were collected in EDTA tubes. Blood samples were processed within 1 h after collection. PBMCs were separated from whole blood by density gradient centrifugation with Ficoll-Hypaque. To analyze the expression of cell surface proteins, 1 × 106 cells were incubated with Fc receptor blocking reagent (Miltenyi Biotec). Subsequently, cells were stained with the fluorescently labeled anti-human mouse monoclonal antibodies CD31-allophycocyanin (Miltenyi Biotec), CD34-fluorescein isothiocyanate (BD Biosciences), CD45–peridinin-chlorophyll-protein (BD Biosciences), and CD133-phycoerythrin (Miltenyi Biotec) or with fluorescently labeled, isotype-matched mouse antibodies (Miltenyi Biotec and BD Biosciences) at 4°C for 30 min in the dark. After triple washing, cells were subjected to flow cytometric analysis with a FACSCalibur cytometer (BD Biosciences). Analysis was done in duplicate and with an isotype control for each patient and a healthy control person. Data were processed with BD CellQuest Pro (BD Biosciences) software.
ELISA.
The levels of Ang-2, EPO, SDF-1α, and VEGF in plasma were quantified by Quantikine enzyme-linked immunosorbent assay (ELISA; R&D Systems). Assays were performed according to the manufacturer's instructions.
Statistical analysis.
Medians of clinical parameters of two groups were compared with the Mann-Whitney U test, and P values of <0.05 were considered significant. Normal distribution was tested with the Kolmogorov-Smirnoff test. Correlation was assessed by calculating Spearman's correlation coefficients.
RESULTS
Patient characteristics.
A total of 36 patients (10 women, 26 men) with acute kidney injury induced by hantavirus infection were included in this study. Their ages ranged from 21 to 70 years. Twenty-nine volunteers (11 women, 18 men) with ages ranging from 23 to 64 years were included as an age-matched healthy control group. Patients infected with hantavirus showed the characteristic initial flu-like symptoms with abdominal pain, nausea, vomiting, headache, fever, and typical signs of vascular damage (hypotension, pleural effusion, pulmonary congestion). Laboratory analysis revealed increased levels of leukocytes, C-reactive protein (CRP), and lactate dehydrogenase (LDH) and decreased serum albumin and platelet levels (Table 1). Renal involvement was revealed by elevated serum creatinine levels, proteinuria, and hematuria. Hemodialysis treatment was not required for any patient. The findings indicate an increased permeability of the vasculature due to endothelial damage in hantavirus-infected patients.
TABLE 1.
Characteristics and mean peak and nadir levels of laboratory parameters of 36 patients with serologically confirmed hantavirus infection during hospitalization
| Characteristic | Mean value ± SD |
|---|---|
| Age (yr) | 42.78 ± 14.16 |
| Duration of hospitalization (days) | 7.889 ± 4.471 |
| Max serum creatinine level (mg/dl) | 6.330 ± 2.833 |
| Min serum albumin level (g/liter) | 32.84 ± 3.774 |
| Max leukocyte no. (G/liter) | 10.69 ± 3.172 |
| Min platelet no. (G/liter) | 141.9 ± 97.54 |
| Max LDH activity (U/liter) | 412.8 ± 137.2 |
| Max CRP level (mg/liter) | 63.31 ± 35.34 |
Increased numbers of cEPCs in hantavirus-infected patients.
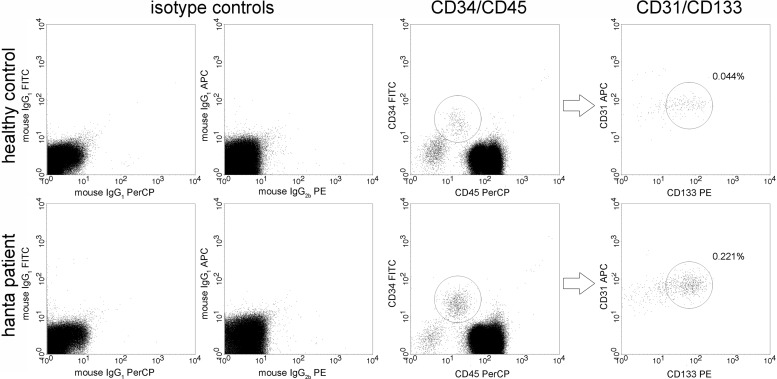
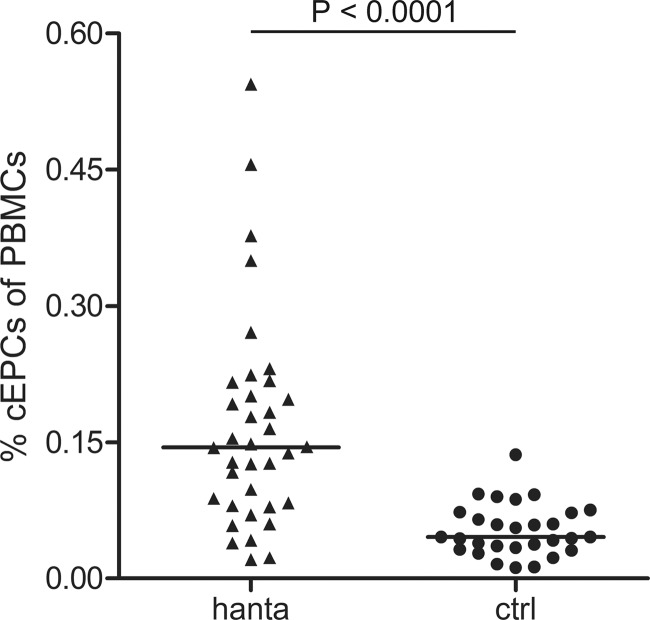
The percentage of cEPCs, defined as cells expressing specific endothelial and hematopoietic progenitor markers, was analyzed by flow cytometry in blood samples of hantavirus-infected patients and healthy control persons. In accordance with the protocol of Duda et al., cEPCs were identified as a population of cells expressing four specific markers (CD31+ CD34+ CD45dim CD133+); cEPCs were positive for CD31 (a marker of monocytes and endothelial cells), CD34 (a marker of hematopoietic progenitor and endothelial cells), and CD133 (a marker of hematopoietic precursor cells) and expressed low levels of CD45 (a marker of leukocyte lineage/leukocyte common antigen) (44). Figure 1 shows representative data from the flow cytometric analysis of blood samples of a healthy control person and a hantavirus-infected patient and demonstrates the gating of cell populations to identify cEPCs. Blood mononuclear cells were analyzed for the presence of CD34 and CD45 and the coexpression of CD31 and CD133. The cEPC levels in hantavirus-infected patients during the acute phase were compared to the number of cEPCs in the age-matched group of healthy control persons (Fig. 2). The median time of cEPC measurement was 10 (range, 5 to 24) days after the onset of symptoms. The analysis revealed that the percentage of cEPCs in the PBMCs of healthy individuals was low. The median percentage of cEPCs in the PBMCs of hantavirus-infected patients (0.145% [range, 0.021 to 0.544%]) was significantly higher than that in the healthy control group (0.046% [range, 0.012 to 0.136%]) (P < 0.0001, two-tailed Mann-Whitney U test). We observed a 3-fold higher level of cEPCs in hantavirus disease patients than in the healthy control group. In septic patients, the number of cEPCs is 2 to 4 times that in healthy controls (31, 32). The increase in cEPC numbers seems to indicate a mobilization of progenitor cells due to hantavirus-induced endothelial damage.
FIG 1.
Representative data from flow cytometric analysis of human PBMCs of a healthy control person (top) and a hantavirus-infected patient (bottom). Human mononuclear cells were separated from whole blood samples and stained for CD31, CD34, CD45, and CD133 or with the respective fluorescently labeled isotype control antibodies. Circulating EPCs were identified as CD31+ CD45dim CD34+ CD133+ cells. FITC, fluorescein isothiocyanate; APC, allophycocyanin; PerCP, peridinin-chlorophyll-protein; PE, phycoerythrin.
FIG 2.
Percentages of circulating EPCs (CD31+ CD45dim CD34+ CD133+ cells) in PBMCs from 36 hantavirus-infected patients (hanta) and 29 age-matched healthy volunteers (ctrl) identified by flow cytometry. Median levels are indicated by horizontal bars. The P value was determined by two-tailed Mann-Whitney U test.
Cytokine levels in hantavirus-infected patients.
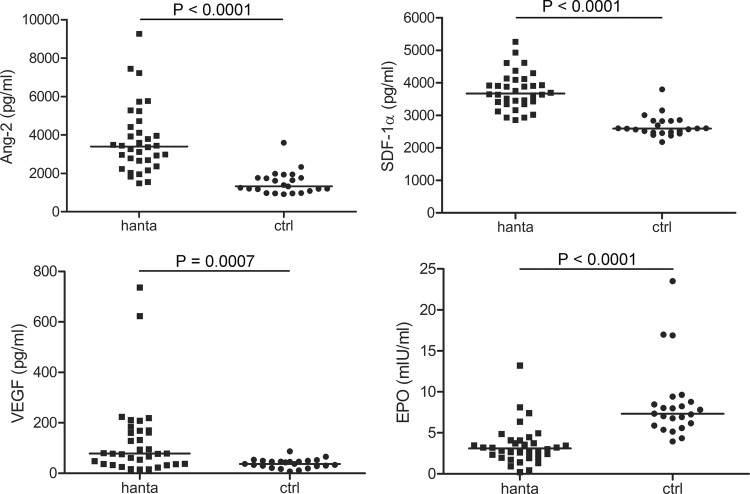
Different cytokines have been found to promote the proliferation and differentiation of circulating EPCs. Levels of cytokines (Ang-2, SDF-1α, VEGF, and EPO) known to play a major role in the mobilization of EPCs were analyzed in plasma samples from hantavirus-infected patients and healthy control subjects (Fig. 3). The analysis revealed that the median levels of Ang-2 (3,400 pg/ml [range, 1,475 to 9,260 pg/ml] versus 1,321 pg/ml [range, 912.5 to 3,588 pg/ml] [P < 0.0001]), SDF-1α (3,672 pg/ml [range, 2,857 to 5,260 pg/ml] versus 2,592 pg/ml [range, 2,178 to 3,799 pg/ml] [P < 0.0001]) and VEGF (78.31 pg/ml [range, 14.76 to 736.3 pg/ml] versus 37.25 pg/ml [range, 7.08 to 87.25 pg/ml] [P = 0.0007]) were higher in the plasma of hantavirus-infected patients than the median levels in the healthy control group. In contrast to the higher levels of Ang-2, SDF-1α, and VEGF, hantavirus-infected patients exhibited lower levels of EPO than healthy controls (3.11 mIU/ml [range, 0.22 to 13.23 mIU/ml] versus 7.33 mIU/ml [range, 3.96 to 23.52 mIU/ml] [P < 0.0001]). EPO deficiency during hantavirus infection probably resulted from acute renal failure and damage to peritubular cells, which are mainly responsible for the production of EPO (45).
FIG 3.
Plasma Ang-2, VEGF, SDF-1α, and EPO levels in 34 hantavirus-infected patients (hanta) and 23 healthy controls (ctrl). Median levels are indicated by horizontal bars. The P values were determined by two-tailed Mann-Whitney U test.
Course of cEPC mobilization.
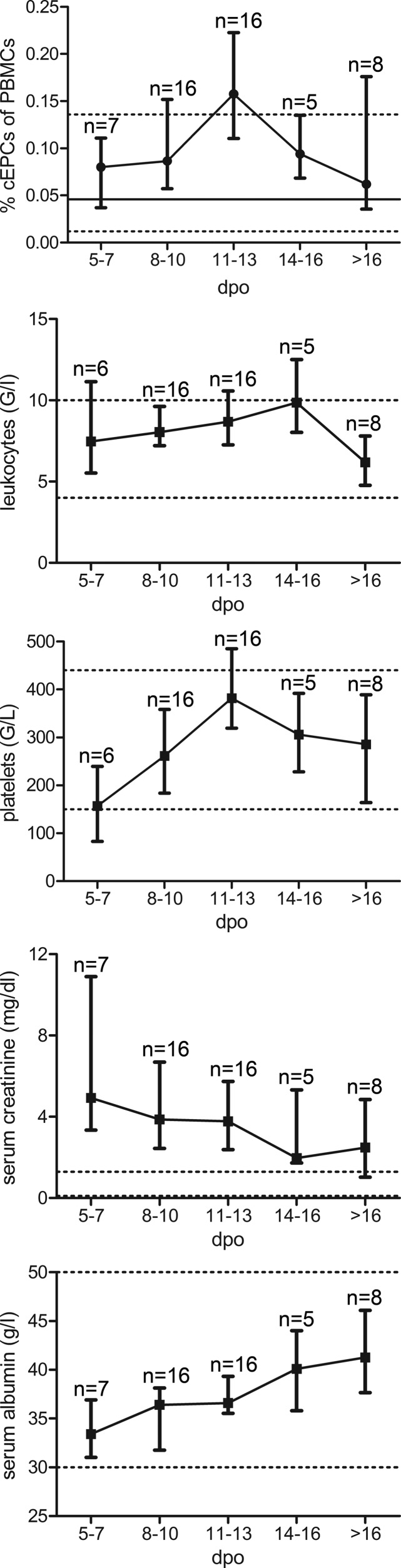
The cEPC number of most patients was measured once during the clinical course and most likely did not reflect the peak level of cEPC mobilization. To analyze the mobilization and role of cEPCs during the course of hantavirus infection in more detail, levels of cEPCs, growth factors, and laboratory parameters were examined over time. Blood samples of hantavirus-infected patients were sampled longitudinally during the acute and recovery phases (>16 days) after symptom onset, and cEPC levels were measured. cEPC numbers increased after symptom onset and decreased rapidly to the baseline level after the peak level was reached. Together with the course of cEPC levels, the courses of various laboratory parameters of patients were analyzed and compared with the course of cEPC numbers (Fig. 4). Leukocyte and serum creatinine levels decreased with the mobilization of cEPCs. Decreased platelet concentrations normalized in parallel with the increase in cEPC numbers. Impaired endothelial barrier function was ameliorated since hypoproteinemia also improved with the mobilization of cEPCs. These findings show that the recovery phase of hantavirus infection starts with the appearance of high levels of cEPCs.
FIG 4.
Time courses of median levels of cEPCs and of laboratory parameters in hantavirus-infected patients after symptom onset. Levels are reported as median values with interquartile ranges. Horizontal lines indicate the median levels of cEPCs (solid) and ranges (dashed) of the healthy control group. Dashed lines indicate reference ranges for healthy adults. dpo, days postonset.
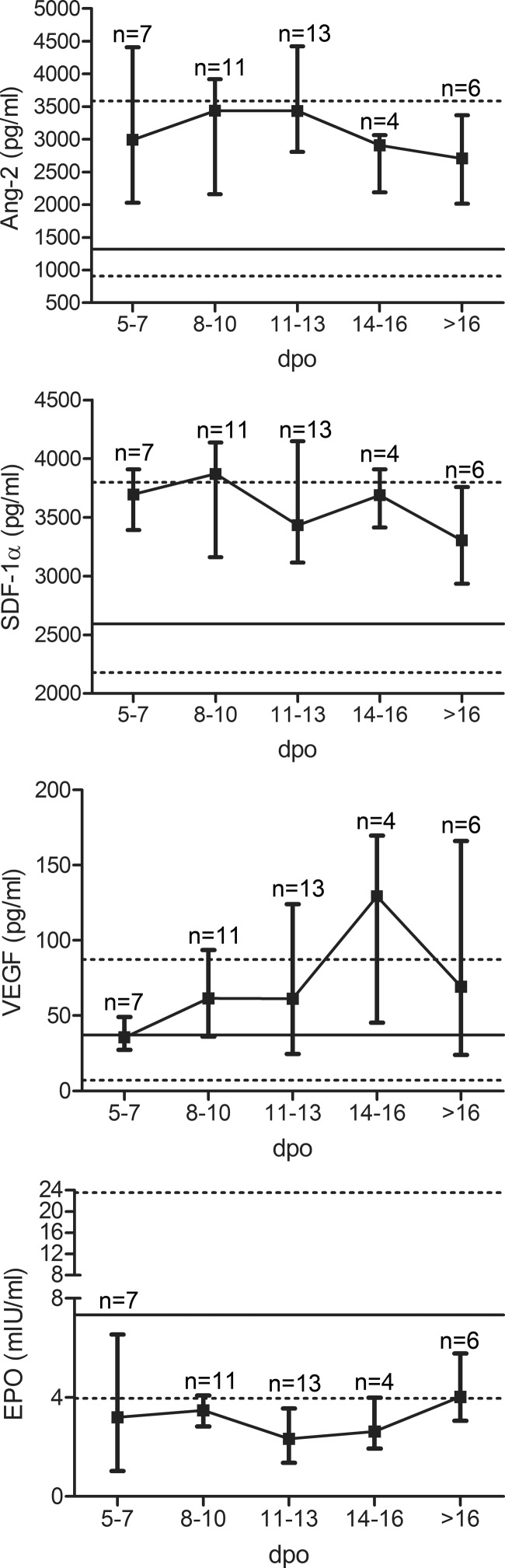
To examine the mobilization of cEPCs, cytokine levels were also analyzed over time (Fig. 5). Levels of Ang-2 and SDF-1α were elevated early in the course of infection. In contrast, levels of VEGF increased later in the clinical course. As mentioned before, EPO levels were lower in hantavirus-infected patients than in control persons. The analysis of EPO levels during the clinical course revealed that levels of EPO increased after the mobilization of cEPCs. We conclude that cEPC-mobilizing cytokines are upregulated during the acute phase of hantavirus infection.
FIG 5.
Time courses of mean levels of cEPCs and the cytokines Ang-2, VEGF, SDF-1α, and EPO in hantavirus-infected patients after symptom onset. Levels are reported as median values with interquartile ranges. Horizontal lines indicate median levels (solid) and ranges (dashed) of healthy control persons. dpo, days postonset.
Correlation of cEPC levels with clinical parameters.
In the next step, we examined the association between cEPC levels and laboratory parameters on the day of cEPC measurement in hantavirus-infected patients (Table 2). During mobilization, levels of cEPCs correlate negatively with CRP levels and positively with serum albumin levels and platelet concentrations. No correlation between cEPCs and age or levels of leukocytes, LDH, or serum creatinine was observed. The correlation between platelet concentrations and serum albumin and cEPC levels indicates the relationship between cEPCs and the repair of vascular damage in hantavirus disease.
TABLE 2.
Correlation between cEPC levels and clinical parameters at day of cEPC measurement in hantavirus-infected patients
| Parameter | ra | CIb | P value |
|---|---|---|---|
| Age (yr) | −0.219 | −0.519–0.127 | 0.1980 |
| CRP level (mg/liter) | −0.510 | −0.734–0.186 | 0.0029 |
| LDH activity (U/liter) | 0.054 | −0.317–0.409 | 0.7745 |
| Leukocyte no. (G/liter) | −0.243 | −0.537–0.103 | 0.1537 |
| Serum creatinine concn (mg/dl) | −0.171 | −0.481–0.177 | 0.3196 |
| Platelet no. (G/liter) | 0.461 | 0.146–0.691 | 0.0047 |
| Serum albumin concn (g/liter) | 0.399 | 0.071–0.649 | 0.0158 |
r, Spearman's correlation coefficient.
CI, 95% confidence interval.
DISCUSSION
Therapy of viral hemorrhagic fever diseases and other viral infections is often limited to the treatment of symptoms because of the lack of a specific antiviral therapy. The clinical picture and severity of infectious diseases vary among different causative agents. However, the common underlying pathogenic mechanisms responsible for the disease are often endothelial damage and increased vascular permeability, which are accompanied by additional pathogen-specific symptoms. In severe infections, capillary leakage leads to hemodynamic alterations that result in hypotension, organ failure, and shock. The broad spectrum of clinical illness, ranging from mild disease to a fatal outcome, is influenced by multiple factors.
Our findings indicate that circulating EPCs may play a role in the clinical course of hantavirus infection. We have shown that the number of circulating EPCs is elevated and that cytokines important in cEPC mobilization are upregulated. The complex process of repair of endothelial injury by progenitor cells is not yet completely understood. Many cytokines are known to be involved in the steps of reendothelialization, namely, mobilization, proliferation, migration, and differentiation. We demonstrate the upregulation of three major determinants that have been found to play a key role in these processes. Levels of Ang-2, VEGF, and SDF-1α are increased during hantavirus infection. Interestingly, levels of Ang-2 and SDF-1α are elevated early in the course of hantavirus infection. In contrast, the VEGF concentration increases at later time points after symptom onset. The effects of the individual cytokines in the course of hantavirus disease and cEPC mobilization, homing, and differentiation remain to be investigated. Cytokines may activate different signaling cascades and cause many effects. VEGF promotes angiogenesis but also induces capillary leakage (46, 47). Previous studies have demonstrated the elevation of VEGF levels in HCPS and HFRS patients (48–51). They observed early and rather localized elevation of VEGF levels and sustained systemic upregulation later in infection. Early in the infection VEGF seems to contribute to hantavirus pathogenesis, whereas VEGF at later time points may contribute to endothelial repair and convalescence (48, 50). Local and systemic effects of cytokines may be involved in pathogenesis and repair (52, 53). The temporospatial regulation of cytokines in hantavirus infection may influence the clinical course and needs further investigation. EPO, a cytokine also important for the mobilization of progenitor cells, is decreased in hantavirus-infected patients. The reason for the downregulation of EPO is the organ-specific manifestation of infections with hantaviruses that cause HFRS. The damage of EPO-producing renal peritubular cells may be responsible for the deregulation of this cytokine (45, 54, 55). Therefore, it would be of great interest to analyze the number of cEPCs and the mobilizing cytokines in infections with New World hantaviruses that cause HCPS. HCPS is characterized by rapidly progressive pulmonary edema, whereas renal involvement is less prominent than in HFRS (56). EPCs may also play a role in the outcome of HCPS. It was shown that an increased number of circulating EPCs is associated with higher survival rates in patients with acute lung injury (57). The mobilization of cEPCs could also be beneficial to patients with HCPS. Furthermore, EPCs probably mediate the repair of endothelial damage in a wide range of infectious diseases. Clinical signs of vascular leakage, such as pleural effusion, ascites, and decreased serum albumin, are common in a wide range of diseases such as viral hemorrhagic fevers (58, 59). Several hemorrhagic fever viruses have been classified as causes of emerging infectious diseases, and the list of viruses that cause organ failure by affecting endothelial function is increasing (60–63). Knowledge of the pathogenesis and epidemiology of these infections has become more and more the focus of multidisciplinary research. As demonstrated here for hantavirus infection, the level of mobilization may be responsible for the clinical course and outcome of other infections that cause severe endothelial damage.
The understanding of the mechanism of endothelial damage and repair will give useful insights into the pathogenesis of hemorrhagic fever diseases that will provide implications for new therapeutic strategies.
ACKNOWLEDGMENTS
This work was supported by a grant from the Bundesministerium für Bildung und Forschung and performed under the umbrella of the Nationalen Forschungsplattform für Zoonosen (grant 01KI1022 to E.K.). N.R. is supported by a research scholarship from the postdoctoral program of the University of Heidelberg.
We thank V. Bollinger for excellent technical assistance, and we thank all of the participants in this study for helping us in this research.
Footnotes
Published ahead of print 23 October 2013
REFERENCES
- 1.Baskerville A, Fisher-Hoch SP, Neild GH, Dowsett AB. 1985. Ultrastructural pathology of experimental Ebola haemorrhagic fever virus infection. J. Pathol. 147:199–209. 10.1002/path.1711470308 [DOI] [PubMed] [Google Scholar]
- 2.Jessie K, Fong MY, Devi S, Lam SK, Wong KT. 2004. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J. Infect. Dis. 189:1411–1418. 10.1086/383043 [DOI] [PubMed] [Google Scholar]
- 3.Schnittler HJ, Mahner F, Drenckhahn D, Klenk HD, Feldmann H. 1993. Replication of Marburg virus in human endothelial cells. A possible mechanism for the development of viral hemorrhagic disease. J. Clin. Invest. 91:1301–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geisbert TW, Young HA, Jahrling PB, Davis KJ, Larsen T, Kagan E, Hensley LE. 2003. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am. J. Pathol. 163:2371–2382. 10.1016/S0002-9440(10)63592-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green S, Rothman A. 2006. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr. Opin. Infect. Dis. 19:429–436. 10.1097/01.qco.0000244047.31135.fa [DOI] [PubMed] [Google Scholar]
- 6.Yang Z, Delgado R, Xu L, Todd RF, Nabel EG, Sanchez A, Nabel GJ. 1998. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science 279:1034–1037. 10.1126/science.279.5353.1034 [DOI] [PubMed] [Google Scholar]
- 7.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. 1999. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 85:221–228. 10.1161/01.RES.85.3.221 [DOI] [PubMed] [Google Scholar]
- 8.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. 1997. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967. 10.1126/science.275.5302.964 [DOI] [PubMed] [Google Scholar]
- 9.Bahlmann FH, De Groot K, Spandau JM, Landry AL, Hertel B, Duckert T, Boehm SM, Menne J, Haller H, Fliser D. 2004. Erythropoietin regulates endothelial progenitor cells. Blood 103:921–926 http://bloodjournal.hematologylibrary.org/content/103/3/921.long [DOI] [PubMed] [Google Scholar]
- 10.Bahlmann FH, DeGroot K, Duckert T, Niemczyk E, Bahlmann E, Boehm SM, Haller H, Fliser D. 2003. Endothelial progenitor cell proliferation and differentiation is regulated by erythropoietin. Kidney Int. 64:1648–1652. 10.1046/j.1523-1755.2003.00279.x [DOI] [PubMed] [Google Scholar]
- 11.Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, Moore MA, Rafii S. 2001. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J. Exp. Med. 193:1005–1014. 10.1084/jem.193.9.1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher AM, Dimmeler S. 2003. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood 102:1340–1346. 10.1182/blood-2003-01-0223 [DOI] [PubMed] [Google Scholar]
- 13.Kim KL, Shin IS, Kim JM, Choi JH, Byun J, Jeon ES, Suh W, Kim DK. 2006. Interaction between Tie receptors modulates angiogenic activity of angiopoietin2 in endothelial progenitor cells. Cardiovasc. Res. 72:394–402. 10.1016/j.cardiores.2006.08.002 [DOI] [PubMed] [Google Scholar]
- 14.Moore MA, Hattori K, Heissig B, Shieh JH, Dias S, Crystal RG, Rafii S. 2001. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann. N. Y. Acad. Sci. 938:36–45; discussion 45–47. 10.1111/j.1749-6632.2001.tb03572.x [DOI] [PubMed] [Google Scholar]
- 15.Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. 2009. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell 4:62–72. 10.1016/j.stem.2008.10.017 [DOI] [PubMed] [Google Scholar]
- 16.Shao H, Tan Y, Eton D, Yang Z, Uberti MG, Li S, Schulick A, Yu H. 2008. Statin and stromal cell-derived factor-1 additively promote angiogenesis by enhancement of progenitor cells incorporation into new vessels. Stem Cells 26:1376–1384. 10.1634/stemcells.2007-0785 [DOI] [PubMed] [Google Scholar]
- 17.Iwakura A, Luedemann C, Shastry S, Hanley A, Kearney M, Aikawa R, Isner JM, Asahara T, Losordo DW. 2003. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation 108:3115–3121. 10.1161/01.CIR.0000106906.56972.83 [DOI] [PubMed] [Google Scholar]
- 18.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, Miche E, Bohm M, Nickenig G. 2004. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 109:220–226. 10.1161/01.CIR.0000109141.48980.37 [DOI] [PubMed] [Google Scholar]
- 19.Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, Laufs K, Ghaeni L, Milosevic M, Bohm M, Nickenig G. 2003. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation 107:3059–3065. 10.1161/01.CIR.0000077911.81151.30 [DOI] [PubMed] [Google Scholar]
- 20.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. 2001. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res. 89:E1–E7. 10.1161/hh1301.093953 [DOI] [PubMed] [Google Scholar]
- 21.Xia WH, Li J, Su C, Yang Z, Chen L, Wu F, Zhang YY, Yu BB, Qiu YX, Wang SM, Tao J. 2012. Physical exercise attenuates age-associated reduction in endothelium-reparative capacity of endothelial progenitor cells by increasing CXCR4/JAK-2 signaling in healthy men. Aging Cell 11:111–119. 10.1111/j.1474-9726.2011.00758.x [DOI] [PubMed] [Google Scholar]
- 22.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. 2001. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J. Clin. Invest. 108:391–397. 10.1172/JCI200113152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, Walsh K, Isner JM, Asahara T. 2001. HMG-CoA reductase inhibitor mobilizes bone marrow-derived endothelial progenitor cells. J. Clin. Invest. 108:399–405. 10.1172/JCI200113131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. 2001. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation 103:2885–2890. 10.1161/hc2401.092816 [DOI] [PubMed] [Google Scholar]
- 25.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. 2005. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension 46:7–18. 10.1161/01.HYP.0000168923.92885.f7 [DOI] [PubMed] [Google Scholar]
- 26.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. 2004. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 53:195–199 [DOI] [PubMed] [Google Scholar]
- 27.Rafii S, Lyden D. 2003. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med. 9:702–712. 10.1038/nm0603-702 [DOI] [PubMed] [Google Scholar]
- 28.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. 2005. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation 111:2981–2987. 10.1161/CIRCULATIONAHA.104.504340 [DOI] [PubMed] [Google Scholar]
- 29.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. 2005. Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med. 353:999–1007. 10.1056/NEJMoa043814 [DOI] [PubMed] [Google Scholar]
- 30.Aird WC. 2003. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 101:3765–3777. 10.1182/blood-2002-06-1887 [DOI] [PubMed] [Google Scholar]
- 31.Patschan SA, Patschan D, Temme J, Korsten P, Wessels JT, Koziolek M, Henze E, Muller GA. 2011. Endothelial progenitor cells (EPC) in sepsis with acute renal dysfunction (ARD). Crit. Care. 15:R94. 10.1186/cc10100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rafat N, Hanusch C, Brinkkoetter PT, Schulte J, Brade J, Zijlstra JG, van der Woude FJ, van Ackern K, Yard BA, Beck G. 2007. Increased circulating endothelial progenitor cells in septic patients: correlation with survival. Crit. Care Med. 35:1677–1684. 10.1097/01.CCM.0000269034.86817.59 [DOI] [PubMed] [Google Scholar]
- 33.Vaheri A, Strandin T, Hepojoki J, Sironen T, Henttonen H, Makela S, Mustonen J. 2013. Uncovering the mysteries of hantavirus infections. Nat. Rev. Microbiol. 11:539–550. 10.1038/nrmicro306 [DOI] [PubMed] [Google Scholar]
- 34.Vapalahti O, Mustonen J, Lundkvist A, Henttonen H, Plyusnin A, Vaheri A. 2003. Hantavirus infections in Europe. Lancet Infect. Dis. 3:653–661. 10.1016/S1473-3099(03)00774-6 [DOI] [PubMed] [Google Scholar]
- 35.Geimonen E, Neff S, Raymond T, Kocer SS, Gavrilovskaya IN, Mackow ER. 2002. Pathogenic and nonpathogenic hantaviruses differentially regulate endothelial cell responses. Proc. Natl. Acad. Sci. U. S. A. 99:13837–13842. 10.1073/pnas.192298899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korva M, Saksida A, Kejzar N, Schmaljohn C, Avsic-Zupanc T. 2013. Viral load and immune response dynamics in patients with haemorrhagic fever with renal syndrome. Clin. Microbiol. Infect. 19:E358–E366. 10.1111/1469-0691.12218 [DOI] [PubMed] [Google Scholar]
- 37.Krautkrämer E, Grouls S, Stein N, Reiser J, Zeier M. 2011. Pathogenic Old World hantaviruses infect renal glomerular and tubular cells and induce disassembling of cell-to-cell contacts. J. Virol. 85:9811–9823. 10.1128/JVI.00568-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindgren T, Ahlm C, Mohamed N, Evander M, Ljunggren HG, Bjorkstrom NK. 2011. Longitudinal analysis of the human T cell response during acute hantavirus infection. J. Virol. 85:10252–10260. 10.1128/JVI.05548-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markotić A, Hensley L, Daddario K, Spik K, Anderson K, Schmaljohn C. 2007. Pathogenic hantaviruses elicit different immunoreactions in THP-1 cells and primary monocytes and induce differentiation of human monocytes to dendritic-like cells. Coll. Antropol. 31:1159–1167 [PubMed] [Google Scholar]
- 40.Pensiero MN, Sharefkin JB, Dieffenbach CW, Hay J. 1992. Hantaan virus infection of human endothelial cells. J. Virol. 66:5929–5936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pettersson L, Thunberg T, Rocklov J, Klingstrom J, Evander M, Ahlm C. 29 April 2013. Viral load and humoral immune response in association with disease severity in Puumala hantavirus-infected patients-implications for treatment. Clin. Microbiol. Infect. 10.1111/1469-0691.12259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schönrich G, Rang A, Lutteke N, Raftery MJ, Charbonnel N, Ulrich RG. 2008. Hantavirus-induced immunity in rodent reservoirs and humans. Immunol. Rev. 225:163–189. 10.1111/j.1600-065X.2008.00694.x [DOI] [PubMed] [Google Scholar]
- 43.Robert-Koch-Institut 2007. Falldefinitionen des Robert Koch-Instituts zur Übermittlung von Erkrankungs- oder Todesfällen und Nachweisen von Krankheitserregern. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 49:1236–1284 (In German.) [DOI] [PubMed] [Google Scholar]
- 44.Duda DG, Cohen KS, Scadden DT, Jain RK. 2007. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat. Protoc. 2:805–810. 10.1038/nprot.2007.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lipkin GW, Kendall R, Haggett P, Turney JH, Brownjohn AM. 1989. Erythropoietin in acute renal failure. Lancet i:1029. [DOI] [PubMed] [Google Scholar]
- 46.Breen EC. 2007. VEGF in biological control. J. Cell. Biochem. 102:1358–1367. 10.1002/jcb.21579 [DOI] [PubMed] [Google Scholar]
- 47.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. 1983. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219:983–985. 10.1126/science.6823562 [DOI] [PubMed] [Google Scholar]
- 48.Gavrilovskaya I, Gorbunova E, Koster F, Mackow E. 2012. Elevated VEGF levels in pulmonary edema fluid and PBMCs from patients with acute hantavirus pulmonary syndrome. Adv. Virol. 2012:674360. 10.1155/2012/674360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Wang W, Wang JP, Pan L, Zhang Y, Yu HT, Jiang W, Wang PZ, Bai XF. 2012. Elevated vascular endothelial growth factor levels induce hyperpermeability of endothelial cells in hantavirus infection. J. Int. Med. Res. 40:1812–1821. 10.1177/030006051204000519 [DOI] [PubMed] [Google Scholar]
- 50.Ma Y, Liu B, Yuan B, Wang J, Yu H, Zhang Y, Xu Z, Yi J, Zhang C, Zhou X, Yang A, Zhuang R, Jin B. 2012. Sustained high level of serum VEGF at convalescent stage contributes to the renal recovery after HTNV infection in patients with hemorrhagic fever with renal syndrome. Clin. Dev. Immunol. 2012:812386. 10.1155/2012/812386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shrivastava-Ranjan P, Rollin PE, Spiropoulou CF. 2010. Andes virus disrupts the endothelial cell barrier by induction of vascular endothelial growth factor and downregulation of VE-cadherin. J. Virol. 84:11227–11234. 10.1128/JVI.01405-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dvorak HF, Sioussat TM, Brown LF, Berse B, Nagy JA, Sotrel A, Manseau EJ, Van de Water L, Senger DR. 1991. Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J. Exp. Med. 174:1275–1278. 10.1084/jem.174.5.1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML, Parente E, Mancina R, Netti GS, Becherucci F, Gacci M, Carini M, Gesualdo L, Rotondi M, Maggi E, Lasagni L, Serio M, Romagnani S, Romagnani P. 2008. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J. Exp. Med. 205:479–490. 10.1084/jem.20071903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clement J, De Bock R, Beguin Y. 1994. Defective Epo production contributes to anemia persistent after ARF in nephropathia epidemica (NE), p 467–473 In Papadimitriou A. (ed), Proceedings of the 3rd International Satellite Symposium on Acute Renal Failure. University Studio Press, Thessaloniki, Greece [Google Scholar]
- 55.Nielsen OJ, Thaysen JH. 1989. Erythropoietin deficiency in acute renal failure. Lancet i:624–625 [DOI] [PubMed] [Google Scholar]
- 56.Duchin JS, Koster FT, Peters CJ, Simpson GL, Tempest B, Zaki SR, Ksiazek TG, Rollin PE, Nichol S, Umland ET, Hantavirus Study Group 1994. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. N. Engl. J. Med. 330:949–955. 10.1056/NEJM199404073301401 [DOI] [PubMed] [Google Scholar]
- 57.Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M. 2005. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am. J. Respir. Crit. Care Med. 172:854–860. 10.1164/rccm.200410-1325OC [DOI] [PubMed] [Google Scholar]
- 58.Mahanty S, Bray M. 2004. Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect. Dis. 4:487–498. 10.1016/S1473-3099(04)01103-X [DOI] [PubMed] [Google Scholar]
- 59.Peters CJ, Zaki SR. 2002. Role of the endothelium in viral hemorrhagic fevers. Crit. Care Med. 30:S268–73. 10.1097/00003246-200205001-00016 [DOI] [PubMed] [Google Scholar]
- 60.Balabanova Y, Gilsdorf A, Buda S, Burger R, Eckmanns T, Gartner B, Gross U, Haas W, Hamouda O, Hubner J, Janisch T, Kist M, Kramer MH, Ledig T, Mielke M, Pulz M, Stark K, Suttorp N, Ulbrich U, Wichmann O, Krause G. 2011. Communicable diseases prioritized for surveillance and epidemiological research: results of a standardized prioritization procedure in Germany, 2011. PLoS One 6:e25691. 10.1371/journal.pone.0025691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klempa B, Witkowski PT, Popugaeva E, Auste B, Koivogui L, Fichet-Calvet E, Strecker T, Ter Meulen J, Krüger DH. 2012. Sangassou virus, the first hantavirus isolate from Africa, displays genetic and functional properties distinct from those of other murinae-associated hantaviruses. J. Virol. 86:3819–3827. 10.1128/JVI.05879-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Memish ZA, Albarrak A, Almazroa MA, Al-Omar I, Alhakeem R, Assiri A, Fagbo S, MacNeil A, Rollin PE, Abdullah N, Stephens G. 2011. Seroprevalence of Alkhurma and other hemorrhagic fever viruses, Saudi Arabia. Emerg. Infect. Dis. 17:2316–2318. 10.3201/eid1712.110658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 364:1523–1532. 10.1056/NEJMoa1010095 [DOI] [PMC free article] [PubMed] [Google Scholar]