Abstract
Lloviu virus (LLOV), a novel filovirus detected in bats, is phylogenetically distinct from viruses in the genera Ebolavirus and Marburgvirus in the family Filoviridae. While filoviruses are known to cause severe hemorrhagic fever in humans and/or nonhuman primates, LLOV is biologically uncharacterized, since infectious LLOV has never been isolated. To examine the properties of LLOV, we characterized its envelope glycoprotein (GP), which likely plays a key role in viral tropism and pathogenicity. We first found that LLOV GP principally has the same primary structure as the other filovirus GPs. Similar to the other filoviruses, virus-like particles (VLPs) produced by transient expression of LLOV GP, matrix protein, and nucleoprotein in 293T cells had densely arrayed GP spikes on a filamentous particle. Mouse antiserum to LLOV VLP was barely cross-reactive to viruses of the other genera, indicating that LLOV is serologically distinct from the other known filoviruses. For functional study of LLOV GP, we utilized a vesicular stomatitis virus (VSV) pseudotype system and found that LLOV GP requires low endosomal pH and cathepsin L, and that human C-type lectins act as attachment factors for LLOV entry into cells. Interestingly, LLOV GP-pseudotyped VSV infected particular bat cell lines more efficiently than viruses bearing other filovirus GPs. These results suggest that LLOV GP mediates cellular entry in a manner similar to that of the other filoviruses while showing preferential tropism for some bat cells.
INTRODUCTION
Filoviruses are nonsegmented, negative-stranded RNA viruses grouped into two genera, Marburgvirus and Ebolavirus. These filoviruses are known to cause severe hemorrhagic fever in human and/or nonhuman primates, with case mortality rates of up to 90% (1). There is one known species of Marburgvirus, Marburg marburgvirus, consisting of two viruses, Marburg virus (MARV) and Ravn virus. On the other hand, five distinct species are known in the genus Ebolavirus, Zaire ebolavirus, Sudan ebolavirus, Taï forest ebolavirus, Bundibugyo ebolavirus, and Reston ebolavirus, represented by Ebola virus (EBOV), Sudan virus (SUDV), Taï forest virus (TAFV), Bundibugyo virus (BDBV), and Reston virus (RESTV), respectively. A difference in pathogenicity was suggested to exist among ebolaviruses. EBOV is thought to be the most pathogenic, killing up to approximately 90% of patients, whereas RESTV has never caused a lethal infection in humans (2) and is less pathogenic in experimentally infected nonhuman primates than EBOV (3).
Recently, a filovirus-like RNA genome was detected in the lungs, livers, rectal swabs, and/or spleens of bat (Miniopterus schreibersii) carcasses found in Cueva del Lloviu, Asturias, Spain. This novel filovirus was designated Lloviu virus (LLOV); its name was derived from the cave in which it was first found (4). LLOV is phylogenetically distinct from other filoviruses; thus, it is proposed to belong to the new genus Cuevavirus, species Lloviu cuevavirus, in the family Filoviridae (4). However, the biological properties of this novel virus are uncharacterized, since infectious LLOV has not been isolated yet.
Filovirus particles consist of at least seven structural proteins, including the nucleoprotein (NP), viral protein (VP) 35, VP40, glycoprotein (GP), VP30, VP24, and polymerase (L) genes (5). The envelope GP is responsible for both receptor binding and fusion of the virus envelope with the host cell membrane (6, 7). GP undergoes proteolytic cleavage by host proteases such as furin, resulting in the two subunits, GP1 and GP2, which are linked by a disulfide bond (7, 8). GP is highly glycosylated with large amounts of N- and O-linked glycans, most of which are located in its middle one-third, designated the mucin-like region (MLR), which plays an important role in attachment to the preferred target cells (9, 10). Although MLR is found in all known filovirus GPs, its highly variable amino acid sequences and sugar chain structures suggest different GP properties among filovirus species. Membrane-anchored cellular C-type lectins have been found to facilitate filovirus infection in vitro through binding to glycans on the MLR (11–13). It was also shown that MLR contains epitopes for antibody-dependent enhancement (ADE) of filovirus infection in vitro (14, 15).
To provide information for estimation of the infectivity and potential pathogenicity of LLOV, this study focused on GP, which likely plays a major role in the replication cycle and the pathogenicity of filoviruses (10, 16). In this study, we investigated the morphology of virus-like particles consisting of LLOV GP, VP40, and NP, compared the antigenicity of GP among filoviruses, and analyzed the ability of GP to mediate virus entry into cells. Here, we show that LLOV GP has the potential to mediate viral entry into cells of various animal species, including primates, in a manner similar to that of the other filoviruses while showing preferential tropism for particular bat cells.
MATERIALS AND METHODS
Cells.
Human embryonic kidney 293 (HEK293), HEK293T, and African green monkey kidney Vero E6 cells were grown in Dulbecco's modified Eagle's medium (DMEM) with 10% fatal calf serum (FCS) and penicillin-streptomycin. Bat cell lines ZFB11-97 and SuBK12-08 were established by transfecting an expression plasmid encoding the Simian virus 40 large T antigen (pCXN2-Flag-SV40LT; kindly provided by H. Sawa and Y. Orba, Hokkaido University Research Center for Zoonosis Control) into primary kidney cells of bats captured in Zambia. The transfected cells were selected by culturing in the presence of G418 (200 μg/ml). ZFB11-97, SuBK12-08, and Madin-Darby canine kidney (MDCK) cells were grown in minimal essential medium (MEM) with 10% FCS, l-glutamine, and penicillin-streptomycin. SK-L cells (17) were cultured in MEM with 10% FCS, l-glutamine, penicillin-streptomycin, and 0.3% tryptose phosphate broth (GIBCO). Bat cell lines BKT1, FBKT1, YubFKT1, IndFSPT1, and DemKT1 were established as described previously (18). Bat species were identified by morphology, habitat, and BLAST searches using the sequences of their cytochrome b genes (nucleotide positions 1 to 400). BKT1, FBKT1, YubFKT1, IndFSPT1, DemKT1, human chronic myelogenous leukemia (K562), and K562 clones expressing human macrophage galactose-type C-type lectin (hMGL) or dendritic cell-specific ICAM-3-grabbing nonintegrin (DC-SIGN) (19, 20) were grown in RPMI 1640 medium with 10% FSC, l-glutamine, and penicillin-streptomycin.
Construction of plasmids expressing GP, NP, and VP40.
Coding regions of the GP, NP, and VP40 genes of LLOV were synthesized in pBS II SK vector (FASMAC) based on the nucleotide sequence of LLOV (GenBank accession number JF828358). The NP and VP40 genes were synthesized according to the coding regions reported in the database. Since the ebolavirus envelope GP is expressed through transcriptional editing (21, 22), the coding region of the GP gene was synthesized with an additional adenosine at the putative editing site. After digestion by restriction enzymes, each gene was cloned into mammalian expression vector pCAGGS (23). The expression plasmids for EBOV (strain Mayinga), SUDV (strain Boniface), TAFV (strain Cote d'Ivoire), BDBV (strain Bundibugyo), RESTV (strain Pennsylvania), and MARV (strains Angola and Musoke) were constructed as described previously (24).
Purification of VLPs.
HEK293T cells were transfected with plasmids encoding GP, VP40, and NP of LLOV, EBOV, SUDV, TAFV, BDBV, RESTV, and MARV (strain Angola) using TransIT LT-1 reagent (Mirus) according to the manufacturer's instructions. Forty-eight hours later, VLPs were purified from culture supernatants by ultracentrifugation at 28,000 × g at 4°C for 1.5 h with a 25% sucrose cushion. Virus-like particle (VLP) pellets were resuspended in phosphate-buffered saline (PBS).
SDS-PAGE and Western blotting.
HEK293T cells were transfected with plasmids encoding filovirus GPs and lysed 48 h after transfection with a lysis buffer (10 mM Tris HCl [pH 7.8], 0.15 M NaCl, 1 mM EDTA, 0.1% Nonidet P-40, and protease inhibitor mixture) (Roche). Cell lysates were mixed with SDS-PAGE sample buffer with or without 5% 2-mercaptoethanol. After electrophoresis on 5 to 20% SuperSep (Wako), separated proteins were blotted on a polyvinylidene difluoride membrane (Millipore). The membrane was incubated with mouse antisera (diluted 1:2,000) to filovirus VLPs (see below), followed by incubation with peroxidase-conjugated goat anti-mouse IgG (H+L) (Jackson ImmunoResearch). The bound antibodies were visualized with Immobilon Western (Millipore).
Mouse antisera and ELISA.
Five-week-old female BALB/c mice were immunized twice intraperitoneally with purified VLPs (100 μg/mouse) at 3-week intervals. Antisera were collected 7 days after the second immunization. Animal studies were carried out in strict accordance with the Guidelines for Proper Conduct of Animal Experiments of the Science Council of Japan. The protocol was approved by the Hokkaido University Animal Care and Use Committee. The GP-based enzyme-linked immunosorbent assay (ELISA) was performed as described previously (25). Serum samples were serially diluted with PBS containing 0.05% Tween 20, 0.5% bovine serum albumin, and 2% FCS. Bound antibodies were visualized by adding peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch) and 3,3′,5,5′-tetramethylbenzidine (Sigma). The reaction was stopped by adding 1 N phosphate acid to the mixture, and the optical density at 450 nm (OD450) was measured.
Electron microscopy.
Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) were carried out as described previously (26, 27). Purified VLPs fixed with 0.25% glutaraldehyde were adsorbed to collodion-carbon-coated copper grids and negatively stained with 2% phosphotungstic acid solution (pH 5.8). For immuno-TEM, we used an anti-LLOV GP monoclonal antibody (LGP14-2), produced in this study as described previously (15), and an immunogold-conjugated goat anti-mouse IgG (H + L) polyclonal antibody (BB International). Samples were examined with an H-7650 electron microscope (Hitachi) at 80 kV. For SEM, cells transfected with plasmids expressing LLOV GP, VP40, and NP were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) and postfixed with 1% osmium tetroxide in the same buffer. The fixed samples were dehydrated with a series of ethanol gradients, replaced with t-butanol, and dried in an ES-2030 freeze dryer (Hitachi). Dried specimens were coated with platinum by using mild spatter E-1046 (Hitachi). The samples were observed with an S-4700 electron microscope (Hitachi) at 15 kV.
VSV pseudotyped with filovirus GPs.
Using vesicular stomatitis virus (VSV) containing the green fluorescent protein (GFP) gene instead of the receptor-binding VSV G protein gene (VSVΔG*-G) (6), pseudotyped viruses with GPs of EBOV, RESTV, MARV strains Angola and Musoke, and LLOV (VSVΔG*-Zaire, VSVΔG*-Reston, VSVΔG*-Angola, VSVΔG*-Musoke, and VSVΔG*-Lloviu, respectively) were generated, and the infectious units (IUs) of stock viruses were determined in Vero E6 cells according to a previous study (6). The genome copy number of each pseudotyped VSV preparation was quantified by real-time reverse transcription-PCR (RT-PCR). Real-time RT-PCR was performed using a One Step SYBR PrimeScript RT-PCR kit II (TaKaRa Bio) and a CFX96 real-time system (Bio-Rad) with primers (GFP498-F, CAAGATCCGCCACAACATCG; GFP-617R, GACTGGGTGCTCAGGTAGTG) to detect the GFP gene in the VSV genome.
Virus titration.
To determine infectivities of VSVs pseudotyped with filovirus GPs, appropriately diluted virus stocks were pretreated with an anti-VSV G monoclonal antibody, VSV-G(N)1-9, to abolish the background infectivity of parental VSVΔG*-G (15). K562 clones expressing hMGL and DC-SIGN grown on 96-well plates were infected with VSV pseudotyped with filovirus GPs (50 to 150 IU; determined in K562), and infectivities were determined by counting the number of GFP-positive cells using flow cytometry as described previously (19, 20). For the assays of antibody-dependent enhancement (ADE) of infection, 10-fold serially diluted mouse antisera were mixed with equal volumes of pseudotyped VSVs (50 to 150 IU; determined in K562). After a 1-h incubation at room temperature, the mixture was inoculated into K562 cells grown on 96-well plates. At 20 h postinoculation, GFP-positive cells were counted with an IN Cell Analyzer 2000 (GE Healthcare). To determine the infectivities in adherent cells of different animal origins, cell monolayers grown on 96-well plates were infected with VSVs pseudotyped with filovirus GPs. Twenty hours later, the virus infectivity in each cell line was determined by counting the number of GFP-expressing cells under a fluorescence microscope, and IUs per 106 genome copies were calculated.
Inhibitor treatments.
Vero E6 cells were pretreated with ammonium chloride (Wako), monensin (Sigma), or cathepsin B or L inhibitors (CA-074Me and FY-dmk, respectively; Calbiochem) for 30 min at 37°C. Treated cells were then infected with VSVΔG*-Zaire, VSVΔG*-Angola, VSVΔG*-Lloviu, and VSVΔG*-G appropriately diluted to yield 200 to 2,000 IUs/106 cells. At 20 h postinoculation, GFP-positive cells were counted with the IN Cell Analyzer 2000 (GE Healthcare).
RESULTS
Characterization of the primary structure of LLOV GP.
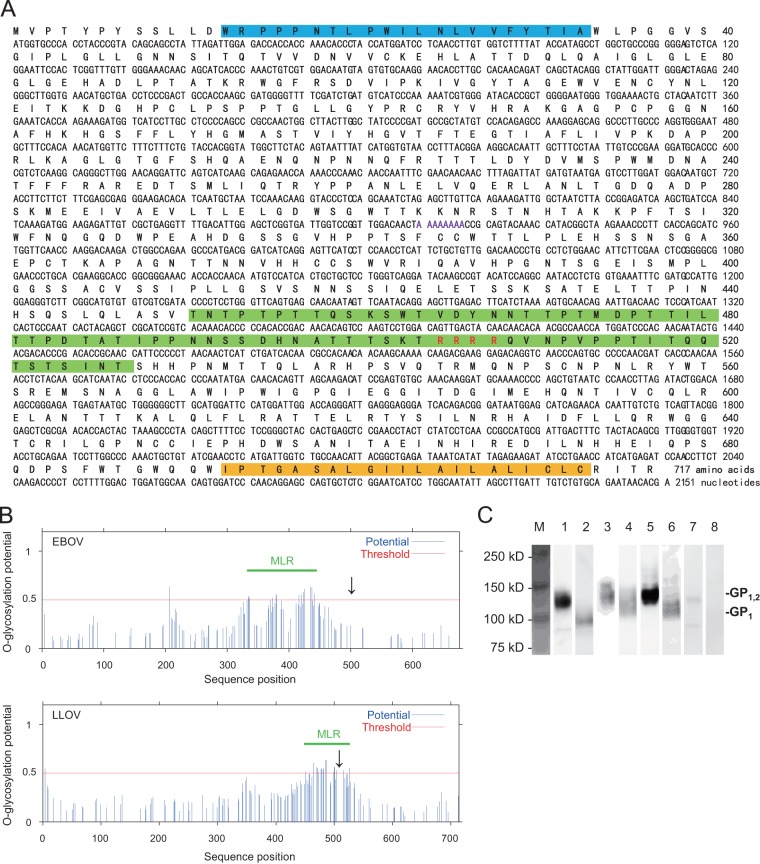
Although the envelope GPs of ebolaviruses are expressed through transcriptional editing (21, 22), similar characteristics were not shown for LLOV GP in a previous study, and the reported LLOV GP gene did not have an open reading frame of the single-transmembrane GP (4). Thus, we first analyzed the nucleotide and deduced amino acid sequences of LLOV GP and found that the nucleotide sequence of LLOV GP had 7 adenosines at positions 910 to 916. Based on sequence comparison to the EBOV genome, which has an editing site at positions 880 to 886, we assumed that this stretch of 7 adenosines is the editing site of the LLOV GP gene. Accordingly, an open reading frame of the full-length transmembrane GP gene was produced by adding an adenosine at this putative editing site (Fig. 1A). We found that LLOV envelope GP had a potential cleavage site (i.e., RRRR; recognized by host ubiquitous proteases, such as furin) and MLR, similar to the other filoviruses. The predicted MLR of LLOV GP differed from that of EBOV GP in length and location (i.e., LLOV MLR was located over the cleavage site and was a little shorter than EBOV MLR) (Fig. 1A and B). We confirmed the approximate molecular size of LLOV GP (GP1,2, approximately 120 to 130 kDa) and its cleavage product, the GP1 subunit (approximately 100 kDa), by Western blotting (Fig. 1C). These results suggested that LLOV GP shares many characteristics with the other filovirus GPs.
FIG 1.
Primary structure of LLOV envelope GP. (A) Nucleotide and deduced amino acid sequences of LLOV GP. Deduced amino acid sequences are shown in boldface letters. Blue, green, and yellow lines represent the signal peptide, MLR, and transmembrane domain predicted by GENETYX Ver.10, NetOGlyc, and TopPred 0.01, respectively. Purple and red letters represent the putative editing and cleavage sites, respectively. (B) Comparison of EBOV and LLOV MLRs. Potential O-glycosylation sites were predicted by NetOGlyc, and MLRs were defined as the regions between the first and last amino acid residues showing a score above the threshold (0.5). Arrows indicate the cleavage sites. (C) Western blotting of filovirus GPs. Proteins in the lysate of HEK293T cells transfected with the plasmid expressing LLOV GP (lanes 1 and 2), EBOV GP (lanes 3 and 4), MARV GP (lanes 5 and 6), or empty vector (lanes 7 and 8) were separated by SDS-PAGE under nonreducing (lanes 1, 3, 5, and 7) or reducing (lanes 2, 4, 6, and 8) conditions.
Morphology of VLPs consisting of LLOV GP, VP40, and NP.
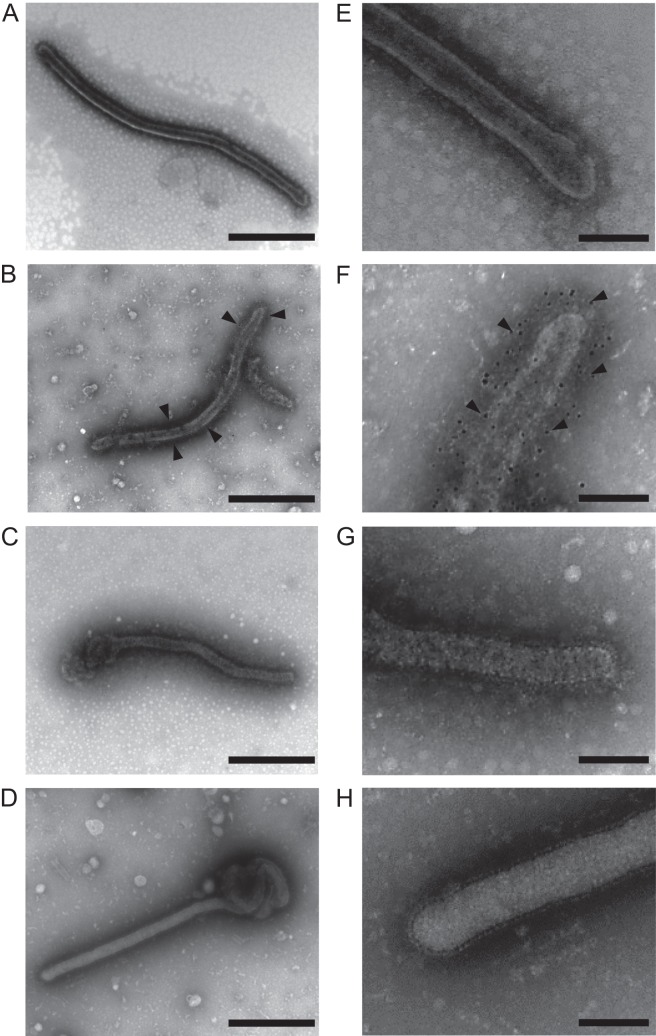
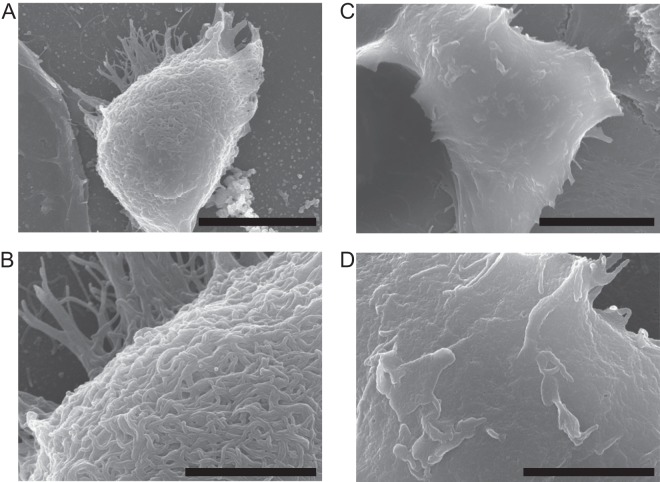
Filoviruses are characterized by their filamentous forms. However, LLOV particles have never been verified morphologically. To determine the possible shape of LLOV particles, we investigated the morphology of VLPs consisting of LLOV GP, VP40, and NP. TEM analyses revealed that LLOV VLPs were filamentous (Fig. 2A and E), like EBOV (Fig. 2C and G) and MARV (Fig. 2D and H) VLPs. Numerous spikes were observed on the VLP surface, and immuno-TEM with an anti-LLOV GP monoclonal antibody confirmed the presence of LLOV GPs on the surface (Fig. 2B and F). Similar to the other filoviruses (28), VLPs with a uniform diameter of approximately 70 nm and various lengths were observed. This was consistent with a previous study showing that the diameters of VLPs were narrower than those reported for actual EBOV particles (80-nm diameter) (1, 29). By SEM, numerous filamentous structures were observed on the surfaces of cells transfected with plasmids expressing LLOV GP, VP40, and NP (Fig. 3), which has similarity to EBOV budding (27). These results suggested that LLOV shared morphological characteristics with the other known filoviruses.
FIG 2.
TEM of filovirus VLPs. Purified VLPs produced from 293T cells transfected with plasmids expressing LLOV (A, B, E, and F), EBOV (Zaire) (C and G), and MARV (Angola) (D and H) proteins were fixed and stained as described in Materials and Methods. For immuno-TEM (B and F), an anti-LLOV GP monoclonal antibody was used. Scale bars represent 500 nm (A to D) and 200 nm (E to H). Arrowheads indicate gold particles.
FIG 3.
SEM of LLOV VLPs. HEK293T cells transfected with pCAGGS expressing LLOV GP, VP40, and NP (A and B) or pCAGGS alone (C and D) were fixed at 48 h after transfection. Samples were observed with an S-4700 scanning electron microscope (Hitachi). Scale bars represent 5 μm (A and C) and 2 μm (B and D).
Antigenic comparison among filovirus GPs.
While LLOV is shown to be phylogenically distinct from the other filovirus species, serological information is lacking. The amino acid sequence of LLOV GP has 35 and 28% similarity to those of EBOV and MARV, respectively. To compare the antigenic relationships among filovirus GPs, we produced antisera to each GP by immunizing mice with VLPs and performed GP-based ELISA (Fig. 4). We found that anti-LLOV GP sera showed exclusive reactivity to the LLOV GP antigen (Fig. 4A). Similarly, anti-MARV GP sera only reacted with the MARV GP antigen (Fig. 4G). Antisera to EBOV, SUDV, TAFV, BDBV, and RESTV GPs showed slight cross-reactivity with ebolavirus antigens at the lowest dilution of the sera but not with the LLOV and MARV GP antigens (Fig. 4B to F). These results indicated that LLOV was distinct not only phylogenically but also serologically from the other known filoviruses.
FIG 4.
Cross-reactivities of anti-GP sera among filoviruses in ELISA. Ten-fold serial dilutions of mouse antisera to EBOV (anti-Zaire), SUDV (anti-Sudan), TAFV (anti-Tai forest), BDBV (anti-Bundibugyo), RESTV (anti-Reston), LLOV (anti-Lloviu), and MARV (anti-Marburg) were tested for IgG reactivities to LLOV (A), EBOV (B), SUDV (C), TAFV (D), BDBV (E), RESTV (F), and MARV (G) GP antigens. Three mice were used for each virus, and averages and standard deviations are shown.
Functional study of LLOV GP with chemical inhibitors.
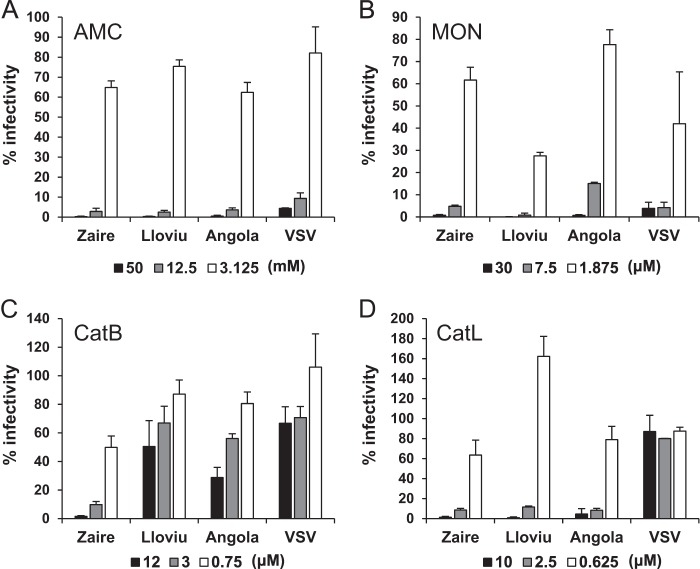
For the functional study of LLOV GP, we produced VSV pseudotyped with LLOV GP, which could infect Vero E6 cells, and confirmed that the full-length GP produced with 8 adenosines at the putative editing sites was fully functional as a single transmembrane GP. It has been shown that endosomal acidification and proteolytic processing with the cellular cysteine proteases cathepsin L and/or B are required for filovirus GP-mediated entry in vitro (6, 7, 30). To investigate the requirement of these factors for the LLOV GP function, we examined the infectivities of pseudotyped VSV in Vero E6 cells pretreated with chemical inhibitors (Fig. 5). Pretreatment of cells with ammonium chloride and monensin markedly reduced the infectivity of VSVΔG*-Lloviu, as was the case with VSVΔG*-Zaire, VSVΔG*-Angola, and VSVΔG*-G, suggesting that LLOV GP requires a low pH for cellular entry. We found that pretreatments with a cathepsin L inhibitor at concentrations of 2.5 and 10 μM significantly reduced the infectivities of VSVΔG*-Lloviu, -Zaire, and -Angola. However, treatments at 0.625 μM did not show any inhibitory effects on the VSVΔG*-Lloviu infectivity; rather, it enhanced the infectivity. Interestingly, when cells were treated with a cathepsin B inhibitor, the infectivity of VSVΔG*-Zaire was reduced significantly in a dose-dependent manner, whereas much less inhibition was observed in the VSVΔG*-Lloviu and -Angola infectivities. These cathepsin inhibitors did not affect the infectivity of VSVΔG*-G.
FIG 5.
Effects of chemical inhibitors on infectivities of pseudotyped VSVs. Vero E6 cells were pretreated with ammonium chloride (AMC) (A), monensin (MON) (B), and cathepsin B (CatB) (C) and L (CatL) (D) inhibitors for 30 min at 37°C. The treated cells were then infected with VSVΔG*-Zaire (Zaire), VSVΔG*-Angola (Angola), VSVΔG*-Lloviu (Lloviu), and VSVΔG*-G (VSV) appropriately diluted to yield 200 to 2,000 IUs/106 cells. At 20 h postinoculation, GFP-positive cells were counted using an IN Cell Analyzer 2000 (GE Healthcare). The percentages of infectivity were determined by setting the number of the untreated cells to 100%. Each experiment was performed three times, and averages and standard deviations are shown.
Human C-type lectin-mediated entry of pseudotyped VSVs.
C-type lectins expressed on the host cell surface are thought to serve as an attachment factor for filovirus GP, and C-type lectin-mediated entry is believed to be one of the important factors responsible for filovirus tropism and pathogenicity (11, 19, 20). Thus, we investigated the potential of LLOV GP to use the human C-type lectins hMGL and DC-SIGN, both of which are known to enhance filovirus infectivity (Fig. 6). VSVΔG*-Lloviu infected DC-SIGN-expressing cells more efficiently than hMGL-expressing cells, which was similar to VSVΔG*-Zaire and VSVΔG*-Reston. VSVΔG*-Lloviu infected hMGL-expressing cells more efficiently than VSVΔG*-Musoke (P < 0.05) but less so than VSVΔG*-Angola (P < 0.05); however, there were no significant differences among VSVΔG*-Lloviu, VSVΔG*-Zaire, and VSVΔG*-Reston. On the other hand, VSVΔG*-Lloviu infected DC-SIGN-expressing cells more efficiently than VSVΔG*-Angola (P < 0.05) and VSVΔG*-Musoke (P < 0.01) and less efficiently than VSVΔG*-Zaire (P < 0.05), but there was no significant difference between VSVΔG*-Lloviu and VSVΔG*-Reston.
FIG 6.
Infectivities of pseudotyped VSVs in C-type lectin-expressing cells. K562 cells expressing hMGL or DC-SIGN were infected with VSVΔG*-Zaire, VSVΔG*-Reston, VSVΔG*-Lloviu, VSVΔG*-Angola, and VSVΔG*-Musoke. The infectivity of each pseudotyped VSV on K562 clones was determined by counting the number of GFP-positive cells using flow cytometry, and the percentages of infectivity in K562-hMGL and K562-DC-SIGN were determined by setting the number of the infected K562 cells as 100%. Each experiment was performed three times, and averages and standard deviations are shown. Statistical significance of the differences was determined by Student's t test (see P values in the text).
Difference in ADE activity between anti-EBOV and anti-LLOVGP antisera.
Antibody-dependent enhancement of infection is also a known in vitro phenomenon observed for comparatively highly lethal filoviruses (e.g., Zaire and Angola) (15, 31, 32). To investigate the potential of LLOV GP to induce ADE antibodies, K562 cells were infected with VSVΔG*-Lloviu or VSVΔG*-Zaire in the presence of mouse antisera specific to the respective viruses (Fig. 7). We confirmed the ADE activity of the anti-Zaire serum as indicated by markedly enhanced infectivities of VSVΔG*-Zaire at serum dilutions of 1:10 and 1:100 (Fig. 7A). In contrast, only minimal ADE activity was seen in the anti-LLOV serum, although similar amounts of specific IgG antibodies were detected in both antisera by ELISA (Fig. 4). Consist with the absence of cross-reactive IgG in ELISA, little cross-ADE activity was observed in the ADE assay.
FIG 7.
ADE activities of antisera to EBOV and LLOV GPs. Ten-fold serially diluted mouse anti-EBOV (A) and anti-LLOV (B) sera were mixed with equal volumes of VSVΔG*-Zaire and VSVΔG*-Lloviu. Relative infectivity was determined by setting the number of infected K562 cells without antisera to 100%. Each experiment was performed three times, and averages and standard deviations are shown.
Cellular tropism of LLOV GP.
To estimate the GP-dependent tropism that is likely reflected by the prevalence of LLOV receptors, we infected various cell lines of different animal origins (Table 1) with pseudotyped VSV, and their infectivities were compared (Fig. 8A). VSVΔG*-Lloviu infected cells that were derived from the human, African green monkey, pig, dog, and bat in a manner similar to that of VSVΔG*-Zaire and VSVΔG*-Reston (Fig. 8A). VSVΔG*-Angola and VSVΔG*-Musoke had higher IUs in these cells than VSVΔG*-Lloviu, VSVΔG*-Zaire, and VSVΔG*-Reston, except in a cell line from the Yaeyama flying fox (Pteropus dasymallus yayeyamae; FBKT1) (Fig. 8A). Since some species of bats are suspected to be natural reservoirs of filoviruses (33–37), we focused on these bat cells and relative infectivities were determined (Fig. 8B). Interestingly, VSVΔG*-Lloviu infected IndFSPT1 and SuBK12-08 more efficiently than the other viruses tested.
TABLE 1.
Origins of cell lines used in this study
| Cell line | Species | Zoological name | Organ |
|---|---|---|---|
| Vero E6 | African green monkey | Chlorocebus sp. | Kidney |
| HEK293 | Human | Homo sapiens | Kidney |
| SK-L | Pig | Sus scrofa domesticus | Kidney |
| MDCK | Dog | Canis lupus familiaris | Kidney |
| BKT1 | Greater horseshoe bata | Rhinolophus ferrumequinum | Kidney |
| FBKT1 | Yaeyama flying foxb | Pteropus dasymallus yayeyamae | Kidney |
| YubFKT1 | Eastern bent-winged batc | Miniopterus fuliginosus | Kidney |
| IndFSPT1 | Indian flying foxd | Pteropus giganteus | Spleen |
| DemKT1 | Leschenault's rousettee | Rousettus leschenaulti | Kidney |
| ZFB11-97 | Gambian epauletted fruit batf | Epomophorus gambianus | Kidney |
| SuBK12-08 | Schreiber's batg | Miniopterus schreibersii | Kidney |
Nucleotide sequence identity is 98% (unpublished data).
Previously described (18).
Nucleotide sequence identity is 99%.
Nucleotide sequence identity is 98%.
Nucleotide sequence identity is 100%.
Nucleotide sequence identity is 89%.
Nucleotide sequence identity is 98%.
FIG 8.
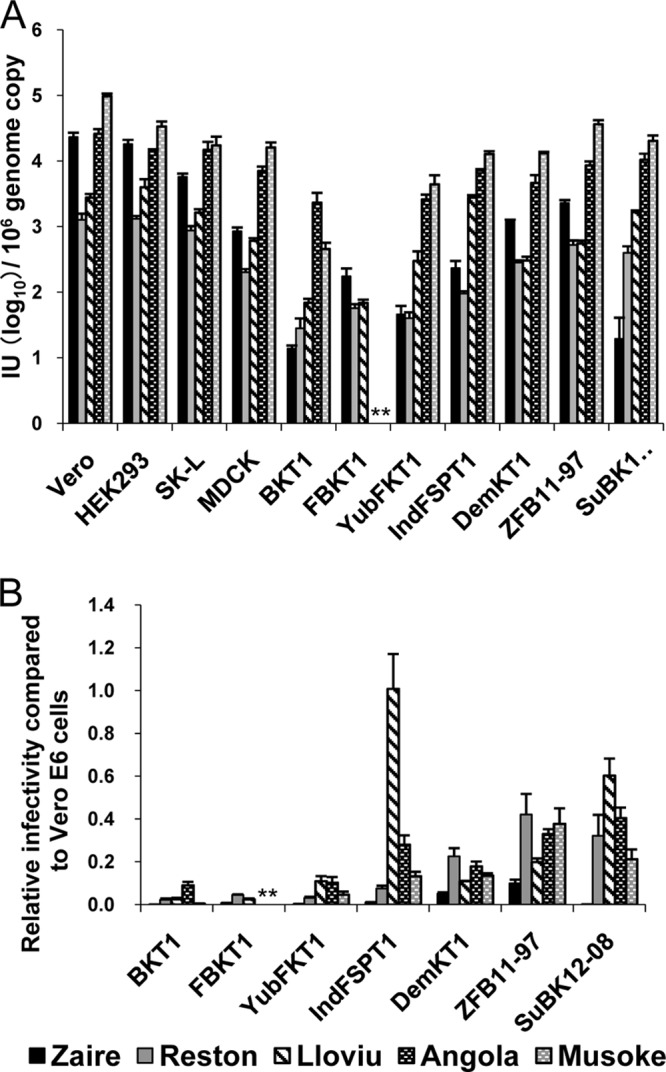
Infectivities of pseudotyped VSVs in mammalian cell lines. VSVΔG*-Zaire, VSVΔG*-Reston, VSVΔG*-Lloviu, VSVΔG*-Angola, and VSVΔG*-Musoke were inoculated into several mammalian cell lines. (A) Infectious units (IUs) of each virus in different cell lines were determined by counting the number of GFP-expressing cells, and each IU value was standardized based on 106 copies of the VSV genome as determined by real-time RT-PCR. (B) Relative infectivities in bat cell lines are given by setting each IU value in Vero E6 cells to 1.0 (i.e., [IU in bat cells]/[IU in Vero E6 cells]). Each experiment was performed three times, and averages and standard deviations are shown. Infectivities of VSVΔG*-Angola and VSVΔG*-Musoke were under the limit of detection (*).
DISCUSSION
This study provides fundamental information on the properties of LLOV GP. The RNA editing that is required to produce the full-length transmembrane GP is a common characteristic of viruses belonging to the genus Ebolavirus (21, 22). The presence of the editing site in the LLOV GP gene supports the notion that this virus is more related to ebolaviruses than marburgviruses. Phylogenic analyses also suggested that LLOV has the same ancestor as ebolaviruses (4). On the other hand, LLOV GP has MLR and a furin cleavage site, both of which are common features shared by previously known filoviruses (38, 39). By TEM and SEM of VLPs, we further found morphological similarity between LLOV and other filoviruses, which have numerous GP spikes located on the filamentous VLP surface. Viral entry assays with chemical inhibitors also suggest that LLOV GP, as well as GPs of the other filoviruses, requires low endosomal pH and proteolytic processing for virus entry into cells. Taken together, these results indicate that the structure and function of LLOV GP are primarily similar to those of the other filovirus GPs.
However, the requirement of cathepsin L might be controversial, since a high concentration (>1 μM) of FY-dmk was suggested to inhibit not only cathepsin L but also cathepsin B and likely other endosomal cysteine proteases (40). FY-dmk at the lowest concentration tested in this study (0.625 μM) did not reduce infectivities of VSVΔG*-Lloviu, VSVΔG*-Zaire, or VSVΔG*-Angola, suggesting that cathepsin L is not essential for LLOV infection, similar to the other filoviruses (40, 41). It was also shown that cathepsin B and cathepsin L activities are not required for EBOV replication in a mouse model (41). Thus, further studies are needed to clarify the in vivo importance of the GP cleavage by cathepsins and some other host proteases for filovirus infection.
Hepatocytes, dendritic cells, monocytes, and macrophages, all of which express cell surface C-type lectins, are thought to be the preferred target cells of filoviruses, and increased infection of these cells might be directly involved in the pathogenesis of filovirus infection (11, 19, 42–44). We demonstrated that LLOV GP utilized human C-type lectins, hMGL and DC-SIGN, most likely as attachment factors, as reported with the other filovirus GPs (13, 19, 20, 45). These C-type lectins have different glycan specificities (i.e., hMGL and DC-SIGN preferentially react with O- and high-mannose-type N-glycans, respectively) (46–48). Like EBOV and RESTV GPs, LLOV GP showed greater preference for DC-SIGN than hMGL, unlike MARV GPs, suggesting that LLOV GP, particularly its MLR, has a carbohydrate structure comparable to that of ebolaviruses. Our data suggest that LLOV GP has a tropism to cells expressing C-type lectins and infect human immune cells, such as dendritic cells and macrophages.
Like C-type lectins, ADE antibodies mostly recognize epitopes on MLR of filovirus GPs, leading to enhanced infectivity (11, 13, 42). Thus, MLR is thought to play important roles for these two attachment functions of GP. In addition to the primary structure of MLR (i.e., the presence of different epitopes and sugar chains among filoviruses), the GP2 region seems to have key amino acid residues (e.g., the amino acid at position 547 in MARV GP) contributing to the efficiency of ADE- and C-type lectin-mediated entry (15, 20). Although detailed functional mapping and structural analysis are still needed, our data indicate that the overall properties of LLOV GP for ADE- and C-type lectin-mediated entry are comparable to those of RESTV GP rather than highly virulent EBOV and MARV (strain Angola) (11, 13, 42).
VSVΔG*-Lloviu infected all cell lines tested, as well as VSVΔG*-Zaire and VSVΔG*-Reston (Fig. 5A). Recently, RESTV was detected in pigs in the Philippines and China, and more recent studies have revealed that EBOV causes respiratory disease in pigs (49–53), suggesting a role of this animal in filovirus ecology. In this study, VSVΔG*-Lloviu infected pig cells (SK-L cells) in a manner similar to that of VSVΔG*-Zaire and VSVΔG*-Reston. Although GP is not the only determinant controlling filovirus pathogenicity, our data suggest that LLOV at least meets the minimum requirements to infect pig cells.
Interestingly, neither VSVΔG*-Angola nor VSVΔG*-Musoke infected FBKT1 cells derived from the Yaeyama flying fox (Pteropus dasymallus yayeyamae), although the infectivities of these viruses in the other cell lines tested were uniformly higher than those of the other viruses (Fig. 5A). This finding suggests the existence of cellular receptors/coreceptors that interact with EBOV, RESTV, and LLOV GPs but not MARV GP. Although several cellular molecules were reported to be involved in filovirus entry (e.g., T-cell immunoglobulin domain and mucin domain 1, Tyro 3 family, C-type lectin, or Niemann-Pick C1) (11–13, 42–47, 54–57), there is only limited information on these molecules in bats. It would be of interest to clarify whether these cellular molecules play critical roles in tissue tropism and/or host range restriction of filoviruses. Alternatively, there might be a new receptor of LLOV in bats.
VSVΔG*-Lloviu infected SuBK12-08 and IndFSPT1 more efficiently than the other viruses (Fig. 5B). It should be noted that SuBK12-08 was derived from the same insectivorous bat species, Schreiber's bat (Miniopterus schreibersii), in which LLOV was first detected in Europe. However, since LLOV likely causes lethal infection of Schreiber's bat, this bat species may not serve as the natural host that can maintain this virus in nature. On the other hand, IndFSPT1 was derived from fruit bats. Considering that some species of fruit bats are suspected to be natural hosts of filoviruses (33, 58), strong tropism to this fruit bat species suggests that fruit bats also play some roles in the ecology of LLOV.
While LLOV seems to be highly pathogenic for some species of bats (e.g., Schreiber's bat), its ability to infect human and nonhuman primates and the pathogenic potential for these hosts can only be hypothesized, since infectious LLOV has never been isolated. In this study, we used a replication-incompetent VSV pseudotype system that enabled us to investigate the cellular tropism mediated by simple interaction between LLOV GP and its cellular ligands. Although a reverse genetics approach and in vivo experiments for infectious LLOV are needed to provide direct evidence of the viral pathogenicity and host specificity, our data suggest that the overall properties of LLOV GP are similar to those of the other filoviruses, and that LLOV has the potential, at least from the aspect of GP-receptor/coreceptor interaction, to infect many mammalian cells, including those of the human, monkey, and pig, with preferential tropism for some bat cells.
ACKNOWLEDGMENTS
We thank Mari Ishijima for technical assistance and Kim Barrymore for editing the manuscript.
This work was supported by KAKENHI, a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), the Japan Society for the Promotion of Science (JSPS), Japan, and partly by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) and the Japan Science and Technology Agency (JST)/Japan International Cooperation Agency (JICA) within the framework of the Science and Technology Research Partnership for Sustainable Development (SATREPS). Funding was also provided by the Program for Leading Graduate Schools from MEXT, Japan.
Footnotes
Published ahead of print 16 October 2013
REFERENCES
- 1.Feldmann H, Geisbert TW. 2011. Ebola haemorrhagic fever. Lancet 377:849–862. 10.1016/S0140-6736(10)60667-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher-Hoch SP, McCormick JB. 1999. Experimental filovirus infections. Curr. Top. Microbiol. Immunol. 235:117–143. 10.1007/978-3-642-59949-1_8 [DOI] [PubMed] [Google Scholar]
- 3.Geisbert TW, Hensley LE. 2004. Ebola virus: new insights into disease aetiopathology and possible therapeutic interventions. Expert. Rev. Mol. Med. 6:1–24. 10.1017/S1462399404008300 [DOI] [PubMed] [Google Scholar]
- 4.Negredo A, Palacios G, Vázquez-Morón S, González F, Dopazo H, Molero F, Juste J, Quetglas J, Savji N, de la Cruz Martínez M, Herrera JE, Pizarro M, Hutchison SK, Echevarría JE, Lipkin WI, Tenorio A. 2011. Discovery of an ebolavirus-like filovirus in Europe. PLoS Pathog. 7:e1002304. 10.1371/journal.ppat.1002304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott LH, Kiley MP, McCormick JB. 1985. Descriptive analysis of Ebola virus proteins. Virology 147:169–176. 10.1016/0042-6822(85)90236-3 [DOI] [PubMed] [Google Scholar]
- 6.Takada A, Robison C, Goto H, Sanchez A, Murti KG, Whitt MA, Kawaoka Y. 1997. A system for functional analysis of Ebola virus glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 94:14764–14769. 10.1073/pnas.94.26.14764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wool-Lewis RJ, Bates P. 1998. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J. Virol. 72:3155–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manicassamy B, Wang J, Rumschlag E, Tymen S, Volchkova V, Volchkov V, Rong L. 2007. Characterization of Marburg virus glycoprotein in viral entry. Virology 358:79–88. 10.1016/j.virol.2006.06.041 [DOI] [PubMed] [Google Scholar]
- 9.Geyer H, Will C, Feldmann H, Klenk HD, Geyer R. 1992. Carbohydrate structure of Marburg virus glycoprotein. Glycobiology 2:299–312. 10.1093/glycob/2.4.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldmann H, Nichol ST, Klenk HD, Peters CJ, Sanchez A. 1994. Characterization of filoviruses based on differences in structure and antigenicity of the virion glycoprotein. Virology 199:469–473. 10.1006/viro.1994.1147 [DOI] [PubMed] [Google Scholar]
- 11.Alvarez CP, Lasala F, Carrillo J, Muñiz O, Corbí AL, Delgado R. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76:6841–6844. 10.1128/JVI.76.13.6841-6844.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baribaud F, Pöhlmann S, Leslie G, Mortari F, Doms RW. 2002. Quantitative expression and virus transmission analysis of DC-SIGN on monocyte-derived dendritic cells. J. Virol. 76:9135–9142. 10.1128/JVI.76.18.9135-9142.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin G, Simmons G, Pöhlmann S, Baribaud F, Ni H, Leslie GJ, Haggarty BS, Bates P, Weissman D, Hoxie JA, Doms RW. 2003. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J. Virol. 77:1337–1346. 10.1128/JVI.77.2.1337-1346.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takada A, Ebihara H, Feldmann H, Geisbert TW, Kawaoka Y. 2007. Epitopes required for antibody-dependent enhancement of Ebola virus infection. J. Infect. Dis. 196(Suppl 2):S347–S356. 10.1086/520581 [DOI] [PubMed] [Google Scholar]
- 15.Nakayama E, Tomabechi D, Matsuno K, Kishida N, Yoshida R, Feldmann H, Takada A. 2011. Antibody-dependent enhancement of Marburg virus infection. J. Infect. Dis. 204(Suppl 3):S978–S985. 10.1093/infdis/jir334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldmann H, Mühlberger E, Randolf A, Will C, Kiley MP, Sanchez A, Klenk HD. 1992. Marburg virus, a filovirus: messenger RNAs, gene order, and regulatory elements of the replication cycle. Virus Res. 24:1–19. 10.1016/0168-1702(92)90027-7 [DOI] [PubMed] [Google Scholar]
- 17.Sakoda Y, Fukusho A. 1998. Establishment and characterization of a porcine kidney cell line, FS-L3, which forms unique multicellular domes in serum-free culture. In Vitro Cell. Dev. Biol. Anim. 34:53–57. 10.1007/s11626-998-0053-6 [DOI] [PubMed] [Google Scholar]
- 18.Maeda K, Hondo E, Terakawa J, Kiso Y, Nakaichi N, Endoh D, Sakai K, Morikawa S, Mizutani T. 2008. Isolation of novel adenovirus from fruit bat (Pteropus dasymallus yayeyamae). Emerg. Infect. Dis. 14:347–349. 10.3201/eid1402.070932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takada A, Fujioka K, Tsuiji M, Morikawa A, Higashi N, Ebihara H, Kobasa D, Feldmann H, Irimura T, Kawaoka Y. 2004. Human macrophage C-type lectin specific for galactose and N-acetylgalactosamine promotes filovirus entry. J. Virol. 78:2943–2947. 10.1128/JVI.78.6.2943-2947.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuno K, Kishida N, Usami K, Igarashi M, Yoshida R, Nakayama E, Shimojima M, Feldmann H, Irimura T, Kawaoka Y, Takada A. 2010. Different potential of C-type lectin-mediated entry between Marburg virus strains. J. Virol. 84:5140–5147. 10.1128/JVI.02021-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez A, Trappier SG, Mahy BW, Peters CJ, Nichol ST. 1996. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc. Natl. Acad. Sci. U. S. A. 93:3602–3607. 10.1073/pnas.93.8.3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volchkov VE, Becker S, Volchkova VA, Ternovoj VA, Kotov AN, Netesov SV, Klenk HD. 1995. GP mRNA of Ebola virus is edited by the Ebola virus polymerase and by T7 and vaccinia virus polymerases. Virology 214:421–430. 10.1006/viro.1995.0052 [DOI] [PubMed] [Google Scholar]
- 23.Niwa H, Yamamura K, Miyazaki J. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199. 10.1016/0378-1119(91)90434-D [DOI] [PubMed] [Google Scholar]
- 24.Changula K, Yoshida R, Noyori O, Marzi A, Miyamoto H, Ishijima M, Yokoyama A, Kajihara M, Feldmann H, Mweene AS, Takada A. Mapping of conserved and species-specific antibody epitopes on the Ebola virus nucleoprotein. Virus Res. 176:83–90. 10.1016/j.virusres.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakayama E, Yokoyama A, Miyamoto H, Igarashi M, Kishida N, Matsuno K, Marzi A, Feldmann H, Ito K, Saijo M, Takada A. 2010. Enzyme-linked immunosorbent assay for detection of filovirus species-specific antibodies. Clin. Vaccine Immunol. 17:1723–1728. 10.1128/CVI.00170-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noda T, Sagara H, Suzuki E, Takada A, Kida H, Kawaoka Y. 2002. Ebola virus VP40 drives the formation of virus-like filamentous particles along with GP. J. Virol. 76:4855–4865. 10.1128/JVI.76.10.4855-4865.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noda T, Ebihara H, Muramoto Y, Fujii K, Takada A, Sagara H, Kim JH, Kida H, Feldmann H, Kawaoka Y. 2006. Assembly and budding of ebolavirus. PLoS Pathog. 2:e99. 10.1371/journal.ppat.0020099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson RF, Bell P, Harty RN. 2006. Effect of Ebola virus proteins GP, NP and VP35 on VP40 VLP morphology. Virol. J. 3:31. 10.1186/1743-422X-3-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geisbert TW, Jahrling PB. 1995. Differentiation of filoviruses by electron microscopy. Virus Res. 39:129–150. 10.1016/0168-1702(95)00080-1 [DOI] [PubMed] [Google Scholar]
- 30.Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. 2005. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 308:1643–1645. 10.1126/science.1110656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takada A, Watanabe S, Okazaki K, Kida H, Kawaoka Y. 2001. Infectivity-enhancing antibodies to Ebola virus glycoprotein. J. Virol. 75:2324–2330. 10.1128/JVI.75.5.2324-2330.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takada A, Kawaoka Y. 2003. Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev. Med. Virol. 13:387–398. 10.1002/rmv.405 [DOI] [PubMed] [Google Scholar]
- 33.Towner JS, Amman BR, Sealy TK, Carroll SA, Comer JA, Kemp A, Swanepoel R, Paddock CD, Balinandi S, Khristova ML, Formenty PB, Albarino CG, Miller DM, Reed ZD, Kayiwa JT, Mills JN, Cannon DL, Greer PW, Byaruhanga E, Farnon EC, Atimnedi P, Okware S, Katongole-Mbidde E, Downing R, Tappero JW, Zaki SR, Ksiazek TG, Nichol ST, Rollin PE. 2009. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog. 5:e1000536. 10.1371/journal.ppat.1000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amman BR, Carroll SA, Reed ZD, Sealy TK, Balinandi S, Swanepoel R, Kemp A, Erickson BR, Comer JA, Campbell S, Cannon DL, Khristova ML, Atimnedi P, Paddock CD, Crockett RJ, Flietstra TD, Warfield KL, Unfer R, Katongole-Mbidde E, Downing R, Tappero JW, Zaki SR, Rollin PE, Ksiazek TG, Nichol ST, Towner JS. 2012. Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathog. 8:e1002877. 10.1371/journal.ppat.1002877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Towner JS, Pourrut X, Albariño CG, Nkogue CN, Bird BH, Grard G, Ksiazek TG, Gonzalez JP, Nichol ST, Leroy EM. 2007. Marburg virus infection detected in a common African bat. PLoS One 2:e764. 10.1371/journal.pone.0000764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, Délicat A, Paweska JT, Gonzalez JP, Swanepoel R. 2005. Fruit bats as reservoirs of Ebola virus. Nature 438:575–576. 10.1038/438575a [DOI] [PubMed] [Google Scholar]
- 37.Pourrut X, Souris M, Towner JS, Rollin PE, Nichol ST, Gonzalez JP, Leroy E. 2009. Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus. BMC Infect. Dis. 9:159. 10.1186/1471-2334-9-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volchkov VE, Feldmann H, Volchkova VA, Klenk HD. 1998. Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc. Natl. Acad. Sci. U. S. A. 95:5762–5767. 10.1073/pnas.95.10.5762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volchkov VE, Volchkova VA, Ströher U, Becker S, Dolnik O, Cieplik M, Garten W, Klenk HD, Feldmann H. 2000. Proteolytic processing of Marburg virus glycoprotein. Virology 268:1–6. 10.1006/viro.1999.0110 [DOI] [PubMed] [Google Scholar]
- 40.Misasi J, Chandran K, Yang JY, Considine B, Filone CM, Cote M, Sullivan N, Fabozzi G, Hensley L. 2012. Filoviruses require endosomal cysteine proteases for entry but exhibit distinct protease preferences. J. Virol. 86:3284–3292. 10.1128/JVI.06346-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marzi A, Reinheckel T, Feldmann H. 2012. Cathepsin B & L are not required for Ebola virus replication. PLoS Negl. Trop. Dis. 6:e1923. 10.1371/journal.pntd.0001923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simmons G, Reeves JD, Grogan CC, Vandenberghe LH, Baribaud F, Whitbeck JC, Burke E, Buchmeier MJ, Soilleux EJ, Riley JL, Doms RW, Bates P, Pöhlmann S. 2003. DC-SIGN and DC-SIGNR bind Ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology 305:115–123. 10.1006/viro.2002.1730 [DOI] [PubMed] [Google Scholar]
- 43.Dominguez-Soto A, Aragoneses-Fenoll L, Martin-Gayo E, Martinez-Prats L, Colmenares M, Naranjo-Gomez M, Borras FE, Munoz P, Zubiaur M, Toribio ML, Delgado R, Corbi AL. 2007. The DC-SIGN-related lectin LSECtin mediates antigen capture and pathogen binding by human myeloid cells. Blood 109:5337–5345. 10.1182/blood-2006-09-048058 [DOI] [PubMed] [Google Scholar]
- 44.Powlesland AS, Fisch T, Taylor ME, Smith DF, Tissot B, Dell A, Pöhlmann S, Drickamer K. 2008. A novel mechanism for LSECtin binding to Ebola virus surface glycoprotein through truncated glycans. J. Biol. Chem. 283:593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuno K, Nakayama E, Noyori O, Marzi A, Ebihara H, Irimura T, Feldmann H, Takada A. 2010. C-type lectins do not act as functional receptors for filovirus entry into cells. Biochem. Biophys. Res. Commun. 403:144–148. 10.1016/j.bbrc.2010.10.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marzi A, Möller P, Hanna SL, Harrer T, Eisemann J, Steinkasserer A, Becker S, Baribaud F, Pöhlmann S. 2007. Analysis of the interaction of Ebola virus glycoprotein with DC-SIGN (dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin) and its homologue DC-SIGNR. J. Infect. Dis. 196(Suppl 2):S237–S246. 10.1086/520607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feinberg H, Mitchell DA, Drickamer K, Weis WI. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294:2163–2166. 10.1126/science.1066371 [DOI] [PubMed] [Google Scholar]
- 48.Higashi N, Fujioka K, Denda-Nagai K, Hashimoto S, Nagai S, Sato T, Fujita Y, Morikawa A, Tsuiji M, Miyata-Takeuchi M, Sano Y, Suzuki N, Yamamoto K, Matsushima K, Irimura T. 2002. The macrophage C-type lectin specific for galactose/N-acetylgalactosamine is an endocytic receptor expressed on monocyte-derived immature dendritic cells. J. Biol. Chem. 277:20686–20693. 10.1074/jbc.M202104200 [DOI] [PubMed] [Google Scholar]
- 49.Suzuki N, Yamamoto K, Toyoshima S, Osawa T, Irimura T. 1996. Molecular cloning and expression of cDNA encoding human macrophage C-type lectin. Its unique carbohydrate binding specificity for Tn antigen. J. Immunol. 156:128–135 [PubMed] [Google Scholar]
- 50.Barrette RW, Metwally SA, Rowland JM, Xu L, Zaki SR, Nichol ST, Rollin PE, Towner JS, Shieh WJ, Batten B, Sealy TK, Carrillo C, Moran KE, Bracht AJ, Mayr GA, Sirios-Cruz M, Catbagan DP, Lautner EA, Ksiazek TG, White WR, McIntosh MT. 2009. Discovery of swine as a host for the Reston ebolavirus. Science 325:204–206. 10.1126/science.1172705 [DOI] [PubMed] [Google Scholar]
- 51.Marsh GA, Haining J, Robinson R, Foord A, Yamada M, Barr JA, Payne J, White J, Yu M, Bingham J, Rollin PE, Nichol ST, Wang LF, Middleton D. 2011. Ebola Reston virus infection of pigs: clinical significance and transmission potential. J. Infect. Dis. 204(Suppl 3):S804–S809. 10.1093/infdis/jir300 [DOI] [PubMed] [Google Scholar]
- 52.Pan Y, Zhang W, Cui L, Hua X, Wang M, Zeng Q. 21 September 2012. Reston virus in domestic pigs in China. Arch. Virol. 10.1007/s00705-012-1477-6 [DOI] [PubMed] [Google Scholar]
- 53.Nfon CK, Leung A, Smith G, Embury-Hyatt C, Kobinger G, Weingartl HM. 2013. Immunopathogenesis of severe acute respiratory disease in Zaire ebolavirus-infected pigs. PLoS One 8:e61904. 10.1371/journal.pone.0061904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kondratowicz AS, Lennemann NJ, Sinn PL, Davey RA, Hunt CL, Moller-Tank S, Meyerholz DK, Rennert P, Mullins RF, Brindley M, Sandersfeld LM, Quinn K, Weller M, McCray PB, Jr, Chiorini J, Maury W. 2011. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc. Natl. Acad. Sci. U. S. A. 108:8426–8431. 10.1073/pnas.1019030108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimojima M, Takada A, Ebihara H, Neumann G, Fujioka K, Irimura T, Jones S, Feldmann H, Kawaoka Y. 2006. Tyro3 family-mediated cell entry of Ebola and Marburg viruses. J. Virol. 80:10109–10116. 10.1128/JVI.01157-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Côté M, Misasi J, Ren T, Bruchez A, Lee K, Filone CM, Hensley L, Li Q, Ory D, Chandran K, Cunningham J. 2011. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature 477:344–348. 10.1038/nature10380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, Mulherkar N, Kuehne AI, Kranzusch PJ, Griffin AM, Ruthel G, Dal Cin P, Dye JM, Whelan SP, Chandran K, Brummelkamp TR. 2011. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 477:340–343. 10.1038/nature10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swanepoel R, Smit SB, Rollin PE, Formenty P, Leman PA, Kemp A, Burt FJ, Grobbelaar AA, Croft J, Bausch DG, Zeller H, Leirs H, Braack LE, Libande ML, Zaki S, Nichol ST, Ksiazek TG, Paweska JT. 2007. Studies of reservoir hosts for Marburg virus. Emerg. Infect. Dis. 13:1847–1851. 10.3201/eid1312.071115 [DOI] [PMC free article] [PubMed] [Google Scholar]