Abstract
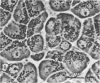
Catecholamines cause rapid release of K+ and formation of vacuoles in acinar gland cells. A high K+ concentration in the medium bathing parotid gland slices prevents vacuole formation by epinephrine and facilitates the secretion of most of the exportable amylase. N6-monobutyryl 3′:5′-cyclic AMP does not cause K+ release and vacuole formation although it efficiently induces amylase secretion. It is suggested that the secretory process is independent of vacuole formation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam A., Ohad I., Schramm M. Dynamic changes in the ultrastructure of the acinar cell of the rat parotid gland during the secretory cycle. J Cell Biol. 1969 Jun;41(3):753–773. doi: 10.1083/jcb.41.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BDOLAH A., SCHRAMM M. THE FUNCTION OF 3'5' CYCLIC AMP IN ENZYME SECRETION. Biochem Biophys Res Commun. 1965 Feb 3;18:452–454. doi: 10.1016/0006-291x(65)90730-8. [DOI] [PubMed] [Google Scholar]
- Babad H., Ben-Zvi R., Bdolah A., Schramm M. The mechanism of enzyme secretion by the cell. 4. Effects of inducers, substrates and inhibitors on amylase secretion by rat parotid slices. Eur J Biochem. 1967 Mar;1(1):96–101. doi: 10.1111/j.1432-1033.1967.tb00049.x. [DOI] [PubMed] [Google Scholar]
- D'Silva J. L. The action of adrenaline on serum potassium. J Physiol. 1936 Feb 8;86(2):219–228. doi: 10.1113/jphysiol.1936.sp003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS S., BECKETT S. B. MECHANISM OF THE POTASSIUM MOBILIZING ACTION OF EPINEPHRINE AND GLUCAGON. J Pharmacol Exp Ther. 1963 Dec;142:318–326. [PubMed] [Google Scholar]
- Feinstein H., Schramm M. Energy production in rat parotid gland. Relation tonzyme secretion and effects of caium. Eur J Biochem. 1970 Mar 1;13(1):158–163. doi: 10.1111/j.1432-1033.1970.tb00912.x. [DOI] [PubMed] [Google Scholar]
- Friedmann N., Park C. R. Early effects of 3',5'-adenosine monophosphate on the fluxes of calcium end potassium in the perfused liver of normal and adrenalectomized rats. Proc Natl Acad Sci U S A. 1968 Oct;61(2):504–508. doi: 10.1073/pnas.61.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm M. Amylase secretion in rat parotid slices by apparent activation of endogenous catecholamine. Biochim Biophys Acta. 1968 Oct 15;165(3):546–549. doi: 10.1016/0304-4165(68)90238-9. [DOI] [PubMed] [Google Scholar]
- Schramm M., Naim E. Adenyl cyclase of rat parotid gland. Activation by fluoride and norepinephrine. J Biol Chem. 1970 Jun;245(12):3225–3231. [PubMed] [Google Scholar]
- Tapp R. L., Trowell O. A. The experimental production of watery vacuolation in the acinar cells of the submandibular gland. J Physiol. 1967 Jan;188(2):191–205. doi: 10.1113/jphysiol.1967.sp008133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowell O. A. The experimental production of watery vacuolation of the liver. J Physiol. 1946 Dec 6;105(3):268–297. [PMC free article] [PubMed] [Google Scholar]